User login
Underlying Mental Illness and Risk of Severe Outcomes Associated With COVID-19
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
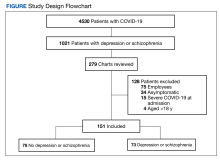
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
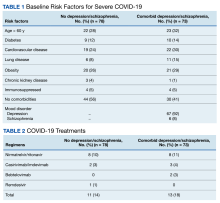
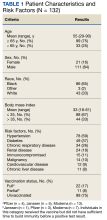
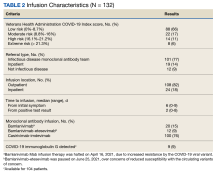
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
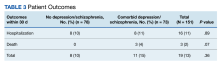
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
How VA Innovative Partnerships and Health Care Systems Can Respond to National Needs: NOSE Trial Example
Traditional manufacturing concentrates capacity into a few discrete locations while applying lean and just-in-time philosophies to maximize profit during times of somewhat predictable supply and demand. This approach exposed nationwide vulnerabilities even during local crises, such as the United States saline shortages following closure of a single plant in Puerto Rico following Hurricane Maria in 2017.1 Interruptions to the supply chain due to pandemic plant closure, weather, politics, or surge demand can cause immediate and lasting shortages. Nasal swabs were a clear example.
At the onset of COVID-19, 2 companies—Puritan in Guilford, Maine, and Copan in Italy—manufactured nearly all of the highly specialized nasopharyngeal (NP) swabs singled out by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) to test patients for COVID-19. Demand for swabs skyrocketed as the virus spread, and they became unattainable. The lack of swabs meant patients went undiagnosed. Without knowing who was positive, people with symptoms and known contacts were presumed positive and quarantined, impacting isolated patients, the health care professionals treating them, and the entire US economy.
3-Dimensional Printing Solutions
Manufacturing NP swabs is not trivial. Their simple shape conceals complexity and requires highly specialized equipment. The lead time for one non-US machine manufacturer was > 6 months at the start of the pandemic.
Digital manufacturing/3-dimensional (3D) printing represented a potential solution to the supply chain crisis.2 Designers created digital blueprints for 3D-printed goods, face masks, face shields, and ventilator splitters were rapidly created and shared.3,4 Scrambling to fill the critical need for NP swabs, many hospitals, businesses, and academic centers began 3D printing swabs. This effort was spearheaded by University of South Florida (USF) and Northwell Health researchers and clinicians, who designed and tested a 3D-printed NP swab from photocurable resin that was printable on 2 models of Formlabs printers.5 Several other 3D-printed NP swab designs soon followed. This innovation and problem-solving renaissance faced several challenges well known to traditional manufacturers of regulated products but novel to newcomers.
The first challlenge was that these NP swabs predate FDA oversight of medical device development and manufacturing and no testing standards existed. Designers began casting prototypes out without guidance about the critical features and clinical functions required. Many of these designs did not have a clinical evaluation pathway to test safety and efficacy.
The second challlenge was that these swabs were being produced by facilities not registered with the FDA. This raised concerns about the quality of unlisted medical products developed and manufactured at novel facilities.
The third challenge was that small-scale novel approaches may offset local shortages but could not address national needs. The self-organized infrastructure for this crisis was ad hoc, local, and lacked coordinated federal support. This led to rolling shortages of these materials for years.
Two studies were performed early in the pandemic. The first study evaluated 4 prototypes of different manufacturer designs, finding excellent concordance among them and their control swab.6 A second study demonstrated the USF swab to be noninferior to the standard of care.7 Both studies acknowledged and addressed the first challenge for their designs.
COLLABORATIONS
Interagency
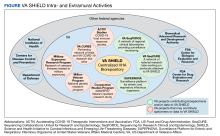
Before the pandemic, the US Department of Veterans Affairs (VA) had been coordinating with the FDA, the National Institutes of Health (NIH), and the nonprofit America Makes to bring medical product development and manufacturing closer to the point of care.
At the outset of the COVID-19 pandemic, the collaboration was formalized to address new challenges.8 The objectives of this collaboration were the following: (1) host a digital repository for 3D-printed digital designs for personal protectice equipment and other medical supplies in or at risk of shortage; (2) provide scientifically based ratings for designs according to clinical and field testing; and (3) offer education to health care workers and the public about the digital manufacturing of medical goods and devices.4,9
A key output of this collaboration was the COVID 3D Trusted Repository For Users And Suppliers Through Testing (COVID 3D TRUST), a curated archive of designs. In most cases, existing FDA standards and guidance formed the basis of testing strategies with deviations due to limited access to traditional testing facilities and reagents.
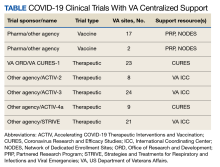
To address novel NP swabs, working with its COVID 3D TRUST partners, the VA gathered a combined list of clinical- and engineering-informed customer requirements and performed a hazard analysis. The result was a list of design inputs for NP swabs and 8 standard test protocols to evaluate key functions (Table).10 These protocols are meant to benchmark novel 3D-printed swabs against the key functions of established, traditionally manufactured swabs, which have a long record of safety and efficacy. The protocols, developed by the VA and undergoing validation by the US Army, empower and inform consumers and provide performance metrics to swab designers and manufacturers. The testing protocols and preliminary test results developed by the VA are publicly available at the NIH.11
Intra-agency
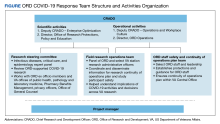
The use of the inputs and verification tests noted in the Table may reduce the risk of poor design but were inadequate to evaluate the clinical safety and efficacy of novel swabs. Recognizing this, the VA Office of Healthcare Innovation and Learning (OHIL) and the Office of Research and Development (ORD) launched the Nasal Swab Objective and Statistical Evaluation (NOSE) study to formally evaluate the safety and efficacy of 3D-printed swabs in the field. This multisite clinical study was a close collaboration between the OHIL and ORD. The OHIL provided the quality system and manufacturing oversight and delivery of the swabs, and the ORD provided scientific review, research infrastructure, human subjects oversight, administrative support, and funding and fiscal oversight. The OHIL/ORD collaboration resulted in the successful completion of the NOSE study.
This study (manuscript under preparation) yielded two 3D-printing production processes and swab designs that had comparable performance to the standard of care, were manufacturable compliant with FDA guidelines, and could be produced at scale in a distributed manner. This approach directly addressed the 3 challenges described earlier.
LESSONS LEARNED
Swabs were an example of supply challenges in the pandemic, but advanced manufacturing (notably, digital designs leading to 3D-printed solutions) also served as a temporary solution to device and product shortages during the COVID-19 pandemic. Digital designs and 3D printing as manufacturing techniques have the following key advantages: (1) they are distributed in nature, both in the breadth of locations that have access to these manufacturing platforms and in the depth of material choice that can be used to fabricate products, which alleviates the threat of a disaster impacting manufacturing capacity or a material stream; (2) they do not require retooling of machinery so new products can deploy rapidly and on demand; and (3) the speed of digital iteration, printing, and revision allows for rapid product development and production.
There also are notable disadvantages to these techniques. First, because 3D printing is a newer technology, there is less general depth of knowledge regarding design and material choice for additive manufacturing. Second, the flexibility of 3D printing means that operators must increase awareness of the factors that might cause the fabrication of a part to fail in either printing or postprocessing. Third, there are significant gaps in understanding how materials and manufacturing processes will perform in high-stakes settings such as health care, where performance and biocompatibility may be critical to support life-sustaining functions. Fourth, digital files are vulnerable to intentional or unintentional alteration. These alterations might weaken design integrity and be imperceptible to the manufacturer or end user. This is a prevalent challenge in all open-source designs.
The pandemic materialized quickly and created vast supply chain challenges. To address this crisis, it was clear that the average 17-year interval between research and translation in the US was unacceptable. The VA was able to accelerate swiftly many existing processes to meet this need, build new capabilities, and establish new practices for the rapid evaluation and deployment of health care products and guidance. This agile and innovative cooperation was critical in the success of the VA’s national support for pandemic solutions.
Finally, although COVID 3D TRUST was able to provide testing of submitted designs, this collaboration was not a substitute for the “peacetime” process of manufacturing site registration with the FDA and product listing. COVID 3D TRUST could evaluate designs only, not the production process, safety, and efficacy.
CALLS TO ACTION
The pandemic's impact on medical supply chain security persists, as does the need for greater foresight and crisis preparation. We must act now to avoid experiencing again the magnitude of fatalities (civilian and veteran) and the devastation to the US economy and livelihoods that occurred during this single biological event. To this end, creating a digital stockpile of federally curated, crisis-ready designs for as-needed distribution across our US industrial base would offer a second line of defense against life-threatening supply chain interruptions. The realization of such a digital stockpile requires calls to action among multiple contributors.
Collaborations
The VA’s Fourth Mission is to improve the nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters. The VA does this by developing plans and taking actions to ensure continued service to veterans, as well as to support national, state, and local emergency management, public health, safety, and homeland security efforts.
The VA partnership with the FDA and NIH during the pandemic enabled successful coordination among federal agencies. Numerous other agencies, including the US Department of Defense (DoD), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Advanced Research Projects Agency (DARPA), also developed and executed successful initiatives.12-14 The joint awareness and management of these efforts, however, could be strengthened through more formal agreements and processes in peacetime. The VA/FDA/NIH Memorandum of Understanding is a prototype example of each agency lending its subject matter expertise to address a host of pandemic challenges collectively, cooperatively, and efficiently.8
Public-private partnerships (eg, VA/FDA/NIH and America Makes) led to coordinated responses for crisis readiness. The Advanced Manufacturing Crisis Product Response Program, a multipartner collaboration that included VA, addressed 7 crisis scenarios, 3 of which were specifically related to COVID-19.15 In addition, both BARDA and DARPA had successful public-private collaborations, and the DoD supported national logistics and other efforts.12-14 Clearly, industry and government both recognize complementary synergies: (1) the depth of resources of US industry; and (2) the national resources, coordination, and clinical insight available through federal agencies that can address the challenges of future crises quickly and efficiently.
When traditional supply chains and manufacturing processes failed during the pandemic, new techniques were exploited to fill the unmet material needs. Novel techniques and product pathways, however, are untested or undeveloped. The collaboration between the ORD and OHIL in support of NP swab testing and production is an example of bringing research insight, regulated product development, and manufacturing together to support a complete product life cycle.
Joint Awareness and Management
The VA continues to refine the joint awareness and management (JAM) process of products from ideation to translation, to shorten the time from research to product delivery. JAM is a VA collaborative committee of partners from ORD research offices and technology transfer program, and the OHIL Office of Advanced Manufacturing, which seeks additional support and guidance from VHA clinical service lines, VA Office of General Council, and VA Office of Acquisitions, Logistics, and Construction.
This team enables the rapid identification of unmet veteran health care product needs. In addition, JAM leverages the resources of each group to support products from problem identification to solution ideation, regulated development, production, and delivery into clinical service lines. While the concept of JAM arose to meet the crisis needs of the pandemic, it persists in delivering advanced health care solutions to veterans.
A Proposed Plan
The next national crisis is likely to involve and threaten national health care security. We propose that federal agencies be brought together to form a federally supported digital stockpile. This digital stockpile must encompass, at minimum, the following features: (1) preservation of novel, scalable medical supplies and products generated during the COVID-19 pandemic, to avoid the loss of this work; (2) clinical maturation of those existing supplies and products to refine their features and functions under the guidance of clinical, regulatory, and manufacturing experts—and validate those outputs with clinical evidence; (3) manufacturing maturation of those existing supplies and products, such that complete design and production processes are developed with the intent to distribute to multiple public manufacturers during the next crisis; (4) a call for new designs/intake portal for new designs to be matured and curated as vulnerabilities are identified; (5) supply chain crisis drills executed to test public-private preparedness to ensure design transfer is turnkey and can be engaged quickly during the next crisis; and (6) public-private engagement to develop strategy, scenarios, and policy to ensure that when supply chains next fail, additional surge capacity can be quickly added to protect American lives and health care, and that when supply chains resume, surge capacity can be redirected or stood down to protect the competitive markets.
This digital stockpile can complement and be part of the Strategic National Stockpile. Whereas the Strategic National Stockpile is a reserve of physical products that may offset product shortages, the digital stockpile is a reserve of turnkey, transferable designs that may offset supply chain disruptions and production-capacity shortages.
CONCLUSIONS
The success of 3D-printed NP swabs is a specific example of the importance of collaborations across industry, government, innovators, and researchers. More important than a sole product, however, these collaborations demonstrated the potential for game-changing approaches to how public-private partnerships support the continuity of health care operations nationally and prevent the potential for unnecessary loss of life due to capacity and supply chain disruptions.
As the largest health care system in the US, the VA has a unique capability to lead in the assessment of other novel 3D-printed medical devices in partnership with the FDA. The VA has a unique patient-centered perspective on medical device efficacy, and as a government institution, it is a trusted independent source for medical device evaluation. The VA’s role in the evaluation of 3D-printed medical devices will benefit veterans and their families, clinicians, hospitals, and the broader public by providing a gold-standard evaluation for the growing medical 3D-printing industry to follow. By creating new pathways and expectations for how federal agencies maintain crisis preparedness—such as establishing a digital stockpile—we can be equipped to serve the US health care system and minimize the effects of supply chain disruptions.
1. Sacks CA, Kesselheim AS, Fralick M. The shortage of normal saline in the wake of Hurricane Maria. JAMA Intern Med. 2018;178(7):885–886. doi:10.1001/jamainternmed.2018.1936
2. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment–a call for ideas. JAMA. 2020;323(19):1911. doi:10.1001/jama.2020.4770
3. Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110(8):1162-1164. doi:10.2105/AJPH.2020.305753
4. McCarthy MC, Di Prima M, Cruz P, et al. Trust in the time of Covid-19: 3D printing and additive manufacturing (3DP/AM) as a solution to supply chain gaps. NEJM Catalyst. 2021;2(6). doi:10.1056/CAT.21.0321
5. Ford J, Goldstein T, Trahan S, Neuwirth A, Tatoris K, Decker S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med. 2020;6(1):21. Published 2020 Aug 15. doi:10.1186/s41205-020-00076-3
6. Callahan CJ, Lee R, Zulauf K, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Preprint. medRxiv. 2020;2020.04.14.20065094. Published 2020 Apr 17. doi:10.1101/2020.04.14.20065094
7. Decker SJ, Goldstein TA, Ford JM, et al. 3-dimensional printed alternative to the standard synthetic flocked nasopharyngeal swabs used for coronavirus disease 2019 testing. Clin Infect Dis. 2021;73(9):e3027-e3032. doi:10.1093/cid/ciaa1366
8. US Food and Drug Administration. Memorandum of understanding: rapid response to Covid-19 using 3d printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration, U.S. Department of Health and Human Services and Veterans Health Administration within the U.S. Department of Veterans Affairs. March 26, 2020. Accessed August 31, 2023. https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008
9. National Institutes of Health, NIH 3D Print Exchange. Covid 3D trust: trusted repository for users and suppliers through testing. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=search
10. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - assessment criteria. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabassessment
11. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - general information. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabinfo
12. US Department of Defense. Coronavirus: DOD response. December 20, 2022. Accessed August 31, 2023. https://www.defense.gov/Spotlights/Coronavirus-DoD-Response
13. US Department of Health and Human Services, Biomedical Advanced Research and Development Authority. BARDA COVID-19 response. Updated May 25, 2023. Accessed August 31, 2023. https://www.medicalcountermeasures.gov/barda/barda-covid-19-response
14. Green S. Pandemic prevention platform (P3). Accessed August 31, 2023. https://www.darpa.mil/program/pandemic-prevention-platform
15. America Makes. America makes completes successful scenario testing for crisis response program [press release]. May 25, 2021. Accessed August 31, 2023. https://www.americamakes.us/america-makes-completes-successful-scenario-testing-for-crisis-response-program
Traditional manufacturing concentrates capacity into a few discrete locations while applying lean and just-in-time philosophies to maximize profit during times of somewhat predictable supply and demand. This approach exposed nationwide vulnerabilities even during local crises, such as the United States saline shortages following closure of a single plant in Puerto Rico following Hurricane Maria in 2017.1 Interruptions to the supply chain due to pandemic plant closure, weather, politics, or surge demand can cause immediate and lasting shortages. Nasal swabs were a clear example.
