User login
VA Big Data Science: A Model for Improved National Pandemic Response Present and Future
The COVID-19 pandemic emphasized the need for rapid response research in health care. The robust enterprise approach used by the US Department of Veterans Affairs (VA), termed VA Research, is meeting these needs by using existing outstanding data resources and interdisciplinary collaborations.1 In the first 7 months of 2021 alone, while many US health care systems struggled with limited data, VA Research published more than 300 unique and instrumental research papers addressing urgent questions about transmission, vaccination, therapeutics, and health impacts of COVID-19 on its high-risk population.1 The ability to leverage the VA electronic health record (EHR) and Corporate Data Warehouse (CDW)—a fully established data system bringing together test results, prescriptions, and complete patient health records, readily accessible and updated daily—was substantial.
With more than 9 million veterans enrolled in care at 171 medical centers and 1113 outpatient facilities across the US and its territories, the CDW provides an unprecedented opportunity to examine outcomes in real time. This allowed research groups such as the VA St Louis Health Care System Research and Education Service to build a cohort of 181,280 veterans with diabetes and positive COVID-19 test results within a 6-month period in 2021 to study the incidence of new diagnoses of diabetes after COVID-19 infection.2 Similarly, the Clinical Epidemiology Program (CEP) at VA White River Junction Health Care System built a cohort of 1,363,180 veterans who received at least 1 COVID-19 vaccine by March 7, 2021, to analyze coverage and effectiveness of those vaccines
The innovation and speed of COVID-19 vaccine development and distribution in the US were unprecedented. The rapid discovery and implementation of multiple preventives and therapeutics for COVID-19 could not have been possible without shared information within a competitive industry. VA studies added significantly to understanding the clinical performance of the messenger RNA (mRNA) COVID-19 vaccines, antivirals, and monoclonal treatments in a real-world setting. For example, a vaccine coverage study by VA Research illustrated how successful vaccination for COVID-19 at the VA has been in protecting a diverse community of patients from hospitalization and death, particularly the highly comorbid, racial and ethnic minorities, and other high-risk populations.3 The study demonstrated the power of the VA system to generate robust and compelling clinical endpoint effectiveness data across a broad range of high-risk groups.
This success is promising. However, the COVID-19 pandemic is not over, and the next could prove even more challenging. For example, through a recent partnership with the US Department of Defense (DoD), the VA was able to rapidly analyze the effectiveness of previous smallpox vaccination efforts in the military for preventing mpox infections.5 We should take this opportunity to think creatively about ways to improve our existing infrastructure based on what we have learned.
A Role for VA Research in Efficacy
The US Food and Drug Administration (FDA) Reauthorization Act of 2017 requires that manufacturers submit evidence establishing a product’s benefits (effectiveness) outweigh its risks (safety) before it can be promoted and distributed.6 As such, the FDA has been obligated by external stakeholders and Congress to be more explicit and transparent about benefit-risk profile supporting its decisions on licensure. This process led to requiring more phase 4 postmarketing observational studies for safety and effectiveness.7 Although the FDA postlicensure system remains vigilant toward safety, effectiveness information is limited due to insufficient reporting (with exceptions of manufacturer studies for new indications or to exhibit superior comparative effectiveness). The agency typically relies on a static set of efficacy data generated prelicensure with a dynamic and evolving set of safety data accrued postlicensure to support its assessment that benefits outweigh risks.
For example, operating in near real time, postauthorization safety monitoring systems, led by the Centers for Disease Control and Prevention and other federal systems, identified a safety signal for thrombosis following the Janssen COVID-19 vaccination. Distribution was quickly paused, the safety signal was investigated, the magnitude of the risk was characterized, new language describing the risk and providing guidance regarding clinical management was included in labeling, and distribution was resumed, all within a few weeks. This remarkable success demonstrated how timely the safety system can operate to evaluate risk.
In contrast, the duration and extent of protection against COVID-19 variants are largely limited to the assessment of immune biomarker surrogates. Such clinical effectiveness data are urgently needed for the FDA’s Center for Biologics Evaluation and Research and Center for Drug Evaluation and Research to make accurate benefit-risk assessments and continue to conclude the balance is favorable. As we prepare for the next pandemic, we must consider plans for monitoring postauthorization/postlicensure effectiveness as well as safety in real time. VA Research is ideally situated for this task.
Published studies on effectiveness at the VA serve as a prototype and could lead the way to initiating those preparations.4,8-11 One of the striking features of the VA system that became apparent in the preparation of the mRNA vaccine study was the speed at which an enormous volume of COVID-19 testing data were produced. This enabled implementation of methodologically sound test-negative and case-control analysis. Analyses sufficiently powered to conclude mRNA vaccines were highly effective when used in real-world conditions among a diverse population from nearly every state and territory during a period in which multiple COVID-19 variants were already circulating.3 This is unique to the VA and would not be possible for any other US health care system. With planning, the VA system could produce product-specific, real-world evidence of effectiveness comparable to the timeliness and quality of the safety data currently produced to support regulatory benefit-risk assessments. For example, the VA conducted an effectiveness study of tixagevimab/cilgavimab for preventing COVID-19 during the initial Omicron surge, which is continually updated while Omicron circulates and repeatable for different subvariants.12
The FDA continues to collaborate with the VA on demonstration projects to evaluate the impact of available vaccines and treatment against COVID-19 variants. The VA has also initiated several large-scale sequencing programs for COVID-19 specimens that will support these efforts, including VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD), VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE).13,14 Successful proof-of-concept studies using these data could provide a template for VA and other medical systems/databases to report effectiveness in near real time.