At the onset of COVID-19, 2 companies—Puritan in Guilford, Maine, and Copan in Italy—manufactured nearly all of the highly specialized nasopharyngeal (NP) swabs singled out by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) to test patients for COVID-19. Demand for swabs skyrocketed as the virus spread, and they became unattainable. The lack of swabs meant patients went undiagnosed. Without knowing who was positive, people with symptoms and known contacts were presumed positive and quarantined, impacting isolated patients, the health care professionals treating them, and the entire US economy.
3-Dimensional Printing Solutions
Manufacturing NP swabs is not trivial. Their simple shape conceals complexity and requires highly specialized equipment. The lead time for one non-US machine manufacturer was > 6 months at the start of the pandemic.
Digital manufacturing/3-dimensional (3D) printing represented a potential solution to the supply chain crisis.2 Designers created digital blueprints for 3D-printed goods, face masks, face shields, and ventilator splitters were rapidly created and shared.3,4 Scrambling to fill the critical need for NP swabs, many hospitals, businesses, and academic centers began 3D printing swabs. This effort was spearheaded by University of South Florida (USF) and Northwell Health researchers and clinicians, who designed and tested a 3D-printed NP swab from photocurable resin that was printable on 2 models of Formlabs printers.5 Several other 3D-printed NP swab designs soon followed. This innovation and problem-solving renaissance faced several challenges well known to traditional manufacturers of regulated products but novel to newcomers.
The first challlenge was that these NP swabs predate FDA oversight of medical device development and manufacturing and no testing standards existed. Designers began casting prototypes out without guidance about the critical features and clinical functions required. Many of these designs did not have a clinical evaluation pathway to test safety and efficacy.
The second challlenge was that these swabs were being produced by facilities not registered with the FDA. This raised concerns about the quality of unlisted medical products developed and manufactured at novel facilities.
The third challenge was that small-scale novel approaches may offset local shortages but could not address national needs. The self-organized infrastructure for this crisis was ad hoc, local, and lacked coordinated federal support. This led to rolling shortages of these materials for years.
Two studies were performed early in the pandemic. The first study evaluated 4 prototypes of different manufacturer designs, finding excellent concordance among them and their control swab.6 A second study demonstrated the USF swab to be noninferior to the standard of care.7 Both studies acknowledged and addressed the first challenge for their designs.
COLLABORATIONS
Interagency
Before the pandemic, the US Department of Veterans Affairs (VA) had been coordinating with the FDA, the National Institutes of Health (NIH), and the nonprofit America Makes to bring medical product development and manufacturing closer to the point of care.
At the outset of the COVID-19 pandemic, the collaboration was formalized to address new challenges.8 The objectives of this collaboration were the following: (1) host a digital repository for 3D-printed digital designs for personal protectice equipment and other medical supplies in or at risk of shortage; (2) provide scientifically based ratings for designs according to clinical and field testing; and (3) offer education to health care workers and the public about the digital manufacturing of medical goods and devices.4,9
A key output of this collaboration was the COVID 3D Trusted Repository For Users And Suppliers Through Testing (COVID 3D TRUST), a curated archive of designs. In most cases, existing FDA standards and guidance formed the basis of testing strategies with deviations due to limited access to traditional testing facilities and reagents.
To address novel NP swabs, working with its COVID 3D TRUST partners, the VA gathered a combined list of clinical- and engineering-informed customer requirements and performed a hazard analysis. The result was a list of design inputs for NP swabs and 8 standard test protocols to evaluate key functions (Table).10 These protocols are meant to benchmark novel 3D-printed swabs against the key functions of established, traditionally manufactured swabs, which have a long record of safety and efficacy. The protocols, developed by the VA and undergoing validation by the US Army, empower and inform consumers and provide performance metrics to swab designers and manufacturers. The testing protocols and preliminary test results developed by the VA are publicly available at the NIH.11
Intra-agency
The use of the inputs and verification tests noted in the Table may reduce the risk of poor design but were inadequate to evaluate the clinical safety and efficacy of novel swabs. Recognizing this, the VA Office of Healthcare Innovation and Learning (OHIL) and the Office of Research and Development (ORD) launched the Nasal Swab Objective and Statistical Evaluation (NOSE) study to formally evaluate the safety and efficacy of 3D-printed swabs in the field. This multisite clinical study was a close collaboration between the OHIL and ORD. The OHIL provided the quality system and manufacturing oversight and delivery of the swabs, and the ORD provided scientific review, research infrastructure, human subjects oversight, administrative support, and funding and fiscal oversight. The OHIL/ORD collaboration resulted in the successful completion of the NOSE study.
This study (manuscript under preparation) yielded two 3D-printing production processes and swab designs that had comparable performance to the standard of care, were manufacturable compliant with FDA guidelines, and could be produced at scale in a distributed manner. This approach directly addressed the 3 challenges described earlier.
LESSONS LEARNED
Swabs were an example of supply challenges in the pandemic, but advanced manufacturing (notably, digital designs leading to 3D-printed solutions) also served as a temporary solution to device and product shortages during the COVID-19 pandemic. Digital designs and 3D printing as manufacturing techniques have the following key advantages: (1) they are distributed in nature, both in the breadth of locations that have access to these manufacturing platforms and in the depth of material choice that can be used to fabricate products, which alleviates the threat of a disaster impacting manufacturing capacity or a material stream; (2) they do not require retooling of machinery so new products can deploy rapidly and on demand; and (3) the speed of digital iteration, printing, and revision allows for rapid product development and production.
There also are notable disadvantages to these techniques. First, because 3D printing is a newer technology, there is less general depth of knowledge regarding design and material choice for additive manufacturing. Second, the flexibility of 3D printing means that operators must increase awareness of the factors that might cause the fabrication of a part to fail in either printing or postprocessing. Third, there are significant gaps in understanding how materials and manufacturing processes will perform in high-stakes settings such as health care, where performance and biocompatibility may be critical to support life-sustaining functions. Fourth, digital files are vulnerable to intentional or unintentional alteration. These alterations might weaken design integrity and be imperceptible to the manufacturer or end user. This is a prevalent challenge in all open-source designs.
The pandemic materialized quickly and created vast supply chain challenges. To address this crisis, it was clear that the average 17-year interval between research and translation in the US was unacceptable. The VA was able to accelerate swiftly many existing processes to meet this need, build new capabilities, and establish new practices for the rapid evaluation and deployment of health care products and guidance. This agile and innovative cooperation was critical in the success of the VA’s national support for pandemic solutions.
Finally, although COVID 3D TRUST was able to provide testing of submitted designs, this collaboration was not a substitute for the “peacetime” process of manufacturing site registration with the FDA and product listing. COVID 3D TRUST could evaluate designs only, not the production process, safety, and efficacy.
CALLS TO ACTION
The pandemic's impact on medical supply chain security persists, as does the need for greater foresight and crisis preparation. We must act now to avoid experiencing again the magnitude of fatalities (civilian and veteran) and the devastation to the US economy and livelihoods that occurred during this single biological event. To this end, creating a digital stockpile of federally curated, crisis-ready designs for as-needed distribution across our US industrial base would offer a second line of defense against life-threatening supply chain interruptions. The realization of such a digital stockpile requires calls to action among multiple contributors.
Collaborations
The VA’s Fourth Mission is to improve the nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters. The VA does this by developing plans and taking actions to ensure continued service to veterans, as well as to support national, state, and local emergency management, public health, safety, and homeland security efforts.
The VA partnership with the FDA and NIH during the pandemic enabled successful coordination among federal agencies. Numerous other agencies, including the US Department of Defense (DoD), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Advanced Research Projects Agency (DARPA), also developed and executed successful initiatives.12-14 The joint awareness and management of these efforts, however, could be strengthened through more formal agreements and processes in peacetime. The VA/FDA/NIH Memorandum of Understanding is a prototype example of each agency lending its subject matter expertise to address a host of pandemic challenges collectively, cooperatively, and efficiently.8
Public-private partnerships (eg, VA/FDA/NIH and America Makes) led to coordinated responses for crisis readiness. The Advanced Manufacturing Crisis Product Response Program, a multipartner collaboration that included VA, addressed 7 crisis scenarios, 3 of which were specifically related to COVID-19.15 In addition, both BARDA and DARPA had successful public-private collaborations, and the DoD supported national logistics and other efforts.12-14 Clearly, industry and government both recognize complementary synergies: (1) the depth of resources of US industry; and (2) the national resources, coordination, and clinical insight available through federal agencies that can address the challenges of future crises quickly and efficiently.
When traditional supply chains and manufacturing processes failed during the pandemic, new techniques were exploited to fill the unmet material needs. Novel techniques and product pathways, however, are untested or undeveloped. The collaboration between the ORD and OHIL in support of NP swab testing and production is an example of bringing research insight, regulated product development, and manufacturing together to support a complete product life cycle.
Joint Awareness and Management
The VA continues to refine the joint awareness and management (JAM) process of products from ideation to translation, to shorten the time from research to product delivery. JAM is a VA collaborative committee of partners from ORD research offices and technology transfer program, and the OHIL Office of Advanced Manufacturing, which seeks additional support and guidance from VHA clinical service lines, VA Office of General Council, and VA Office of Acquisitions, Logistics, and Construction.
This team enables the rapid identification of unmet veteran health care product needs. In addition, JAM leverages the resources of each group to support products from problem identification to solution ideation, regulated development, production, and delivery into clinical service lines. While the concept of JAM arose to meet the crisis needs of the pandemic, it persists in delivering advanced health care solutions to veterans.
A Proposed Plan
The next national crisis is likely to involve and threaten national health care security. We propose that federal agencies be brought together to form a federally supported digital stockpile. This digital stockpile must encompass, at minimum, the following features: (1) preservation of novel, scalable medical supplies and products generated during the COVID-19 pandemic, to avoid the loss of this work; (2) clinical maturation of those existing supplies and products to refine their features and functions under the guidance of clinical, regulatory, and manufacturing experts—and validate those outputs with clinical evidence; (3) manufacturing maturation of those existing supplies and products, such that complete design and production processes are developed with the intent to distribute to multiple public manufacturers during the next crisis; (4) a call for new designs/intake portal for new designs to be matured and curated as vulnerabilities are identified; (5) supply chain crisis drills executed to test public-private preparedness to ensure design transfer is turnkey and can be engaged quickly during the next crisis; and (6) public-private engagement to develop strategy, scenarios, and policy to ensure that when supply chains next fail, additional surge capacity can be quickly added to protect American lives and health care, and that when supply chains resume, surge capacity can be redirected or stood down to protect the competitive markets.
This digital stockpile can complement and be part of the Strategic National Stockpile. Whereas the Strategic National Stockpile is a reserve of physical products that may offset product shortages, the digital stockpile is a reserve of turnkey, transferable designs that may offset supply chain disruptions and production-capacity shortages.
CONCLUSIONS
The success of 3D-printed NP swabs is a specific example of the importance of collaborations across industry, government, innovators, and researchers. More important than a sole product, however, these collaborations demonstrated the potential for game-changing approaches to how public-private partnerships support the continuity of health care operations nationally and prevent the potential for unnecessary loss of life due to capacity and supply chain disruptions.
As the largest health care system in the US, the VA has a unique capability to lead in the assessment of other novel 3D-printed medical devices in partnership with the FDA. The VA has a unique patient-centered perspective on medical device efficacy, and as a government institution, it is a trusted independent source for medical device evaluation. The VA’s role in the evaluation of 3D-printed medical devices will benefit veterans and their families, clinicians, hospitals, and the broader public by providing a gold-standard evaluation for the growing medical 3D-printing industry to follow. By creating new pathways and expectations for how federal agencies maintain crisis preparedness—such as establishing a digital stockpile—we can be equipped to serve the US health care system and minimize the effects of supply chain disruptions.
Traditional manufacturing concentrates capacity into a few discrete locations while applying lean and just-in-time philosophies to maximize profit during times of somewhat predictable supply and demand. This approach exposed nationwide vulnerabilities even during local crises, such as the United States saline shortages following closure of a single plant in Puerto Rico following Hurricane Maria in 2017.1 Interruptions to the supply chain due to pandemic plant closure, weather, politics, or surge demand can cause immediate and lasting shortages. Nasal swabs were a clear example.
At the onset of COVID-19, 2 companies—Puritan in Guilford, Maine, and Copan in Italy—manufactured nearly all of the highly specialized nasopharyngeal (NP) swabs singled out by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) to test patients for COVID-19. Demand for swabs skyrocketed as the virus spread, and they became unattainable. The lack of swabs meant patients went undiagnosed. Without knowing who was positive, people with symptoms and known contacts were presumed positive and quarantined, impacting isolated patients, the health care professionals treating them, and the entire US economy.
3-Dimensional Printing Solutions
Manufacturing NP swabs is not trivial. Their simple shape conceals complexity and requires highly specialized equipment. The lead time for one non-US machine manufacturer was > 6 months at the start of the pandemic.
Digital manufacturing/3-dimensional (3D) printing represented a potential solution to the supply chain crisis.2 Designers created digital blueprints for 3D-printed goods, face masks, face shields, and ventilator splitters were rapidly created and shared.3,4 Scrambling to fill the critical need for NP swabs, many hospitals, businesses, and academic centers began 3D printing swabs. This effort was spearheaded by University of South Florida (USF) and Northwell Health researchers and clinicians, who designed and tested a 3D-printed NP swab from photocurable resin that was printable on 2 models of Formlabs printers.5 Several other 3D-printed NP swab designs soon followed. This innovation and problem-solving renaissance faced several challenges well known to traditional manufacturers of regulated products but novel to newcomers.
The first challlenge was that these NP swabs predate FDA oversight of medical device development and manufacturing and no testing standards existed. Designers began casting prototypes out without guidance about the critical features and clinical functions required. Many of these designs did not have a clinical evaluation pathway to test safety and efficacy.
The second challlenge was that these swabs were being produced by facilities not registered with the FDA. This raised concerns about the quality of unlisted medical products developed and manufactured at novel facilities.
The third challenge was that small-scale novel approaches may offset local shortages but could not address national needs. The self-organized infrastructure for this crisis was ad hoc, local, and lacked coordinated federal support. This led to rolling shortages of these materials for years.
Two studies were performed early in the pandemic. The first study evaluated 4 prototypes of different manufacturer designs, finding excellent concordance among them and their control swab.6 A second study demonstrated the USF swab to be noninferior to the standard of care.7 Both studies acknowledged and addressed the first challenge for their designs.
COLLABORATIONS
Interagency
Before the pandemic, the US Department of Veterans Affairs (VA) had been coordinating with the FDA, the National Institutes of Health (NIH), and the nonprofit America Makes to bring medical product development and manufacturing closer to the point of care.
At the outset of the COVID-19 pandemic, the collaboration was formalized to address new challenges.8 The objectives of this collaboration were the following: (1) host a digital repository for 3D-printed digital designs for personal protectice equipment and other medical supplies in or at risk of shortage; (2) provide scientifically based ratings for designs according to clinical and field testing; and (3) offer education to health care workers and the public about the digital manufacturing of medical goods and devices.4,9
A key output of this collaboration was the COVID 3D Trusted Repository For Users And Suppliers Through Testing (COVID 3D TRUST), a curated archive of designs. In most cases, existing FDA standards and guidance formed the basis of testing strategies with deviations due to limited access to traditional testing facilities and reagents.
To address novel NP swabs, working with its COVID 3D TRUST partners, the VA gathered a combined list of clinical- and engineering-informed customer requirements and performed a hazard analysis. The result was a list of design inputs for NP swabs and 8 standard test protocols to evaluate key functions (Table).10 These protocols are meant to benchmark novel 3D-printed swabs against the key functions of established, traditionally manufactured swabs, which have a long record of safety and efficacy. The protocols, developed by the VA and undergoing validation by the US Army, empower and inform consumers and provide performance metrics to swab designers and manufacturers. The testing protocols and preliminary test results developed by the VA are publicly available at the NIH.11
Intra-agency
The use of the inputs and verification tests noted in the Table may reduce the risk of poor design but were inadequate to evaluate the clinical safety and efficacy of novel swabs. Recognizing this, the VA Office of Healthcare Innovation and Learning (OHIL) and the Office of Research and Development (ORD) launched the Nasal Swab Objective and Statistical Evaluation (NOSE) study to formally evaluate the safety and efficacy of 3D-printed swabs in the field. This multisite clinical study was a close collaboration between the OHIL and ORD. The OHIL provided the quality system and manufacturing oversight and delivery of the swabs, and the ORD provided scientific review, research infrastructure, human subjects oversight, administrative support, and funding and fiscal oversight. The OHIL/ORD collaboration resulted in the successful completion of the NOSE study.
This study (manuscript under preparation) yielded two 3D-printing production processes and swab designs that had comparable performance to the standard of care, were manufacturable compliant with FDA guidelines, and could be produced at scale in a distributed manner. This approach directly addressed the 3 challenges described earlier.
LESSONS LEARNED
Swabs were an example of supply challenges in the pandemic, but advanced manufacturing (notably, digital designs leading to 3D-printed solutions) also served as a temporary solution to device and product shortages during the COVID-19 pandemic. Digital designs and 3D printing as manufacturing techniques have the following key advantages: (1) they are distributed in nature, both in the breadth of locations that have access to these manufacturing platforms and in the depth of material choice that can be used to fabricate products, which alleviates the threat of a disaster impacting manufacturing capacity or a material stream; (2) they do not require retooling of machinery so new products can deploy rapidly and on demand; and (3) the speed of digital iteration, printing, and revision allows for rapid product development and production.
There also are notable disadvantages to these techniques. First, because 3D printing is a newer technology, there is less general depth of knowledge regarding design and material choice for additive manufacturing. Second, the flexibility of 3D printing means that operators must increase awareness of the factors that might cause the fabrication of a part to fail in either printing or postprocessing. Third, there are significant gaps in understanding how materials and manufacturing processes will perform in high-stakes settings such as health care, where performance and biocompatibility may be critical to support life-sustaining functions. Fourth, digital files are vulnerable to intentional or unintentional alteration. These alterations might weaken design integrity and be imperceptible to the manufacturer or end user. This is a prevalent challenge in all open-source designs.
The pandemic materialized quickly and created vast supply chain challenges. To address this crisis, it was clear that the average 17-year interval between research and translation in the US was unacceptable. The VA was able to accelerate swiftly many existing processes to meet this need, build new capabilities, and establish new practices for the rapid evaluation and deployment of health care products and guidance. This agile and innovative cooperation was critical in the success of the VA’s national support for pandemic solutions.
Finally, although COVID 3D TRUST was able to provide testing of submitted designs, this collaboration was not a substitute for the “peacetime” process of manufacturing site registration with the FDA and product listing. COVID 3D TRUST could evaluate designs only, not the production process, safety, and efficacy.
CALLS TO ACTION
The pandemic's impact on medical supply chain security persists, as does the need for greater foresight and crisis preparation. We must act now to avoid experiencing again the magnitude of fatalities (civilian and veteran) and the devastation to the US economy and livelihoods that occurred during this single biological event. To this end, creating a digital stockpile of federally curated, crisis-ready designs for as-needed distribution across our US industrial base would offer a second line of defense against life-threatening supply chain interruptions. The realization of such a digital stockpile requires calls to action among multiple contributors.
Collaborations
The VA’s Fourth Mission is to improve the nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters. The VA does this by developing plans and taking actions to ensure continued service to veterans, as well as to support national, state, and local emergency management, public health, safety, and homeland security efforts.
The VA partnership with the FDA and NIH during the pandemic enabled successful coordination among federal agencies. Numerous other agencies, including the US Department of Defense (DoD), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Advanced Research Projects Agency (DARPA), also developed and executed successful initiatives.12-14 The joint awareness and management of these efforts, however, could be strengthened through more formal agreements and processes in peacetime. The VA/FDA/NIH Memorandum of Understanding is a prototype example of each agency lending its subject matter expertise to address a host of pandemic challenges collectively, cooperatively, and efficiently.8
Public-private partnerships (eg, VA/FDA/NIH and America Makes) led to coordinated responses for crisis readiness. The Advanced Manufacturing Crisis Product Response Program, a multipartner collaboration that included VA, addressed 7 crisis scenarios, 3 of which were specifically related to COVID-19.15 In addition, both BARDA and DARPA had successful public-private collaborations, and the DoD supported national logistics and other efforts.12-14 Clearly, industry and government both recognize complementary synergies: (1) the depth of resources of US industry; and (2) the national resources, coordination, and clinical insight available through federal agencies that can address the challenges of future crises quickly and efficiently.
When traditional supply chains and manufacturing processes failed during the pandemic, new techniques were exploited to fill the unmet material needs. Novel techniques and product pathways, however, are untested or undeveloped. The collaboration between the ORD and OHIL in support of NP swab testing and production is an example of bringing research insight, regulated product development, and manufacturing together to support a complete product life cycle.
Joint Awareness and Management
The VA continues to refine the joint awareness and management (JAM) process of products from ideation to translation, to shorten the time from research to product delivery. JAM is a VA collaborative committee of partners from ORD research offices and technology transfer program, and the OHIL Office of Advanced Manufacturing, which seeks additional support and guidance from VHA clinical service lines, VA Office of General Council, and VA Office of Acquisitions, Logistics, and Construction.
This team enables the rapid identification of unmet veteran health care product needs. In addition, JAM leverages the resources of each group to support products from problem identification to solution ideation, regulated development, production, and delivery into clinical service lines. While the concept of JAM arose to meet the crisis needs of the pandemic, it persists in delivering advanced health care solutions to veterans.
A Proposed Plan
The next national crisis is likely to involve and threaten national health care security. We propose that federal agencies be brought together to form a federally supported digital stockpile. This digital stockpile must encompass, at minimum, the following features: (1) preservation of novel, scalable medical supplies and products generated during the COVID-19 pandemic, to avoid the loss of this work; (2) clinical maturation of those existing supplies and products to refine their features and functions under the guidance of clinical, regulatory, and manufacturing experts—and validate those outputs with clinical evidence; (3) manufacturing maturation of those existing supplies and products, such that complete design and production processes are developed with the intent to distribute to multiple public manufacturers during the next crisis; (4) a call for new designs/intake portal for new designs to be matured and curated as vulnerabilities are identified; (5) supply chain crisis drills executed to test public-private preparedness to ensure design transfer is turnkey and can be engaged quickly during the next crisis; and (6) public-private engagement to develop strategy, scenarios, and policy to ensure that when supply chains next fail, additional surge capacity can be quickly added to protect American lives and health care, and that when supply chains resume, surge capacity can be redirected or stood down to protect the competitive markets.
This digital stockpile can complement and be part of the Strategic National Stockpile. Whereas the Strategic National Stockpile is a reserve of physical products that may offset product shortages, the digital stockpile is a reserve of turnkey, transferable designs that may offset supply chain disruptions and production-capacity shortages.
CONCLUSIONS
The success of 3D-printed NP swabs is a specific example of the importance of collaborations across industry, government, innovators, and researchers. More important than a sole product, however, these collaborations demonstrated the potential for game-changing approaches to how public-private partnerships support the continuity of health care operations nationally and prevent the potential for unnecessary loss of life due to capacity and supply chain disruptions.
As the largest health care system in the US, the VA has a unique capability to lead in the assessment of other novel 3D-printed medical devices in partnership with the FDA. The VA has a unique patient-centered perspective on medical device efficacy, and as a government institution, it is a trusted independent source for medical device evaluation. The VA’s role in the evaluation of 3D-printed medical devices will benefit veterans and their families, clinicians, hospitals, and the broader public by providing a gold-standard evaluation for the growing medical 3D-printing industry to follow. By creating new pathways and expectations for how federal agencies maintain crisis preparedness—such as establishing a digital stockpile—we can be equipped to serve the US health care system and minimize the effects of supply chain disruptions.
1. Sacks CA, Kesselheim AS, Fralick M. The shortage of normal saline in the wake of Hurricane Maria. JAMA Intern Med. 2018;178(7):885–886. doi:10.1001/jamainternmed.2018.1936
2. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment–a call for ideas. JAMA. 2020;323(19):1911. doi:10.1001/jama.2020.4770
3. Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110(8):1162-1164. doi:10.2105/AJPH.2020.305753
4. McCarthy MC, Di Prima M, Cruz P, et al. Trust in the time of Covid-19: 3D printing and additive manufacturing (3DP/AM) as a solution to supply chain gaps. NEJM Catalyst. 2021;2(6). doi:10.1056/CAT.21.0321
5. Ford J, Goldstein T, Trahan S, Neuwirth A, Tatoris K, Decker S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med. 2020;6(1):21. Published 2020 Aug 15. doi:10.1186/s41205-020-00076-3
6. Callahan CJ, Lee R, Zulauf K, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Preprint. medRxiv. 2020;2020.04.14.20065094. Published 2020 Apr 17. doi:10.1101/2020.04.14.20065094
7. Decker SJ, Goldstein TA, Ford JM, et al. 3-dimensional printed alternative to the standard synthetic flocked nasopharyngeal swabs used for coronavirus disease 2019 testing. Clin Infect Dis. 2021;73(9):e3027-e3032. doi:10.1093/cid/ciaa1366
8. US Food and Drug Administration. Memorandum of understanding: rapid response to Covid-19 using 3d printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration, U.S. Department of Health and Human Services and Veterans Health Administration within the U.S. Department of Veterans Affairs. March 26, 2020. Accessed August 31, 2023. https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008
9. National Institutes of Health, NIH 3D Print Exchange. Covid 3D trust: trusted repository for users and suppliers through testing. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=search
10. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - assessment criteria. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabassessment
11. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - general information. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabinfo
12. US Department of Defense. Coronavirus: DOD response. December 20, 2022. Accessed August 31, 2023. https://www.defense.gov/Spotlights/Coronavirus-DoD-Response
13. US Department of Health and Human Services, Biomedical Advanced Research and Development Authority. BARDA COVID-19 response. Updated May 25, 2023. Accessed August 31, 2023. https://www.medicalcountermeasures.gov/barda/barda-covid-19-response
14. Green S. Pandemic prevention platform (P3). Accessed August 31, 2023. https://www.darpa.mil/program/pandemic-prevention-platform
15. America Makes. America makes completes successful scenario testing for crisis response program [press release]. May 25, 2021. Accessed August 31, 2023. https://www.americamakes.us/america-makes-completes-successful-scenario-testing-for-crisis-response-program
1. Sacks CA, Kesselheim AS, Fralick M. The shortage of normal saline in the wake of Hurricane Maria. JAMA Intern Med. 2018;178(7):885–886. doi:10.1001/jamainternmed.2018.1936
2. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment–a call for ideas. JAMA. 2020;323(19):1911. doi:10.1001/jama.2020.4770
3. Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110(8):1162-1164. doi:10.2105/AJPH.2020.305753
4. McCarthy MC, Di Prima M, Cruz P, et al. Trust in the time of Covid-19: 3D printing and additive manufacturing (3DP/AM) as a solution to supply chain gaps. NEJM Catalyst. 2021;2(6). doi:10.1056/CAT.21.0321
5. Ford J, Goldstein T, Trahan S, Neuwirth A, Tatoris K, Decker S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med. 2020;6(1):21. Published 2020 Aug 15. doi:10.1186/s41205-020-00076-3
6. Callahan CJ, Lee R, Zulauf K, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Preprint. medRxiv. 2020;2020.04.14.20065094. Published 2020 Apr 17. doi:10.1101/2020.04.14.20065094
7. Decker SJ, Goldstein TA, Ford JM, et al. 3-dimensional printed alternative to the standard synthetic flocked nasopharyngeal swabs used for coronavirus disease 2019 testing. Clin Infect Dis. 2021;73(9):e3027-e3032. doi:10.1093/cid/ciaa1366
8. US Food and Drug Administration. Memorandum of understanding: rapid response to Covid-19 using 3d printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration, U.S. Department of Health and Human Services and Veterans Health Administration within the U.S. Department of Veterans Affairs. March 26, 2020. Accessed August 31, 2023. https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008
9. National Institutes of Health, NIH 3D Print Exchange. Covid 3D trust: trusted repository for users and suppliers through testing. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=search
10. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - assessment criteria. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabassessment
11. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - general information. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabinfo
12. US Department of Defense. Coronavirus: DOD response. December 20, 2022. Accessed August 31, 2023. https://www.defense.gov/Spotlights/Coronavirus-DoD-Response
13. US Department of Health and Human Services, Biomedical Advanced Research and Development Authority. BARDA COVID-19 response. Updated May 25, 2023. Accessed August 31, 2023. https://www.medicalcountermeasures.gov/barda/barda-covid-19-response
14. Green S. Pandemic prevention platform (P3). Accessed August 31, 2023. https://www.darpa.mil/program/pandemic-prevention-platform
15. America Makes. America makes completes successful scenario testing for crisis response program [press release]. May 25, 2021. Accessed August 31, 2023. https://www.americamakes.us/america-makes-completes-successful-scenario-testing-for-crisis-response-program
VA SHIELD: A Biorepository for Veterans and the Nation
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
Nationwide Genomic Surveillance and Response to COVID-19: The VA SeqFORCE and SeqCURE Consortiums
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
VA Big Data Science: A Model for Improved National Pandemic Response Present and Future
The COVID-19 pandemic emphasized the need for rapid response research in health care. The robust enterprise approach used by the US Department of Veterans Affairs (VA), termed VA Research, is meeting these needs by using existing outstanding data resources and interdisciplinary collaborations.1 In the first 7 months of 2021 alone, while many US health care systems struggled with limited data, VA Research published more than 300 unique and instrumental research papers addressing urgent questions about transmission, vaccination, therapeutics, and health impacts of COVID-19 on its high-risk population.1 The ability to leverage the VA electronic health record (EHR) and Corporate Data Warehouse (CDW)—a fully established data system bringing together test results, prescriptions, and complete patient health records, readily accessible and updated daily—was substantial.