Interagency Collaboration
The potential advantages of federal agencies working with the VA to build an infrastructure capable of generating real-world evidence effectiveness analyses in near real time is not limited to needs that will arise in the next pandemic. For example, generating randomized, placebo-controlled, clinical trial endpoint data on the effectiveness of new variant vaccines will be difficult from a feasibility and ethical standpoint. Combining the VA’s robust virus sequencing program with preexisting mechanisms, such as expanded access studies (allowed under FDA Investigational New Drug regulations), researchers could enable a large-scale effective evaluation program of vaccination with variant or universal COVID-19 vaccines, using rapidly accruing effectiveness data.
The pandemic created opportunities to advance innovative approaches to medical product development. Some have advocated these innovative approaches should proceed together toward a seamless convergence between the domains of medical research and clinical care. A shift toward expecting, as a matter of routine, effectiveness data to be generated in near real time and made available for benefit-risk assessment would be a useful step in that direction.
Expanding and sharing analytical platforms, including methodology and programming codes, will allow increased access to rapidly refreshed real-world data. A common adaptive platform of complete and continuously updated data will also enable a wider community of researchers to create multiple investigatory groups simultaneously accessing fully de-identified data for concurrent observational studies. In turn, researchers need to have programming, study design, and methodology ready in an open-source platform. An efficient platform would also require the adoption of artificial intelligence, natural language processing, imaging processing, and quantum computing for validation and improved data quality.
COVID-19 has demonstrated the need for open science data synchronization with universal access for faster action and improved outcomes able to gain public confidence. OpenSafely (UK), a software platform for analysis of EHR data that is shared automatically and openly for scientific review and efficient reuse, created a cohort of about 23.4 million records for observational review of monoclonal COVID-19 treatments. To keep pace with the UK, Israel, and other nationalized systems, the US would benefit from duplicating this example of coordination between federal agencies and their data repositories. For example, combining data between the DoD, which captures active military health care data through TRICARE, and VA, which follows postmilitary discharge, would create datasets encompassing complete life spans. Additionally, expanding the National COVID Cohort Collaborative (N3C) program—one of the largest collections of clinical data related to COVID-19 symptoms and patient outcomes in the US—to include EHR data from DoD, VA, Medicare, and Test to Treat initiative partners would further expand research capabilities. This could be accomplished through a framework of anonymized, readily available, harmonized data. EHRs with synchronized datasets from every health care practitioner—independent pharmacies, primary care physicians, and hospitals—could all work to create a de-identified, comprehensive, continuously updated, near real-time dataset accessible to all federal researchers.
Conclusions
The VA has been lauded for its rapid, effective response to the current pandemic. The successful management and prescription of vaccines and treatment to the largely high-risk veteran population was possible because of the existing data framework within the VA. VA Research continues to build and refine infrastructure to improve speed, quality, and value of data analytics. We can do more. Expanding partnerships to use existing VA data strategies in designing a cooperative national data alliance would deliver necessary progress to research and public health.
Acknowledgments
The authors thank Jeff Roberts, MD, for his insight on the US Food and Drug Administration, its responsibilities, and the potential benefit of real world data to its missions.
1. US Department of Veterans Affairs, Veterans Health Administration. Third report details VA’s continued efforts addressing COVID-19 pandemic. Accessed August 15, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5748
2. Xie Y, Ziyad A. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi:10.1016/S2213-8587(22)00044-4
3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4(10):e2128391. doi:10.1001/jamanetworkopen.2021.28391
4. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi:10.1056/NEJMoa2115463
5. Titanji BK, Eick-Cost A, Partan ES, et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. N Engl J Med. 2023;389(12):1147-1148. doi:10.1056/NEJMc2300805
6. Sarata AK, Dabrowska A, Johnson JA, Thaul S. FDA Reauthorization Act of 2017. Accessed August 15, 2023. https://sgp.fas.org/crs/misc/R44961.pdf
7. US Food and Drug Administration. FDA’s sentinel initiative–background. February 2, 2022. Updated February 4, 2022. Accessed August 15, 2023. https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
8. Bajema KL, Dahl RM, Prill MM, et al; SUPERNOVA COVID-19; Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly. 2021;70(37):1294-1299. doi:10.15585/mmwr.mm7037e3
9. Sharma A, Oda G, Holodniy M. COVID-19 vaccine breakthrough infections in Veterans Health Administration. medRxiv. Posted September 26, 2021. doi:10.1101/2021.09.23.21263864
10. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of third doses of mRNA-based COVID-19 vaccines in US veterans. Nat Microbiol. 2023;8(1):55-63. doi:10.1038/s41564-022-01272-z
11. Tang F, Hammel IS, Andrew MK, Ruiz JG. Frailty reduces vaccine effectiveness against SARS-CoV-2 infection: a test-negative case control study using national VA data. J Nutr Health Aging. 2023;27(2):81-88. doi:10.1007/s12603-023-1885-1
12. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023;14(4):e0102423. doi:10.1128/mbio.01024-23
13. US Department of Veterans Affairs. VA science and health initiative to combat infectious and emerging life-threatening diseases. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac64
14. Bilal MY. Similarity index–probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia. 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
The COVID-19 pandemic emphasized the need for rapid response research in health care. The robust enterprise approach used by the US Department of Veterans Affairs (VA), termed VA Research, is meeting these needs by using existing outstanding data resources and interdisciplinary collaborations.1 In the first 7 months of 2021 alone, while many US health care systems struggled with limited data, VA Research published more than 300 unique and instrumental research papers addressing urgent questions about transmission, vaccination, therapeutics, and health impacts of COVID-19 on its high-risk population.1 The ability to leverage the VA electronic health record (EHR) and Corporate Data Warehouse (CDW)—a fully established data system bringing together test results, prescriptions, and complete patient health records, readily accessible and updated daily—was substantial.