With more than 9 million veterans enrolled in care at 171 medical centers and 1113 outpatient facilities across the US and its territories, the CDW provides an unprecedented opportunity to examine outcomes in real time. This allowed research groups such as the VA St Louis Health Care System Research and Education Service to build a cohort of 181,280 veterans with diabetes and positive COVID-19 test results within a 6-month period in 2021 to study the incidence of new diagnoses of diabetes after COVID-19 infection.2 Similarly, the Clinical Epidemiology Program (CEP) at VA White River Junction Health Care System built a cohort of 1,363,180 veterans who received at least 1 COVID-19 vaccine by March 7, 2021, to analyze coverage and effectiveness of those vaccines
The innovation and speed of COVID-19 vaccine development and distribution in the US were unprecedented. The rapid discovery and implementation of multiple preventives and therapeutics for COVID-19 could not have been possible without shared information within a competitive industry. VA studies added significantly to understanding the clinical performance of the messenger RNA (mRNA) COVID-19 vaccines, antivirals, and monoclonal treatments in a real-world setting. For example, a vaccine coverage study by VA Research illustrated how successful vaccination for COVID-19 at the VA has been in protecting a diverse community of patients from hospitalization and death, particularly the highly comorbid, racial and ethnic minorities, and other high-risk populations.3 The study demonstrated the power of the VA system to generate robust and compelling clinical endpoint effectiveness data across a broad range of high-risk groups.
This success is promising. However, the COVID-19 pandemic is not over, and the next could prove even more challenging. For example, through a recent partnership with the US Department of Defense (DoD), the VA was able to rapidly analyze the effectiveness of previous smallpox vaccination efforts in the military for preventing mpox infections.5 We should take this opportunity to think creatively about ways to improve our existing infrastructure based on what we have learned.
A Role for VA Research in Efficacy
The US Food and Drug Administration (FDA) Reauthorization Act of 2017 requires that manufacturers submit evidence establishing a product’s benefits (effectiveness) outweigh its risks (safety) before it can be promoted and distributed.6 As such, the FDA has been obligated by external stakeholders and Congress to be more explicit and transparent about benefit-risk profile supporting its decisions on licensure. This process led to requiring more phase 4 postmarketing observational studies for safety and effectiveness.7 Although the FDA postlicensure system remains vigilant toward safety, effectiveness information is limited due to insufficient reporting (with exceptions of manufacturer studies for new indications or to exhibit superior comparative effectiveness). The agency typically relies on a static set of efficacy data generated prelicensure with a dynamic and evolving set of safety data accrued postlicensure to support its assessment that benefits outweigh risks.
For example, operating in near real time, postauthorization safety monitoring systems, led by the Centers for Disease Control and Prevention and other federal systems, identified a safety signal for thrombosis following the Janssen COVID-19 vaccination. Distribution was quickly paused, the safety signal was investigated, the magnitude of the risk was characterized, new language describing the risk and providing guidance regarding clinical management was included in labeling, and distribution was resumed, all within a few weeks. This remarkable success demonstrated how timely the safety system can operate to evaluate risk.
In contrast, the duration and extent of protection against COVID-19 variants are largely limited to the assessment of immune biomarker surrogates. Such clinical effectiveness data are urgently needed for the FDA’s Center for Biologics Evaluation and Research and Center for Drug Evaluation and Research to make accurate benefit-risk assessments and continue to conclude the balance is favorable. As we prepare for the next pandemic, we must consider plans for monitoring postauthorization/postlicensure effectiveness as well as safety in real time. VA Research is ideally situated for this task.
Published studies on effectiveness at the VA serve as a prototype and could lead the way to initiating those preparations.4,8-11 One of the striking features of the VA system that became apparent in the preparation of the mRNA vaccine study was the speed at which an enormous volume of COVID-19 testing data were produced. This enabled implementation of methodologically sound test-negative and case-control analysis. Analyses sufficiently powered to conclude mRNA vaccines were highly effective when used in real-world conditions among a diverse population from nearly every state and territory during a period in which multiple COVID-19 variants were already circulating.3 This is unique to the VA and would not be possible for any other US health care system. With planning, the VA system could produce product-specific, real-world evidence of effectiveness comparable to the timeliness and quality of the safety data currently produced to support regulatory benefit-risk assessments. For example, the VA conducted an effectiveness study of tixagevimab/cilgavimab for preventing COVID-19 during the initial Omicron surge, which is continually updated while Omicron circulates and repeatable for different subvariants.12
The FDA continues to collaborate with the VA on demonstration projects to evaluate the impact of available vaccines and treatment against COVID-19 variants. The VA has also initiated several large-scale sequencing programs for COVID-19 specimens that will support these efforts, including VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD), VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE).13,14 Successful proof-of-concept studies using these data could provide a template for VA and other medical systems/databases to report effectiveness in near real time.
Interagency Collaboration
The potential advantages of federal agencies working with the VA to build an infrastructure capable of generating real-world evidence effectiveness analyses in near real time is not limited to needs that will arise in the next pandemic. For example, generating randomized, placebo-controlled, clinical trial endpoint data on the effectiveness of new variant vaccines will be difficult from a feasibility and ethical standpoint. Combining the VA’s robust virus sequencing program with preexisting mechanisms, such as expanded access studies (allowed under FDA Investigational New Drug regulations), researchers could enable a large-scale effective evaluation program of vaccination with variant or universal COVID-19 vaccines, using rapidly accruing effectiveness data.
The pandemic created opportunities to advance innovative approaches to medical product development. Some have advocated these innovative approaches should proceed together toward a seamless convergence between the domains of medical research and clinical care. A shift toward expecting, as a matter of routine, effectiveness data to be generated in near real time and made available for benefit-risk assessment would be a useful step in that direction.
Expanding and sharing analytical platforms, including methodology and programming codes, will allow increased access to rapidly refreshed real-world data. A common adaptive platform of complete and continuously updated data will also enable a wider community of researchers to create multiple investigatory groups simultaneously accessing fully de-identified data for concurrent observational studies. In turn, researchers need to have programming, study design, and methodology ready in an open-source platform. An efficient platform would also require the adoption of artificial intelligence, natural language processing, imaging processing, and quantum computing for validation and improved data quality.
COVID-19 has demonstrated the need for open science data synchronization with universal access for faster action and improved outcomes able to gain public confidence. OpenSafely (UK), a software platform for analysis of EHR data that is shared automatically and openly for scientific review and efficient reuse, created a cohort of about 23.4 million records for observational review of monoclonal COVID-19 treatments. To keep pace with the UK, Israel, and other nationalized systems, the US would benefit from duplicating this example of coordination between federal agencies and their data repositories. For example, combining data between the DoD, which captures active military health care data through TRICARE, and VA, which follows postmilitary discharge, would create datasets encompassing complete life spans. Additionally, expanding the National COVID Cohort Collaborative (N3C) program—one of the largest collections of clinical data related to COVID-19 symptoms and patient outcomes in the US—to include EHR data from DoD, VA, Medicare, and Test to Treat initiative partners would further expand research capabilities. This could be accomplished through a framework of anonymized, readily available, harmonized data. EHRs with synchronized datasets from every health care practitioner—independent pharmacies, primary care physicians, and hospitals—could all work to create a de-identified, comprehensive, continuously updated, near real-time dataset accessible to all federal researchers.
Conclusions
The VA has been lauded for its rapid, effective response to the current pandemic. The successful management and prescription of vaccines and treatment to the largely high-risk veteran population was possible because of the existing data framework within the VA. VA Research continues to build and refine infrastructure to improve speed, quality, and value of data analytics. We can do more. Expanding partnerships to use existing VA data strategies in designing a cooperative national data alliance would deliver necessary progress to research and public health.
Acknowledgments
The authors thank Jeff Roberts, MD, for his insight on the US Food and Drug Administration, its responsibilities, and the potential benefit of real world data to its missions.
1. US Department of Veterans Affairs, Veterans Health Administration. Third report details VA’s continued efforts addressing COVID-19 pandemic. Accessed August 15, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5748
2. Xie Y, Ziyad A. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi:10.1016/S2213-8587(22)00044-4
3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4(10):e2128391. doi:10.1001/jamanetworkopen.2021.28391
4. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi:10.1056/NEJMoa2115463
5. Titanji BK, Eick-Cost A, Partan ES, et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. N Engl J Med. 2023;389(12):1147-1148. doi:10.1056/NEJMc2300805
6. Sarata AK, Dabrowska A, Johnson JA, Thaul S. FDA Reauthorization Act of 2017. Accessed August 15, 2023. https://sgp.fas.org/crs/misc/R44961.pdf
7. US Food and Drug Administration. FDA’s sentinel initiative–background. February 2, 2022. Updated February 4, 2022. Accessed August 15, 2023. https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
8. Bajema KL, Dahl RM, Prill MM, et al; SUPERNOVA COVID-19; Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly. 2021;70(37):1294-1299. doi:10.15585/mmwr.mm7037e3
9. Sharma A, Oda G, Holodniy M. COVID-19 vaccine breakthrough infections in Veterans Health Administration. medRxiv. Posted September 26, 2021. doi:10.1101/2021.09.23.21263864
10. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of third doses of mRNA-based COVID-19 vaccines in US veterans. Nat Microbiol. 2023;8(1):55-63. doi:10.1038/s41564-022-01272-z
11. Tang F, Hammel IS, Andrew MK, Ruiz JG. Frailty reduces vaccine effectiveness against SARS-CoV-2 infection: a test-negative case control study using national VA data. J Nutr Health Aging. 2023;27(2):81-88. doi:10.1007/s12603-023-1885-1
12. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023;14(4):e0102423. doi:10.1128/mbio.01024-23
13. US Department of Veterans Affairs. VA science and health initiative to combat infectious and emerging life-threatening diseases. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac64
14. Bilal MY. Similarity index–probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia. 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
The COVID-19 pandemic emphasized the need for rapid response research in health care. The robust enterprise approach used by the US Department of Veterans Affairs (VA), termed VA Research, is meeting these needs by using existing outstanding data resources and interdisciplinary collaborations.1 In the first 7 months of 2021 alone, while many US health care systems struggled with limited data, VA Research published more than 300 unique and instrumental research papers addressing urgent questions about transmission, vaccination, therapeutics, and health impacts of COVID-19 on its high-risk population.1 The ability to leverage the VA electronic health record (EHR) and Corporate Data Warehouse (CDW)—a fully established data system bringing together test results, prescriptions, and complete patient health records, readily accessible and updated daily—was substantial.
With more than 9 million veterans enrolled in care at 171 medical centers and 1113 outpatient facilities across the US and its territories, the CDW provides an unprecedented opportunity to examine outcomes in real time. This allowed research groups such as the VA St Louis Health Care System Research and Education Service to build a cohort of 181,280 veterans with diabetes and positive COVID-19 test results within a 6-month period in 2021 to study the incidence of new diagnoses of diabetes after COVID-19 infection.2 Similarly, the Clinical Epidemiology Program (CEP) at VA White River Junction Health Care System built a cohort of 1,363,180 veterans who received at least 1 COVID-19 vaccine by March 7, 2021, to analyze coverage and effectiveness of those vaccines
The innovation and speed of COVID-19 vaccine development and distribution in the US were unprecedented. The rapid discovery and implementation of multiple preventives and therapeutics for COVID-19 could not have been possible without shared information within a competitive industry. VA studies added significantly to understanding the clinical performance of the messenger RNA (mRNA) COVID-19 vaccines, antivirals, and monoclonal treatments in a real-world setting. For example, a vaccine coverage study by VA Research illustrated how successful vaccination for COVID-19 at the VA has been in protecting a diverse community of patients from hospitalization and death, particularly the highly comorbid, racial and ethnic minorities, and other high-risk populations.3 The study demonstrated the power of the VA system to generate robust and compelling clinical endpoint effectiveness data across a broad range of high-risk groups.
This success is promising. However, the COVID-19 pandemic is not over, and the next could prove even more challenging. For example, through a recent partnership with the US Department of Defense (DoD), the VA was able to rapidly analyze the effectiveness of previous smallpox vaccination efforts in the military for preventing mpox infections.5 We should take this opportunity to think creatively about ways to improve our existing infrastructure based on what we have learned.
A Role for VA Research in Efficacy
The US Food and Drug Administration (FDA) Reauthorization Act of 2017 requires that manufacturers submit evidence establishing a product’s benefits (effectiveness) outweigh its risks (safety) before it can be promoted and distributed.6 As such, the FDA has been obligated by external stakeholders and Congress to be more explicit and transparent about benefit-risk profile supporting its decisions on licensure. This process led to requiring more phase 4 postmarketing observational studies for safety and effectiveness.7 Although the FDA postlicensure system remains vigilant toward safety, effectiveness information is limited due to insufficient reporting (with exceptions of manufacturer studies for new indications or to exhibit superior comparative effectiveness). The agency typically relies on a static set of efficacy data generated prelicensure with a dynamic and evolving set of safety data accrued postlicensure to support its assessment that benefits outweigh risks.
For example, operating in near real time, postauthorization safety monitoring systems, led by the Centers for Disease Control and Prevention and other federal systems, identified a safety signal for thrombosis following the Janssen COVID-19 vaccination. Distribution was quickly paused, the safety signal was investigated, the magnitude of the risk was characterized, new language describing the risk and providing guidance regarding clinical management was included in labeling, and distribution was resumed, all within a few weeks. This remarkable success demonstrated how timely the safety system can operate to evaluate risk.
In contrast, the duration and extent of protection against COVID-19 variants are largely limited to the assessment of immune biomarker surrogates. Such clinical effectiveness data are urgently needed for the FDA’s Center for Biologics Evaluation and Research and Center for Drug Evaluation and Research to make accurate benefit-risk assessments and continue to conclude the balance is favorable. As we prepare for the next pandemic, we must consider plans for monitoring postauthorization/postlicensure effectiveness as well as safety in real time. VA Research is ideally situated for this task.
Published studies on effectiveness at the VA serve as a prototype and could lead the way to initiating those preparations.4,8-11 One of the striking features of the VA system that became apparent in the preparation of the mRNA vaccine study was the speed at which an enormous volume of COVID-19 testing data were produced. This enabled implementation of methodologically sound test-negative and case-control analysis. Analyses sufficiently powered to conclude mRNA vaccines were highly effective when used in real-world conditions among a diverse population from nearly every state and territory during a period in which multiple COVID-19 variants were already circulating.3 This is unique to the VA and would not be possible for any other US health care system. With planning, the VA system could produce product-specific, real-world evidence of effectiveness comparable to the timeliness and quality of the safety data currently produced to support regulatory benefit-risk assessments. For example, the VA conducted an effectiveness study of tixagevimab/cilgavimab for preventing COVID-19 during the initial Omicron surge, which is continually updated while Omicron circulates and repeatable for different subvariants.12
The FDA continues to collaborate with the VA on demonstration projects to evaluate the impact of available vaccines and treatment against COVID-19 variants. The VA has also initiated several large-scale sequencing programs for COVID-19 specimens that will support these efforts, including VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD), VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE).13,14 Successful proof-of-concept studies using these data could provide a template for VA and other medical systems/databases to report effectiveness in near real time.
Interagency Collaboration
The potential advantages of federal agencies working with the VA to build an infrastructure capable of generating real-world evidence effectiveness analyses in near real time is not limited to needs that will arise in the next pandemic. For example, generating randomized, placebo-controlled, clinical trial endpoint data on the effectiveness of new variant vaccines will be difficult from a feasibility and ethical standpoint. Combining the VA’s robust virus sequencing program with preexisting mechanisms, such as expanded access studies (allowed under FDA Investigational New Drug regulations), researchers could enable a large-scale effective evaluation program of vaccination with variant or universal COVID-19 vaccines, using rapidly accruing effectiveness data.
The pandemic created opportunities to advance innovative approaches to medical product development. Some have advocated these innovative approaches should proceed together toward a seamless convergence between the domains of medical research and clinical care. A shift toward expecting, as a matter of routine, effectiveness data to be generated in near real time and made available for benefit-risk assessment would be a useful step in that direction.
Expanding and sharing analytical platforms, including methodology and programming codes, will allow increased access to rapidly refreshed real-world data. A common adaptive platform of complete and continuously updated data will also enable a wider community of researchers to create multiple investigatory groups simultaneously accessing fully de-identified data for concurrent observational studies. In turn, researchers need to have programming, study design, and methodology ready in an open-source platform. An efficient platform would also require the adoption of artificial intelligence, natural language processing, imaging processing, and quantum computing for validation and improved data quality.
COVID-19 has demonstrated the need for open science data synchronization with universal access for faster action and improved outcomes able to gain public confidence. OpenSafely (UK), a software platform for analysis of EHR data that is shared automatically and openly for scientific review and efficient reuse, created a cohort of about 23.4 million records for observational review of monoclonal COVID-19 treatments. To keep pace with the UK, Israel, and other nationalized systems, the US would benefit from duplicating this example of coordination between federal agencies and their data repositories. For example, combining data between the DoD, which captures active military health care data through TRICARE, and VA, which follows postmilitary discharge, would create datasets encompassing complete life spans. Additionally, expanding the National COVID Cohort Collaborative (N3C) program—one of the largest collections of clinical data related to COVID-19 symptoms and patient outcomes in the US—to include EHR data from DoD, VA, Medicare, and Test to Treat initiative partners would further expand research capabilities. This could be accomplished through a framework of anonymized, readily available, harmonized data. EHRs with synchronized datasets from every health care practitioner—independent pharmacies, primary care physicians, and hospitals—could all work to create a de-identified, comprehensive, continuously updated, near real-time dataset accessible to all federal researchers.
Conclusions
The VA has been lauded for its rapid, effective response to the current pandemic. The successful management and prescription of vaccines and treatment to the largely high-risk veteran population was possible because of the existing data framework within the VA. VA Research continues to build and refine infrastructure to improve speed, quality, and value of data analytics. We can do more. Expanding partnerships to use existing VA data strategies in designing a cooperative national data alliance would deliver necessary progress to research and public health.
Acknowledgments
The authors thank Jeff Roberts, MD, for his insight on the US Food and Drug Administration, its responsibilities, and the potential benefit of real world data to its missions.
The COVID-19 pandemic emphasized the need for rapid response research in health care. The robust enterprise approach used by the US Department of Veterans Affairs (VA), termed VA Research, is meeting these needs by using existing outstanding data resources and interdisciplinary collaborations.1 In the first 7 months of 2021 alone, while many US health care systems struggled with limited data, VA Research published more than 300 unique and instrumental research papers addressing urgent questions about transmission, vaccination, therapeutics, and health impacts of COVID-19 on its high-risk population.1 The ability to leverage the VA electronic health record (EHR) and Corporate Data Warehouse (CDW)—a fully established data system bringing together test results, prescriptions, and complete patient health records, readily accessible and updated daily—was substantial.
With more than 9 million veterans enrolled in care at 171 medical centers and 1113 outpatient facilities across the US and its territories, the CDW provides an unprecedented opportunity to examine outcomes in real time. This allowed research groups such as the VA St Louis Health Care System Research and Education Service to build a cohort of 181,280 veterans with diabetes and positive COVID-19 test results within a 6-month period in 2021 to study the incidence of new diagnoses of diabetes after COVID-19 infection.2 Similarly, the Clinical Epidemiology Program (CEP) at VA White River Junction Health Care System built a cohort of 1,363,180 veterans who received at least 1 COVID-19 vaccine by March 7, 2021, to analyze coverage and effectiveness of those vaccines
The innovation and speed of COVID-19 vaccine development and distribution in the US were unprecedented. The rapid discovery and implementation of multiple preventives and therapeutics for COVID-19 could not have been possible without shared information within a competitive industry. VA studies added significantly to understanding the clinical performance of the messenger RNA (mRNA) COVID-19 vaccines, antivirals, and monoclonal treatments in a real-world setting. For example, a vaccine coverage study by VA Research illustrated how successful vaccination for COVID-19 at the VA has been in protecting a diverse community of patients from hospitalization and death, particularly the highly comorbid, racial and ethnic minorities, and other high-risk populations.3 The study demonstrated the power of the VA system to generate robust and compelling clinical endpoint effectiveness data across a broad range of high-risk groups.
This success is promising. However, the COVID-19 pandemic is not over, and the next could prove even more challenging. For example, through a recent partnership with the US Department of Defense (DoD), the VA was able to rapidly analyze the effectiveness of previous smallpox vaccination efforts in the military for preventing mpox infections.5 We should take this opportunity to think creatively about ways to improve our existing infrastructure based on what we have learned.
A Role for VA Research in Efficacy
The US Food and Drug Administration (FDA) Reauthorization Act of 2017 requires that manufacturers submit evidence establishing a product’s benefits (effectiveness) outweigh its risks (safety) before it can be promoted and distributed.6 As such, the FDA has been obligated by external stakeholders and Congress to be more explicit and transparent about benefit-risk profile supporting its decisions on licensure. This process led to requiring more phase 4 postmarketing observational studies for safety and effectiveness.7 Although the FDA postlicensure system remains vigilant toward safety, effectiveness information is limited due to insufficient reporting (with exceptions of manufacturer studies for new indications or to exhibit superior comparative effectiveness). The agency typically relies on a static set of efficacy data generated prelicensure with a dynamic and evolving set of safety data accrued postlicensure to support its assessment that benefits outweigh risks.
For example, operating in near real time, postauthorization safety monitoring systems, led by the Centers for Disease Control and Prevention and other federal systems, identified a safety signal for thrombosis following the Janssen COVID-19 vaccination. Distribution was quickly paused, the safety signal was investigated, the magnitude of the risk was characterized, new language describing the risk and providing guidance regarding clinical management was included in labeling, and distribution was resumed, all within a few weeks. This remarkable success demonstrated how timely the safety system can operate to evaluate risk.
In contrast, the duration and extent of protection against COVID-19 variants are largely limited to the assessment of immune biomarker surrogates. Such clinical effectiveness data are urgently needed for the FDA’s Center for Biologics Evaluation and Research and Center for Drug Evaluation and Research to make accurate benefit-risk assessments and continue to conclude the balance is favorable. As we prepare for the next pandemic, we must consider plans for monitoring postauthorization/postlicensure effectiveness as well as safety in real time. VA Research is ideally situated for this task.
Published studies on effectiveness at the VA serve as a prototype and could lead the way to initiating those preparations.4,8-11 One of the striking features of the VA system that became apparent in the preparation of the mRNA vaccine study was the speed at which an enormous volume of COVID-19 testing data were produced. This enabled implementation of methodologically sound test-negative and case-control analysis. Analyses sufficiently powered to conclude mRNA vaccines were highly effective when used in real-world conditions among a diverse population from nearly every state and territory during a period in which multiple COVID-19 variants were already circulating.3 This is unique to the VA and would not be possible for any other US health care system. With planning, the VA system could produce product-specific, real-world evidence of effectiveness comparable to the timeliness and quality of the safety data currently produced to support regulatory benefit-risk assessments. For example, the VA conducted an effectiveness study of tixagevimab/cilgavimab for preventing COVID-19 during the initial Omicron surge, which is continually updated while Omicron circulates and repeatable for different subvariants.12
The FDA continues to collaborate with the VA on demonstration projects to evaluate the impact of available vaccines and treatment against COVID-19 variants. The VA has also initiated several large-scale sequencing programs for COVID-19 specimens that will support these efforts, including VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD), VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE).13,14 Successful proof-of-concept studies using these data could provide a template for VA and other medical systems/databases to report effectiveness in near real time.
Interagency Collaboration
The potential advantages of federal agencies working with the VA to build an infrastructure capable of generating real-world evidence effectiveness analyses in near real time is not limited to needs that will arise in the next pandemic. For example, generating randomized, placebo-controlled, clinical trial endpoint data on the effectiveness of new variant vaccines will be difficult from a feasibility and ethical standpoint. Combining the VA’s robust virus sequencing program with preexisting mechanisms, such as expanded access studies (allowed under FDA Investigational New Drug regulations), researchers could enable a large-scale effective evaluation program of vaccination with variant or universal COVID-19 vaccines, using rapidly accruing effectiveness data.
The pandemic created opportunities to advance innovative approaches to medical product development. Some have advocated these innovative approaches should proceed together toward a seamless convergence between the domains of medical research and clinical care. A shift toward expecting, as a matter of routine, effectiveness data to be generated in near real time and made available for benefit-risk assessment would be a useful step in that direction.
Expanding and sharing analytical platforms, including methodology and programming codes, will allow increased access to rapidly refreshed real-world data. A common adaptive platform of complete and continuously updated data will also enable a wider community of researchers to create multiple investigatory groups simultaneously accessing fully de-identified data for concurrent observational studies. In turn, researchers need to have programming, study design, and methodology ready in an open-source platform. An efficient platform would also require the adoption of artificial intelligence, natural language processing, imaging processing, and quantum computing for validation and improved data quality.
COVID-19 has demonstrated the need for open science data synchronization with universal access for faster action and improved outcomes able to gain public confidence. OpenSafely (UK), a software platform for analysis of EHR data that is shared automatically and openly for scientific review and efficient reuse, created a cohort of about 23.4 million records for observational review of monoclonal COVID-19 treatments. To keep pace with the UK, Israel, and other nationalized systems, the US would benefit from duplicating this example of coordination between federal agencies and their data repositories. For example, combining data between the DoD, which captures active military health care data through TRICARE, and VA, which follows postmilitary discharge, would create datasets encompassing complete life spans. Additionally, expanding the National COVID Cohort Collaborative (N3C) program—one of the largest collections of clinical data related to COVID-19 symptoms and patient outcomes in the US—to include EHR data from DoD, VA, Medicare, and Test to Treat initiative partners would further expand research capabilities. This could be accomplished through a framework of anonymized, readily available, harmonized data. EHRs with synchronized datasets from every health care practitioner—independent pharmacies, primary care physicians, and hospitals—could all work to create a de-identified, comprehensive, continuously updated, near real-time dataset accessible to all federal researchers.
Conclusions
The VA has been lauded for its rapid, effective response to the current pandemic. The successful management and prescription of vaccines and treatment to the largely high-risk veteran population was possible because of the existing data framework within the VA. VA Research continues to build and refine infrastructure to improve speed, quality, and value of data analytics. We can do more. Expanding partnerships to use existing VA data strategies in designing a cooperative national data alliance would deliver necessary progress to research and public health.
Acknowledgments
The authors thank Jeff Roberts, MD, for his insight on the US Food and Drug Administration, its responsibilities, and the potential benefit of real world data to its missions.
1. US Department of Veterans Affairs, Veterans Health Administration. Third report details VA’s continued efforts addressing COVID-19 pandemic. Accessed August 15, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5748
2. Xie Y, Ziyad A. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi:10.1016/S2213-8587(22)00044-4
3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4(10):e2128391. doi:10.1001/jamanetworkopen.2021.28391
4. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi:10.1056/NEJMoa2115463
5. Titanji BK, Eick-Cost A, Partan ES, et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. N Engl J Med. 2023;389(12):1147-1148. doi:10.1056/NEJMc2300805
6. Sarata AK, Dabrowska A, Johnson JA, Thaul S. FDA Reauthorization Act of 2017. Accessed August 15, 2023. https://sgp.fas.org/crs/misc/R44961.pdf
7. US Food and Drug Administration. FDA’s sentinel initiative–background. February 2, 2022. Updated February 4, 2022. Accessed August 15, 2023. https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
8. Bajema KL, Dahl RM, Prill MM, et al; SUPERNOVA COVID-19; Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly. 2021;70(37):1294-1299. doi:10.15585/mmwr.mm7037e3
9. Sharma A, Oda G, Holodniy M. COVID-19 vaccine breakthrough infections in Veterans Health Administration. medRxiv. Posted September 26, 2021. doi:10.1101/2021.09.23.21263864
10. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of third doses of mRNA-based COVID-19 vaccines in US veterans. Nat Microbiol. 2023;8(1):55-63. doi:10.1038/s41564-022-01272-z
11. Tang F, Hammel IS, Andrew MK, Ruiz JG. Frailty reduces vaccine effectiveness against SARS-CoV-2 infection: a test-negative case control study using national VA data. J Nutr Health Aging. 2023;27(2):81-88. doi:10.1007/s12603-023-1885-1
12. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023;14(4):e0102423. doi:10.1128/mbio.01024-23
13. US Department of Veterans Affairs. VA science and health initiative to combat infectious and emerging life-threatening diseases. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac64
14. Bilal MY. Similarity index–probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia. 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
1. US Department of Veterans Affairs, Veterans Health Administration. Third report details VA’s continued efforts addressing COVID-19 pandemic. Accessed August 15, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5748
2. Xie Y, Ziyad A. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi:10.1016/S2213-8587(22)00044-4
3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4(10):e2128391. doi:10.1001/jamanetworkopen.2021.28391
4. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi:10.1056/NEJMoa2115463
5. Titanji BK, Eick-Cost A, Partan ES, et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. N Engl J Med. 2023;389(12):1147-1148. doi:10.1056/NEJMc2300805
6. Sarata AK, Dabrowska A, Johnson JA, Thaul S. FDA Reauthorization Act of 2017. Accessed August 15, 2023. https://sgp.fas.org/crs/misc/R44961.pdf
7. US Food and Drug Administration. FDA’s sentinel initiative–background. February 2, 2022. Updated February 4, 2022. Accessed August 15, 2023. https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
8. Bajema KL, Dahl RM, Prill MM, et al; SUPERNOVA COVID-19; Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly. 2021;70(37):1294-1299. doi:10.15585/mmwr.mm7037e3
9. Sharma A, Oda G, Holodniy M. COVID-19 vaccine breakthrough infections in Veterans Health Administration. medRxiv. Posted September 26, 2021. doi:10.1101/2021.09.23.21263864
10. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of third doses of mRNA-based COVID-19 vaccines in US veterans. Nat Microbiol. 2023;8(1):55-63. doi:10.1038/s41564-022-01272-z
11. Tang F, Hammel IS, Andrew MK, Ruiz JG. Frailty reduces vaccine effectiveness against SARS-CoV-2 infection: a test-negative case control study using national VA data. J Nutr Health Aging. 2023;27(2):81-88. doi:10.1007/s12603-023-1885-1
12. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023;14(4):e0102423. doi:10.1128/mbio.01024-23
13. US Department of Veterans Affairs. VA science and health initiative to combat infectious and emerging life-threatening diseases. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac64
14. Bilal MY. Similarity index–probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia. 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
Leveraging the Million Veteran Program Infrastructure and Data for a Rapid Research Response to COVID-19
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
VA Lessons From Partnering in COVID-19 Clinical Trials
The US Department of Veterans Affairs (VA), through its Office of Research and Development (ORD), supports an extensive and experienced clinical research enterprise, including the first multisite trials in the US.1 These resources contribute to the ORD support for the largest US integrated health care system, with a primary focus on the care and well-being of veterans. While the history of VA research has facilitated the creation of an experienced and organized research enterprise, the COVID-19 pandemic challenged VA to contribute even more significantly. These challenges became pronounced given the urgency associated with standing up VA sites for both therapeutic and vaccine trials.
VA Clinical Research Enterprise
The VA recognized an early need for an organized research response not only to address operational challenges resulting from COVID-19 but also ensure that the agency would be ready to support new scientific efforts focused specifically on the virus and related outcomes.2 As a result, the ORD took decisive action first by establishing itself as a central headquarters for VA COVID-19 research activities, and second, by leveraging existing resources, initiatives, and infrastructure to develop new mechanisms that would ensure that the VA was well positioned to develop or participate in research endeavors being driven by the VA as well federal, industry, and non-VA partners.
Prior to the pandemic, the ORD, through its Cooperative Studies Program (CSP), had strategies to address challenges associated with clinical trial startup and improved efficient conduct.3 For example, the VA Network of Dedicated Enrollment Sites (NODES) is a consortium of 23 VA medical centers (VAMCs) dedicated to rapid startup and recruitment into VA-sponsored clinical trials. NODES provides site-level expertise on clinical trial management, including troubleshooting challenges that may occur during clinical research execution.4 Another initiative, Access to Clinical Trials (ACT) for Veterans, engaged industry, academic, patient advocacy, and other partners to identify potential regulatory and operational hurdles to efficient startup activities specific to externally sponsored multisite clinical trials. Under ACT for Veterans, stakeholders emphasized the importance of developing a single VA point of contact for external partners to work with to more efficiently understand and navigate the VA system. In turn, such a resource could be designed to facilitate substantive research and long-term relationships with compatible external partners. Targeted to launch in April 2020, the Partnered Research Program (PRP) was expedited to respond to the pandemic.
During the pandemic, new VA efforts included the creation of the VA CoronavirUs Research and Efficacy Studies (VA CURES) network, initially established as a clinical trial master protocol framework to support and maximize VA-funded COVID-19 trial efficiency.5 VA CURES joined the consortium of trials networks funded by the National Heart, Lung, and Blood Institute. It began treatment trials under Accelerating COVID-19 Therapeutic Interventions and Vaccination (ACTIV), specifically ACTIV-4. The VA also partnered with the National Institutes of Allergy and Infectious Diseases (NIAID) by organizing the VA International Coordinating Center (VA ICC) for other ACTIV trials (ACTIV-2 and -3). When approached to startup studies that included veterans and the VA health care system, these capabilities comprised the VA research response.
A Need for a New Approach
As the impact of the pandemic expanded and the need for effective treatments and vaccines grew, national calls were made to assess the capabilities and readiness of available clinical trials networks. Additionally, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority, ACTIV, NIAID Division of Clinical Research and Division of AIDS, and many pharmaceutical companies were starting to roll out trials of new therapeutics and vaccines. These groups approached the VA to help evaluate the safety and efficacy of several therapeutics and vaccines because they recognized several advantages of the VA enterprise, including its position as the nation’s largest integrated health care system, its diverse patient population, and its expertise in conducting clinical trials.
Although the VA was well positioned as an important player in a collaborative investigational approach to COVID-19 research, these trials required startup approaches that were significantly different from those it had employed in traditional, prepandemic, clinical research. Despite the VA being a single federal agency, each VAMC conducting research establishes its own practices to address both operational and regulatory requirements. This structure results in individual units that operate under different standard operating procedures. Efforts must be taken centrally to organize them into a singular network for the entire health care system. During a national crisis, when there was a need for rapid trial startup to answer safety and efficacy questions and participate under a common approach to protocol execution, this variability was neither manageable nor acceptable. Additionally, the intense resource demands associated with such research, coupled with frequent reporting requirements by VA leaders, Congress, and the White House, required that VAMCs function more like a single unit. Therefore, the ORD needed to develop VAMCs’ abilities to work collectively toward a common goal, share knowledge and experience, and capitalize on potential efficiencies concerning legal, regulatory, and operational processes.
Beginning August 2020, 39 VAMCs joined 7 large-scale collaborative COVID-19 therapeutic and vaccine trials. Through its COVID-19 Research Response Team, the ORD identified, engaged, and directed appropriate resources to support the VAMC under a centralized framework for study management (Table). Centralized management not only afforded VAMCs the opportunity to work more collectively and efficiently but also provided an important advantage by enabling the VA to collect and organize its experiences (and on occasion data) to provide a base for continual learning and improvement efforts. While others have described efforts undertaken across networks to advance learning health systems, the VA’s national scope and integration of research and clinical care allow greater opportunities to learn in a practical setting.6
Challenges and Best Practices
Using surveys, webinars, interviews, and observation from site and VA Central Office personnel, the ORD identified specific variables that prevented the VAMCs from quickly starting up as a clinical trial site. We also documented strategies, solutions, and recommendations for improving startup time lines. These were organized into 8 categories: (1) site infrastructure needs and capabilities; (2) study management roles and responsibilities; (3) educational resources and training; (4) local review requirements and procedures; (5) study design demands; (6) contracting and budgeting; (7) central-level systems and processes; and (8) communication between external partners and within the VA.
Site Infrastructure Needs and Capabilities
A primary impediment to rapid study startup was a lack of basic infrastructure, including staff, space, and the agility necessary for the changing demands of high-priority, high-enrolling trials. This observation is not unique to the VA.7 Initially, certain facilities located in hot spots where COVID-19 was more prevalent became high-interest targets for study placement, despite varying degrees of available research infrastructure. Furthermore, pandemic shutdowns and quarantines permitted fewer employees onsite. This resulted in inadequate staffing in personnel needed to support required startup activities and those needed to handle the high volume of study participants who were being recruited, screened, enrolled, and followed. Additionally, as clinical care needs and infection control practices were prioritized, clinical research space was often appropriated for these needs, making it difficult to find the space to conduct trials. Lastly, supply chain issues also posed unique challenges, sometimes making it difficult for participating VAMCs to obtain needed materials, such as IV solution bags of specific sizes and contents, safety injection needles, and IV line filters.
The VA was able to use central purchasing/contracting at coordinating centers or the VA Central Office to support investigators and assist with finding supplies and clinical research space. VAMCs with research operating budgets to cover startup costs were better positioned to handle funding delays. During the pandemic, the ORD further contracted to supply administrative support to research offices to address regulatory and other requirements needed for startup activities. The ability to expand such central contracts to procure clinical research staff and outpatient clinical research space may also prove useful in meeting key needs at a site.
Management Roles and Responsibilities
Ambiguous and variable roles and responsibilities among the various partners and stakeholders represented a challenge given the large-scale, national, or international operations involved in the trials. VA attempts to operate uniformly were further limited given that each sponsor or group had preferred methods for operating and/or organizing work under urgent time lines. For example, one trial involved a coordinating center, a contract research organization, and federal partners that each worked with individual sites. Consequently, VA study teams would receive messages that were conflicting or unclear.
The VA learned that studies need a single “source of truth” and/or central command structure in times of urgency. To mitigate conflicting messages, vaccine trials relied on a clearinghouse through the PRP to interpret requirements or work on behalf of all sites before key actions were taken. For studies with the NIAID, the VA relied on experienced staff at the CSP coordinating center at the Perry Point, Maryland, VAMC before beginning. This approach especially helped with the challenges of understaffing and sites’ lack of familiarity with complex platform trial designs and already-established network practices within the ACTIV-2 and ACTIV-3 studies.
Educational Resources and Training
Since VA participation in externally sponsored, multisite clinical trials traditionally relies on an individual VAMC study team and its local resources, transitioning to centralized approaches for COVID-19 multisite studies created barriers. Many VAMCs were unfamiliar with newer capabilities for more rapid regulatory reviews and approvals involving commercial institutional review boards (IRBs) and central VA information security and privacy reviews. While tools and resources were available to facilitate these processes, real-time use had not been fully tested. As a result, everyone had to learn as they went along.
The simultaneous establishment of workflows required the ORD to centralize operations and provide training and guidance to field personnel. Although many principal investigators and clinical research coordinators had trial experience, training required unlearning previous understandings of requirements to meet urgent time lines. ORD enterprise road maps, central tools, and training materials also were made available on a study-by-study basis. Open communication was vital to train on central study materials while opportunities to discuss, question, and share experiences and ideas were promoted. The ORD also sent regular emails to prepare for upcoming work and/or raise awareness of identified challenges.
Local Review Requirements/Procedures
The clinical trials were impacted by varying VAMC review requirements and approval processes. Although VA policy defines standard requirements, the timing and procedures are left to the individual facility to determine any local factors to accommodate and/or resource availability. While such an approach is well understood within the VA, external sponsors were not as familiar and assumed a more uniform approach across all sites. In response, some VAMCs established ad hoc research and development committee review procedures, allowing study teams to obtain the necessary reviews in a timely fashion. However, not all VAMCs had the infrastructure (especially when clinical personnel had been redeployed to other priorities) to respond with such agility. One critical role of the VA Central Office coordinating entities was to communicate and manage external sponsor and group expectations surrounding individual site review time lines. However, establishing policies and procedures that focus on streamlining local review processes helped to broadly mitigate the COVID-19 trial challenges.
Study Design Demands
The design of COVID-19 studies combined with the uncertainty of the pandemic required rapid protocol changes and adaptations that were often difficult to deliver. The multinetwork trials that the VA collaborated on were platform or master protocol designs. These designs emphasized overall goals (eg, treating patients requiring intensive care unit care). However, because this trial strategy also introduces complexities that may impact review and execution among those unfamiliar with it, there is a need for increased discussion and understanding of this methodology.8 For example, there can be shared control groups, reliance on specific criteria for halting because of safety or futility concerns, or continuation and expansion applied through an external review board. Delays may arise when changes to study protocols occur rapidly or frequently and necessitate new regulatory reviews, negotiation of new agreements, modifications to contracts, changes to entry criteria, etc.
While the VA has adopted a quality by design framework, VA investigators noted many missed opportunities related to looking at outcomes with new diagnostics, studies of serology, outcomes related to vaccinations, and understanding the natural history of disease in these trials.9 The limited opportunities for investigator input suggested that the advantages offered by platform designs were not maximized during pandemic-focused urgencies. It was unclear whether this barrier was created by a general lack of awareness by sponsors or a lack of opportunities. At the very least, quality by design approaches may help avoid redundancies in documentation or study processes at the central and site levels.
Contracting and Budgeting
Given external sponsorship of COVID-19 trials, efficient contracting and budgeting were critical for a rapid start up. The variability of processes associated with these trials created several challenges that were compounded by issues, such as site sub-agreements and budget documents that did not always go to the correct groups and individuals. Furthermore, the VA’s ability to use contracted resources (eg, tents, trailers, personnel) that external sponsors had built into their contracts was more difficult for VA as a federal agency governed by other statues and policies. This also put VAMCs at a disadvantage from a timing perspective, as the VA often required additional time to find equivalent solutions that met federal regulations.
Although the VA was able to establish contract solutions to some issues, time was still lost while working to secure initial funding. Additionally, for needs such as home phlebotomy—commended for convenience to veterans and research staff—and engaging a specialized research team in the Office of General Counsel, early awareness of protocol needs and sponsor solutions could allow VA to pursue alternatives sooner.
Central-Level Systems and Processes
Not all challenges were at the VAMC level. As the ORD explored solutions, it learned that various tools and study platforms were available but not considered. Applications, such as eConsent, and file-sharing platforms that met existing information security and privacy requirements were needed but had to comply with the Privacy Act of 1974, Federal Information Security Modernization Act, and other requirements. Using sponsor-provided devices, such as drug temperature monitoring equipment, required additional review to ensure that they met system requirements for a national health care system. In addition, the VA uses a clinical trials management review system; however, its implementation was new at the time these trials began. Furthermore, the system engaged with some commercial IRBs but not all. This resulted in additional delays as VAMCs and central resources worked to familiarize themselves with the system and procedures.
The ability to work collaboratively across the VA includes having a framework in which key startup processes are standardized. This allows for efficiency and minimizes variability. Also, all stakeholders should understand the importance of holding discussions to identify appropriate solutions, guidance, and instruction. Finally, the VA must strive to be more nimble when adapting technological, regulatory, and financial processes.
Internal and External Communication
The value of communication—both internal and external—cannot be understated. Minimizing confusion, managing expectations, and ensuring consistent messaging were essential for rapid trial execution. Despite being the second largest federal agency, the VA did not have a seat at the study leadership table for several protocols. When it joined later, several study aspects were set and/or difficult to revise. Challenges affecting time and securing resources have been noted. The ability to plan and then share expectations and responsibilities across and within the respective participating organizations early in the process was perhaps the single factor that was most addressable. The VA enterprise organization and integration with other units could accentuate key communications that would be essential in time-sensitive activities.
VA as a Partner for Future Research
Before the pandemic, the VA had already undertaken a path to enhance its ability to partner as part of the national biomedical research enterprise. The need for COVID-19 therapeutic and vaccine trials accelerated opportunities to plan and develop processes and capabilities to advance this path. As a key strength for VA scientific activities, clinical trials represent a primary medium by which to develop its partnerships. Learning and development have become part of a culture that expedites opportunities for veterans who actively seek ways to contribute to medical knowledge and treatments for their peers and the nation.
CONCLUSIONS
Challenges associated with rapid startup and completion of clinical trials have been discussed for some time. During the pandemic, needs and barriers were magnified because of the heightened urgency for evidence-based therapeutics and vaccines. While the VA faced similar problems as well as those specific to it as a health care system, it had the opportunity to learn and more systematically implement solutions to help in its partnered efforts.10 As an enterprise, the VA hopes to apply lessons learned, strategies, and best practices to further its goals to enhance veteran access to clinical trials and respond to any future need to quickly establish evidence bases in pandemics and other health emergencies that warrant the rapid implementation of research.
Acknowledgments
The activities reported here were supported by the US Department of Veterans Affairs, Office of Research and Development.
1. Hays MT; US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. A historical look at the establishment of the Department of Veterans Affairs Research & Development Program. Accessed August 28, 2023. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
2. Garcia AP, Huang GD, Arnheim L, Ramoni R, Clancy C. The VA research enterprise: a platform for national partnerships toward evidence building and scientific innovation. Fed Pract. 2023;40(suppl 5):S12-S17. doi:10.12788/fp.0425
3. Johnston SC, Lewis-Hall F, Bajpai A, et al. It’s time to harmonize clinical trial site standards. NAM Perspectives. October 9, 2017. Accessed August 28, 2023. https://nam.edu/wp-content/uploads/2017/10/Its-Time-to-Harmonize-Clinical-Trial-Site-1.pdf
4. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
5. US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. March 2022. Accessed August 23, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and
6. IOM Roundtable on Value & Science-Driven Care; Institute of Medicine. Continuous learning and improvement in health care. In: Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. National Academies Press (US); 2015:chap 2. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK284654 7. Institute of Medicine (US). Building an infrastructure to support clinical trials. In: Envisioning a Transformed Clinical Trials Enterprise in the United States. National Academies Press (US); 2012:chap 5. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK114656
8. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1-8. doi:10.1016/j.jclinepi.2020.04.025
9. Meeker-O’Connell A, Glessner C, Behm M, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439-444. doi:10.1177/1740774516643491
10. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
The US Department of Veterans Affairs (VA), through its Office of Research and Development (ORD), supports an extensive and experienced clinical research enterprise, including the first multisite trials in the US.1 These resources contribute to the ORD support for the largest US integrated health care system, with a primary focus on the care and well-being of veterans. While the history of VA research has facilitated the creation of an experienced and organized research enterprise, the COVID-19 pandemic challenged VA to contribute even more significantly. These challenges became pronounced given the urgency associated with standing up VA sites for both therapeutic and vaccine trials.
VA Clinical Research Enterprise
The VA recognized an early need for an organized research response not only to address operational challenges resulting from COVID-19 but also ensure that the agency would be ready to support new scientific efforts focused specifically on the virus and related outcomes.2 As a result, the ORD took decisive action first by establishing itself as a central headquarters for VA COVID-19 research activities, and second, by leveraging existing resources, initiatives, and infrastructure to develop new mechanisms that would ensure that the VA was well positioned to develop or participate in research endeavors being driven by the VA as well federal, industry, and non-VA partners.
Prior to the pandemic, the ORD, through its Cooperative Studies Program (CSP), had strategies to address challenges associated with clinical trial startup and improved efficient conduct.3 For example, the VA Network of Dedicated Enrollment Sites (NODES) is a consortium of 23 VA medical centers (VAMCs) dedicated to rapid startup and recruitment into VA-sponsored clinical trials. NODES provides site-level expertise on clinical trial management, including troubleshooting challenges that may occur during clinical research execution.4 Another initiative, Access to Clinical Trials (ACT) for Veterans, engaged industry, academic, patient advocacy, and other partners to identify potential regulatory and operational hurdles to efficient startup activities specific to externally sponsored multisite clinical trials. Under ACT for Veterans, stakeholders emphasized the importance of developing a single VA point of contact for external partners to work with to more efficiently understand and navigate the VA system. In turn, such a resource could be designed to facilitate substantive research and long-term relationships with compatible external partners. Targeted to launch in April 2020, the Partnered Research Program (PRP) was expedited to respond to the pandemic.
During the pandemic, new VA efforts included the creation of the VA CoronavirUs Research and Efficacy Studies (VA CURES) network, initially established as a clinical trial master protocol framework to support and maximize VA-funded COVID-19 trial efficiency.5 VA CURES joined the consortium of trials networks funded by the National Heart, Lung, and Blood Institute. It began treatment trials under Accelerating COVID-19 Therapeutic Interventions and Vaccination (ACTIV), specifically ACTIV-4. The VA also partnered with the National Institutes of Allergy and Infectious Diseases (NIAID) by organizing the VA International Coordinating Center (VA ICC) for other ACTIV trials (ACTIV-2 and -3). When approached to startup studies that included veterans and the VA health care system, these capabilities comprised the VA research response.
A Need for a New Approach
As the impact of the pandemic expanded and the need for effective treatments and vaccines grew, national calls were made to assess the capabilities and readiness of available clinical trials networks. Additionally, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority, ACTIV, NIAID Division of Clinical Research and Division of AIDS, and many pharmaceutical companies were starting to roll out trials of new therapeutics and vaccines. These groups approached the VA to help evaluate the safety and efficacy of several therapeutics and vaccines because they recognized several advantages of the VA enterprise, including its position as the nation’s largest integrated health care system, its diverse patient population, and its expertise in conducting clinical trials.
Although the VA was well positioned as an important player in a collaborative investigational approach to COVID-19 research, these trials required startup approaches that were significantly different from those it had employed in traditional, prepandemic, clinical research. Despite the VA being a single federal agency, each VAMC conducting research establishes its own practices to address both operational and regulatory requirements. This structure results in individual units that operate under different standard operating procedures. Efforts must be taken centrally to organize them into a singular network for the entire health care system. During a national crisis, when there was a need for rapid trial startup to answer safety and efficacy questions and participate under a common approach to protocol execution, this variability was neither manageable nor acceptable. Additionally, the intense resource demands associated with such research, coupled with frequent reporting requirements by VA leaders, Congress, and the White House, required that VAMCs function more like a single unit. Therefore, the ORD needed to develop VAMCs’ abilities to work collectively toward a common goal, share knowledge and experience, and capitalize on potential efficiencies concerning legal, regulatory, and operational processes.
Beginning August 2020, 39 VAMCs joined 7 large-scale collaborative COVID-19 therapeutic and vaccine trials. Through its COVID-19 Research Response Team, the ORD identified, engaged, and directed appropriate resources to support the VAMC under a centralized framework for study management (Table). Centralized management not only afforded VAMCs the opportunity to work more collectively and efficiently but also provided an important advantage by enabling the VA to collect and organize its experiences (and on occasion data) to provide a base for continual learning and improvement efforts. While others have described efforts undertaken across networks to advance learning health systems, the VA’s national scope and integration of research and clinical care allow greater opportunities to learn in a practical setting.6
Challenges and Best Practices
Using surveys, webinars, interviews, and observation from site and VA Central Office personnel, the ORD identified specific variables that prevented the VAMCs from quickly starting up as a clinical trial site. We also documented strategies, solutions, and recommendations for improving startup time lines. These were organized into 8 categories: (1) site infrastructure needs and capabilities; (2) study management roles and responsibilities; (3) educational resources and training; (4) local review requirements and procedures; (5) study design demands; (6) contracting and budgeting; (7) central-level systems and processes; and (8) communication between external partners and within the VA.
Site Infrastructure Needs and Capabilities
A primary impediment to rapid study startup was a lack of basic infrastructure, including staff, space, and the agility necessary for the changing demands of high-priority, high-enrolling trials. This observation is not unique to the VA.7 Initially, certain facilities located in hot spots where COVID-19 was more prevalent became high-interest targets for study placement, despite varying degrees of available research infrastructure. Furthermore, pandemic shutdowns and quarantines permitted fewer employees onsite. This resulted in inadequate staffing in personnel needed to support required startup activities and those needed to handle the high volume of study participants who were being recruited, screened, enrolled, and followed. Additionally, as clinical care needs and infection control practices were prioritized, clinical research space was often appropriated for these needs, making it difficult to find the space to conduct trials. Lastly, supply chain issues also posed unique challenges, sometimes making it difficult for participating VAMCs to obtain needed materials, such as IV solution bags of specific sizes and contents, safety injection needles, and IV line filters.
The VA was able to use central purchasing/contracting at coordinating centers or the VA Central Office to support investigators and assist with finding supplies and clinical research space. VAMCs with research operating budgets to cover startup costs were better positioned to handle funding delays. During the pandemic, the ORD further contracted to supply administrative support to research offices to address regulatory and other requirements needed for startup activities. The ability to expand such central contracts to procure clinical research staff and outpatient clinical research space may also prove useful in meeting key needs at a site.
Management Roles and Responsibilities
Ambiguous and variable roles and responsibilities among the various partners and stakeholders represented a challenge given the large-scale, national, or international operations involved in the trials. VA attempts to operate uniformly were further limited given that each sponsor or group had preferred methods for operating and/or organizing work under urgent time lines. For example, one trial involved a coordinating center, a contract research organization, and federal partners that each worked with individual sites. Consequently, VA study teams would receive messages that were conflicting or unclear.
The VA learned that studies need a single “source of truth” and/or central command structure in times of urgency. To mitigate conflicting messages, vaccine trials relied on a clearinghouse through the PRP to interpret requirements or work on behalf of all sites before key actions were taken. For studies with the NIAID, the VA relied on experienced staff at the CSP coordinating center at the Perry Point, Maryland, VAMC before beginning. This approach especially helped with the challenges of understaffing and sites’ lack of familiarity with complex platform trial designs and already-established network practices within the ACTIV-2 and ACTIV-3 studies.
Educational Resources and Training
Since VA participation in externally sponsored, multisite clinical trials traditionally relies on an individual VAMC study team and its local resources, transitioning to centralized approaches for COVID-19 multisite studies created barriers. Many VAMCs were unfamiliar with newer capabilities for more rapid regulatory reviews and approvals involving commercial institutional review boards (IRBs) and central VA information security and privacy reviews. While tools and resources were available to facilitate these processes, real-time use had not been fully tested. As a result, everyone had to learn as they went along.
The simultaneous establishment of workflows required the ORD to centralize operations and provide training and guidance to field personnel. Although many principal investigators and clinical research coordinators had trial experience, training required unlearning previous understandings of requirements to meet urgent time lines. ORD enterprise road maps, central tools, and training materials also were made available on a study-by-study basis. Open communication was vital to train on central study materials while opportunities to discuss, question, and share experiences and ideas were promoted. The ORD also sent regular emails to prepare for upcoming work and/or raise awareness of identified challenges.
Local Review Requirements/Procedures
The clinical trials were impacted by varying VAMC review requirements and approval processes. Although VA policy defines standard requirements, the timing and procedures are left to the individual facility to determine any local factors to accommodate and/or resource availability. While such an approach is well understood within the VA, external sponsors were not as familiar and assumed a more uniform approach across all sites. In response, some VAMCs established ad hoc research and development committee review procedures, allowing study teams to obtain the necessary reviews in a timely fashion. However, not all VAMCs had the infrastructure (especially when clinical personnel had been redeployed to other priorities) to respond with such agility. One critical role of the VA Central Office coordinating entities was to communicate and manage external sponsor and group expectations surrounding individual site review time lines. However, establishing policies and procedures that focus on streamlining local review processes helped to broadly mitigate the COVID-19 trial challenges.
Study Design Demands
The design of COVID-19 studies combined with the uncertainty of the pandemic required rapid protocol changes and adaptations that were often difficult to deliver. The multinetwork trials that the VA collaborated on were platform or master protocol designs. These designs emphasized overall goals (eg, treating patients requiring intensive care unit care). However, because this trial strategy also introduces complexities that may impact review and execution among those unfamiliar with it, there is a need for increased discussion and understanding of this methodology.8 For example, there can be shared control groups, reliance on specific criteria for halting because of safety or futility concerns, or continuation and expansion applied through an external review board. Delays may arise when changes to study protocols occur rapidly or frequently and necessitate new regulatory reviews, negotiation of new agreements, modifications to contracts, changes to entry criteria, etc.
While the VA has adopted a quality by design framework, VA investigators noted many missed opportunities related to looking at outcomes with new diagnostics, studies of serology, outcomes related to vaccinations, and understanding the natural history of disease in these trials.9 The limited opportunities for investigator input suggested that the advantages offered by platform designs were not maximized during pandemic-focused urgencies. It was unclear whether this barrier was created by a general lack of awareness by sponsors or a lack of opportunities. At the very least, quality by design approaches may help avoid redundancies in documentation or study processes at the central and site levels.
Contracting and Budgeting
Given external sponsorship of COVID-19 trials, efficient contracting and budgeting were critical for a rapid start up. The variability of processes associated with these trials created several challenges that were compounded by issues, such as site sub-agreements and budget documents that did not always go to the correct groups and individuals. Furthermore, the VA’s ability to use contracted resources (eg, tents, trailers, personnel) that external sponsors had built into their contracts was more difficult for VA as a federal agency governed by other statues and policies. This also put VAMCs at a disadvantage from a timing perspective, as the VA often required additional time to find equivalent solutions that met federal regulations.
Although the VA was able to establish contract solutions to some issues, time was still lost while working to secure initial funding. Additionally, for needs such as home phlebotomy—commended for convenience to veterans and research staff—and engaging a specialized research team in the Office of General Counsel, early awareness of protocol needs and sponsor solutions could allow VA to pursue alternatives sooner.
Central-Level Systems and Processes
Not all challenges were at the VAMC level. As the ORD explored solutions, it learned that various tools and study platforms were available but not considered. Applications, such as eConsent, and file-sharing platforms that met existing information security and privacy requirements were needed but had to comply with the Privacy Act of 1974, Federal Information Security Modernization Act, and other requirements. Using sponsor-provided devices, such as drug temperature monitoring equipment, required additional review to ensure that they met system requirements for a national health care system. In addition, the VA uses a clinical trials management review system; however, its implementation was new at the time these trials began. Furthermore, the system engaged with some commercial IRBs but not all. This resulted in additional delays as VAMCs and central resources worked to familiarize themselves with the system and procedures.
The ability to work collaboratively across the VA includes having a framework in which key startup processes are standardized. This allows for efficiency and minimizes variability. Also, all stakeholders should understand the importance of holding discussions to identify appropriate solutions, guidance, and instruction. Finally, the VA must strive to be more nimble when adapting technological, regulatory, and financial processes.
Internal and External Communication
The value of communication—both internal and external—cannot be understated. Minimizing confusion, managing expectations, and ensuring consistent messaging were essential for rapid trial execution. Despite being the second largest federal agency, the VA did not have a seat at the study leadership table for several protocols. When it joined later, several study aspects were set and/or difficult to revise. Challenges affecting time and securing resources have been noted. The ability to plan and then share expectations and responsibilities across and within the respective participating organizations early in the process was perhaps the single factor that was most addressable. The VA enterprise organization and integration with other units could accentuate key communications that would be essential in time-sensitive activities.
VA as a Partner for Future Research
Before the pandemic, the VA had already undertaken a path to enhance its ability to partner as part of the national biomedical research enterprise. The need for COVID-19 therapeutic and vaccine trials accelerated opportunities to plan and develop processes and capabilities to advance this path. As a key strength for VA scientific activities, clinical trials represent a primary medium by which to develop its partnerships. Learning and development have become part of a culture that expedites opportunities for veterans who actively seek ways to contribute to medical knowledge and treatments for their peers and the nation.
CONCLUSIONS
Challenges associated with rapid startup and completion of clinical trials have been discussed for some time. During the pandemic, needs and barriers were magnified because of the heightened urgency for evidence-based therapeutics and vaccines. While the VA faced similar problems as well as those specific to it as a health care system, it had the opportunity to learn and more systematically implement solutions to help in its partnered efforts.10 As an enterprise, the VA hopes to apply lessons learned, strategies, and best practices to further its goals to enhance veteran access to clinical trials and respond to any future need to quickly establish evidence bases in pandemics and other health emergencies that warrant the rapid implementation of research.
Acknowledgments
The activities reported here were supported by the US Department of Veterans Affairs, Office of Research and Development.
The US Department of Veterans Affairs (VA), through its Office of Research and Development (ORD), supports an extensive and experienced clinical research enterprise, including the first multisite trials in the US.1 These resources contribute to the ORD support for the largest US integrated health care system, with a primary focus on the care and well-being of veterans. While the history of VA research has facilitated the creation of an experienced and organized research enterprise, the COVID-19 pandemic challenged VA to contribute even more significantly. These challenges became pronounced given the urgency associated with standing up VA sites for both therapeutic and vaccine trials.
VA Clinical Research Enterprise
The VA recognized an early need for an organized research response not only to address operational challenges resulting from COVID-19 but also ensure that the agency would be ready to support new scientific efforts focused specifically on the virus and related outcomes.2 As a result, the ORD took decisive action first by establishing itself as a central headquarters for VA COVID-19 research activities, and second, by leveraging existing resources, initiatives, and infrastructure to develop new mechanisms that would ensure that the VA was well positioned to develop or participate in research endeavors being driven by the VA as well federal, industry, and non-VA partners.
Prior to the pandemic, the ORD, through its Cooperative Studies Program (CSP), had strategies to address challenges associated with clinical trial startup and improved efficient conduct.3 For example, the VA Network of Dedicated Enrollment Sites (NODES) is a consortium of 23 VA medical centers (VAMCs) dedicated to rapid startup and recruitment into VA-sponsored clinical trials. NODES provides site-level expertise on clinical trial management, including troubleshooting challenges that may occur during clinical research execution.4 Another initiative, Access to Clinical Trials (ACT) for Veterans, engaged industry, academic, patient advocacy, and other partners to identify potential regulatory and operational hurdles to efficient startup activities specific to externally sponsored multisite clinical trials. Under ACT for Veterans, stakeholders emphasized the importance of developing a single VA point of contact for external partners to work with to more efficiently understand and navigate the VA system. In turn, such a resource could be designed to facilitate substantive research and long-term relationships with compatible external partners. Targeted to launch in April 2020, the Partnered Research Program (PRP) was expedited to respond to the pandemic.
During the pandemic, new VA efforts included the creation of the VA CoronavirUs Research and Efficacy Studies (VA CURES) network, initially established as a clinical trial master protocol framework to support and maximize VA-funded COVID-19 trial efficiency.5 VA CURES joined the consortium of trials networks funded by the National Heart, Lung, and Blood Institute. It began treatment trials under Accelerating COVID-19 Therapeutic Interventions and Vaccination (ACTIV), specifically ACTIV-4. The VA also partnered with the National Institutes of Allergy and Infectious Diseases (NIAID) by organizing the VA International Coordinating Center (VA ICC) for other ACTIV trials (ACTIV-2 and -3). When approached to startup studies that included veterans and the VA health care system, these capabilities comprised the VA research response.
A Need for a New Approach
As the impact of the pandemic expanded and the need for effective treatments and vaccines grew, national calls were made to assess the capabilities and readiness of available clinical trials networks. Additionally, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority, ACTIV, NIAID Division of Clinical Research and Division of AIDS, and many pharmaceutical companies were starting to roll out trials of new therapeutics and vaccines. These groups approached the VA to help evaluate the safety and efficacy of several therapeutics and vaccines because they recognized several advantages of the VA enterprise, including its position as the nation’s largest integrated health care system, its diverse patient population, and its expertise in conducting clinical trials.
Although the VA was well positioned as an important player in a collaborative investigational approach to COVID-19 research, these trials required startup approaches that were significantly different from those it had employed in traditional, prepandemic, clinical research. Despite the VA being a single federal agency, each VAMC conducting research establishes its own practices to address both operational and regulatory requirements. This structure results in individual units that operate under different standard operating procedures. Efforts must be taken centrally to organize them into a singular network for the entire health care system. During a national crisis, when there was a need for rapid trial startup to answer safety and efficacy questions and participate under a common approach to protocol execution, this variability was neither manageable nor acceptable. Additionally, the intense resource demands associated with such research, coupled with frequent reporting requirements by VA leaders, Congress, and the White House, required that VAMCs function more like a single unit. Therefore, the ORD needed to develop VAMCs’ abilities to work collectively toward a common goal, share knowledge and experience, and capitalize on potential efficiencies concerning legal, regulatory, and operational processes.
Beginning August 2020, 39 VAMCs joined 7 large-scale collaborative COVID-19 therapeutic and vaccine trials. Through its COVID-19 Research Response Team, the ORD identified, engaged, and directed appropriate resources to support the VAMC under a centralized framework for study management (Table). Centralized management not only afforded VAMCs the opportunity to work more collectively and efficiently but also provided an important advantage by enabling the VA to collect and organize its experiences (and on occasion data) to provide a base for continual learning and improvement efforts. While others have described efforts undertaken across networks to advance learning health systems, the VA’s national scope and integration of research and clinical care allow greater opportunities to learn in a practical setting.6
Challenges and Best Practices
Using surveys, webinars, interviews, and observation from site and VA Central Office personnel, the ORD identified specific variables that prevented the VAMCs from quickly starting up as a clinical trial site. We also documented strategies, solutions, and recommendations for improving startup time lines. These were organized into 8 categories: (1) site infrastructure needs and capabilities; (2) study management roles and responsibilities; (3) educational resources and training; (4) local review requirements and procedures; (5) study design demands; (6) contracting and budgeting; (7) central-level systems and processes; and (8) communication between external partners and within the VA.
Site Infrastructure Needs and Capabilities
A primary impediment to rapid study startup was a lack of basic infrastructure, including staff, space, and the agility necessary for the changing demands of high-priority, high-enrolling trials. This observation is not unique to the VA.7 Initially, certain facilities located in hot spots where COVID-19 was more prevalent became high-interest targets for study placement, despite varying degrees of available research infrastructure. Furthermore, pandemic shutdowns and quarantines permitted fewer employees onsite. This resulted in inadequate staffing in personnel needed to support required startup activities and those needed to handle the high volume of study participants who were being recruited, screened, enrolled, and followed. Additionally, as clinical care needs and infection control practices were prioritized, clinical research space was often appropriated for these needs, making it difficult to find the space to conduct trials. Lastly, supply chain issues also posed unique challenges, sometimes making it difficult for participating VAMCs to obtain needed materials, such as IV solution bags of specific sizes and contents, safety injection needles, and IV line filters.
The VA was able to use central purchasing/contracting at coordinating centers or the VA Central Office to support investigators and assist with finding supplies and clinical research space. VAMCs with research operating budgets to cover startup costs were better positioned to handle funding delays. During the pandemic, the ORD further contracted to supply administrative support to research offices to address regulatory and other requirements needed for startup activities. The ability to expand such central contracts to procure clinical research staff and outpatient clinical research space may also prove useful in meeting key needs at a site.
Management Roles and Responsibilities
Ambiguous and variable roles and responsibilities among the various partners and stakeholders represented a challenge given the large-scale, national, or international operations involved in the trials. VA attempts to operate uniformly were further limited given that each sponsor or group had preferred methods for operating and/or organizing work under urgent time lines. For example, one trial involved a coordinating center, a contract research organization, and federal partners that each worked with individual sites. Consequently, VA study teams would receive messages that were conflicting or unclear.
The VA learned that studies need a single “source of truth” and/or central command structure in times of urgency. To mitigate conflicting messages, vaccine trials relied on a clearinghouse through the PRP to interpret requirements or work on behalf of all sites before key actions were taken. For studies with the NIAID, the VA relied on experienced staff at the CSP coordinating center at the Perry Point, Maryland, VAMC before beginning. This approach especially helped with the challenges of understaffing and sites’ lack of familiarity with complex platform trial designs and already-established network practices within the ACTIV-2 and ACTIV-3 studies.
Educational Resources and Training
Since VA participation in externally sponsored, multisite clinical trials traditionally relies on an individual VAMC study team and its local resources, transitioning to centralized approaches for COVID-19 multisite studies created barriers. Many VAMCs were unfamiliar with newer capabilities for more rapid regulatory reviews and approvals involving commercial institutional review boards (IRBs) and central VA information security and privacy reviews. While tools and resources were available to facilitate these processes, real-time use had not been fully tested. As a result, everyone had to learn as they went along.
The simultaneous establishment of workflows required the ORD to centralize operations and provide training and guidance to field personnel. Although many principal investigators and clinical research coordinators had trial experience, training required unlearning previous understandings of requirements to meet urgent time lines. ORD enterprise road maps, central tools, and training materials also were made available on a study-by-study basis. Open communication was vital to train on central study materials while opportunities to discuss, question, and share experiences and ideas were promoted. The ORD also sent regular emails to prepare for upcoming work and/or raise awareness of identified challenges.
Local Review Requirements/Procedures
The clinical trials were impacted by varying VAMC review requirements and approval processes. Although VA policy defines standard requirements, the timing and procedures are left to the individual facility to determine any local factors to accommodate and/or resource availability. While such an approach is well understood within the VA, external sponsors were not as familiar and assumed a more uniform approach across all sites. In response, some VAMCs established ad hoc research and development committee review procedures, allowing study teams to obtain the necessary reviews in a timely fashion. However, not all VAMCs had the infrastructure (especially when clinical personnel had been redeployed to other priorities) to respond with such agility. One critical role of the VA Central Office coordinating entities was to communicate and manage external sponsor and group expectations surrounding individual site review time lines. However, establishing policies and procedures that focus on streamlining local review processes helped to broadly mitigate the COVID-19 trial challenges.
Study Design Demands
The design of COVID-19 studies combined with the uncertainty of the pandemic required rapid protocol changes and adaptations that were often difficult to deliver. The multinetwork trials that the VA collaborated on were platform or master protocol designs. These designs emphasized overall goals (eg, treating patients requiring intensive care unit care). However, because this trial strategy also introduces complexities that may impact review and execution among those unfamiliar with it, there is a need for increased discussion and understanding of this methodology.8 For example, there can be shared control groups, reliance on specific criteria for halting because of safety or futility concerns, or continuation and expansion applied through an external review board. Delays may arise when changes to study protocols occur rapidly or frequently and necessitate new regulatory reviews, negotiation of new agreements, modifications to contracts, changes to entry criteria, etc.
While the VA has adopted a quality by design framework, VA investigators noted many missed opportunities related to looking at outcomes with new diagnostics, studies of serology, outcomes related to vaccinations, and understanding the natural history of disease in these trials.9 The limited opportunities for investigator input suggested that the advantages offered by platform designs were not maximized during pandemic-focused urgencies. It was unclear whether this barrier was created by a general lack of awareness by sponsors or a lack of opportunities. At the very least, quality by design approaches may help avoid redundancies in documentation or study processes at the central and site levels.
Contracting and Budgeting
Given external sponsorship of COVID-19 trials, efficient contracting and budgeting were critical for a rapid start up. The variability of processes associated with these trials created several challenges that were compounded by issues, such as site sub-agreements and budget documents that did not always go to the correct groups and individuals. Furthermore, the VA’s ability to use contracted resources (eg, tents, trailers, personnel) that external sponsors had built into their contracts was more difficult for VA as a federal agency governed by other statues and policies. This also put VAMCs at a disadvantage from a timing perspective, as the VA often required additional time to find equivalent solutions that met federal regulations.
Although the VA was able to establish contract solutions to some issues, time was still lost while working to secure initial funding. Additionally, for needs such as home phlebotomy—commended for convenience to veterans and research staff—and engaging a specialized research team in the Office of General Counsel, early awareness of protocol needs and sponsor solutions could allow VA to pursue alternatives sooner.
Central-Level Systems and Processes
Not all challenges were at the VAMC level. As the ORD explored solutions, it learned that various tools and study platforms were available but not considered. Applications, such as eConsent, and file-sharing platforms that met existing information security and privacy requirements were needed but had to comply with the Privacy Act of 1974, Federal Information Security Modernization Act, and other requirements. Using sponsor-provided devices, such as drug temperature monitoring equipment, required additional review to ensure that they met system requirements for a national health care system. In addition, the VA uses a clinical trials management review system; however, its implementation was new at the time these trials began. Furthermore, the system engaged with some commercial IRBs but not all. This resulted in additional delays as VAMCs and central resources worked to familiarize themselves with the system and procedures.
The ability to work collaboratively across the VA includes having a framework in which key startup processes are standardized. This allows for efficiency and minimizes variability. Also, all stakeholders should understand the importance of holding discussions to identify appropriate solutions, guidance, and instruction. Finally, the VA must strive to be more nimble when adapting technological, regulatory, and financial processes.
Internal and External Communication
The value of communication—both internal and external—cannot be understated. Minimizing confusion, managing expectations, and ensuring consistent messaging were essential for rapid trial execution. Despite being the second largest federal agency, the VA did not have a seat at the study leadership table for several protocols. When it joined later, several study aspects were set and/or difficult to revise. Challenges affecting time and securing resources have been noted. The ability to plan and then share expectations and responsibilities across and within the respective participating organizations early in the process was perhaps the single factor that was most addressable. The VA enterprise organization and integration with other units could accentuate key communications that would be essential in time-sensitive activities.
VA as a Partner for Future Research
Before the pandemic, the VA had already undertaken a path to enhance its ability to partner as part of the national biomedical research enterprise. The need for COVID-19 therapeutic and vaccine trials accelerated opportunities to plan and develop processes and capabilities to advance this path. As a key strength for VA scientific activities, clinical trials represent a primary medium by which to develop its partnerships. Learning and development have become part of a culture that expedites opportunities for veterans who actively seek ways to contribute to medical knowledge and treatments for their peers and the nation.
CONCLUSIONS
Challenges associated with rapid startup and completion of clinical trials have been discussed for some time. During the pandemic, needs and barriers were magnified because of the heightened urgency for evidence-based therapeutics and vaccines. While the VA faced similar problems as well as those specific to it as a health care system, it had the opportunity to learn and more systematically implement solutions to help in its partnered efforts.10 As an enterprise, the VA hopes to apply lessons learned, strategies, and best practices to further its goals to enhance veteran access to clinical trials and respond to any future need to quickly establish evidence bases in pandemics and other health emergencies that warrant the rapid implementation of research.
Acknowledgments
The activities reported here were supported by the US Department of Veterans Affairs, Office of Research and Development.
1. Hays MT; US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. A historical look at the establishment of the Department of Veterans Affairs Research & Development Program. Accessed August 28, 2023. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
2. Garcia AP, Huang GD, Arnheim L, Ramoni R, Clancy C. The VA research enterprise: a platform for national partnerships toward evidence building and scientific innovation. Fed Pract. 2023;40(suppl 5):S12-S17. doi:10.12788/fp.0425
3. Johnston SC, Lewis-Hall F, Bajpai A, et al. It’s time to harmonize clinical trial site standards. NAM Perspectives. October 9, 2017. Accessed August 28, 2023. https://nam.edu/wp-content/uploads/2017/10/Its-Time-to-Harmonize-Clinical-Trial-Site-1.pdf
4. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
5. US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. March 2022. Accessed August 23, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and
6. IOM Roundtable on Value & Science-Driven Care; Institute of Medicine. Continuous learning and improvement in health care. In: Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. National Academies Press (US); 2015:chap 2. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK284654 7. Institute of Medicine (US). Building an infrastructure to support clinical trials. In: Envisioning a Transformed Clinical Trials Enterprise in the United States. National Academies Press (US); 2012:chap 5. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK114656
8. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1-8. doi:10.1016/j.jclinepi.2020.04.025
9. Meeker-O’Connell A, Glessner C, Behm M, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439-444. doi:10.1177/1740774516643491
10. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
1. Hays MT; US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. A historical look at the establishment of the Department of Veterans Affairs Research & Development Program. Accessed August 28, 2023. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
2. Garcia AP, Huang GD, Arnheim L, Ramoni R, Clancy C. The VA research enterprise: a platform for national partnerships toward evidence building and scientific innovation. Fed Pract. 2023;40(suppl 5):S12-S17. doi:10.12788/fp.0425
3. Johnston SC, Lewis-Hall F, Bajpai A, et al. It’s time to harmonize clinical trial site standards. NAM Perspectives. October 9, 2017. Accessed August 28, 2023. https://nam.edu/wp-content/uploads/2017/10/Its-Time-to-Harmonize-Clinical-Trial-Site-1.pdf
4. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
5. US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. March 2022. Accessed August 23, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and
6. IOM Roundtable on Value & Science-Driven Care; Institute of Medicine. Continuous learning and improvement in health care. In: Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. National Academies Press (US); 2015:chap 2. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK284654 7. Institute of Medicine (US). Building an infrastructure to support clinical trials. In: Envisioning a Transformed Clinical Trials Enterprise in the United States. National Academies Press (US); 2012:chap 5. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK114656
8. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1-8. doi:10.1016/j.jclinepi.2020.04.025
9. Meeker-O’Connell A, Glessner C, Behm M, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439-444. doi:10.1177/1740774516643491
10. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
The VA Research Enterprise: A Platform for National Partnerships Toward Evidence Building and Scientific Innovation
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
Using Active Surveillance to Identify Monoclonal Antibody Candidates Among COVID-19–Positive Veterans in the Atlanta VA Health Care System
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Olfactory Hallucinations Following COVID-19 Vaccination
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9