With more than 9 million veterans enrolled in care at 171 medical centers and 1113 outpatient facilities across the US and its territories, the CDW provides an unprecedented opportunity to examine outcomes in real time. This allowed research groups such as the VA St Louis Health Care System Research and Education Service to build a cohort of 181,280 veterans with diabetes and positive COVID-19 test results within a 6-month period in 2021 to study the incidence of new diagnoses of diabetes after COVID-19 infection.2 Similarly, the Clinical Epidemiology Program (CEP) at VA White River Junction Health Care System built a cohort of 1,363,180 veterans who received at least 1 COVID-19 vaccine by March 7, 2021, to analyze coverage and effectiveness of those vaccines
The innovation and speed of COVID-19 vaccine development and distribution in the US were unprecedented. The rapid discovery and implementation of multiple preventives and therapeutics for COVID-19 could not have been possible without shared information within a competitive industry. VA studies added significantly to understanding the clinical performance of the messenger RNA (mRNA) COVID-19 vaccines, antivirals, and monoclonal treatments in a real-world setting. For example, a vaccine coverage study by VA Research illustrated how successful vaccination for COVID-19 at the VA has been in protecting a diverse community of patients from hospitalization and death, particularly the highly comorbid, racial and ethnic minorities, and other high-risk populations.3 The study demonstrated the power of the VA system to generate robust and compelling clinical endpoint effectiveness data across a broad range of high-risk groups.
This success is promising. However, the COVID-19 pandemic is not over, and the next could prove even more challenging. For example, through a recent partnership with the US Department of Defense (DoD), the VA was able to rapidly analyze the effectiveness of previous smallpox vaccination efforts in the military for preventing mpox infections.5 We should take this opportunity to think creatively about ways to improve our existing infrastructure based on what we have learned.
A Role for VA Research in Efficacy
The US Food and Drug Administration (FDA) Reauthorization Act of 2017 requires that manufacturers submit evidence establishing a product’s benefits (effectiveness) outweigh its risks (safety) before it can be promoted and distributed.6 As such, the FDA has been obligated by external stakeholders and Congress to be more explicit and transparent about benefit-risk profile supporting its decisions on licensure. This process led to requiring more phase 4 postmarketing observational studies for safety and effectiveness.7 Although the FDA postlicensure system remains vigilant toward safety, effectiveness information is limited due to insufficient reporting (with exceptions of manufacturer studies for new indications or to exhibit superior comparative effectiveness). The agency typically relies on a static set of efficacy data generated prelicensure with a dynamic and evolving set of safety data accrued postlicensure to support its assessment that benefits outweigh risks.
For example, operating in near real time, postauthorization safety monitoring systems, led by the Centers for Disease Control and Prevention and other federal systems, identified a safety signal for thrombosis following the Janssen COVID-19 vaccination. Distribution was quickly paused, the safety signal was investigated, the magnitude of the risk was characterized, new language describing the risk and providing guidance regarding clinical management was included in labeling, and distribution was resumed, all within a few weeks. This remarkable success demonstrated how timely the safety system can operate to evaluate risk.
In contrast, the duration and extent of protection against COVID-19 variants are largely limited to the assessment of immune biomarker surrogates. Such clinical effectiveness data are urgently needed for the FDA’s Center for Biologics Evaluation and Research and Center for Drug Evaluation and Research to make accurate benefit-risk assessments and continue to conclude the balance is favorable. As we prepare for the next pandemic, we must consider plans for monitoring postauthorization/postlicensure effectiveness as well as safety in real time. VA Research is ideally situated for this task.
Published studies on effectiveness at the VA serve as a prototype and could lead the way to initiating those preparations.4,8-11 One of the striking features of the VA system that became apparent in the preparation of the mRNA vaccine study was the speed at which an enormous volume of COVID-19 testing data were produced. This enabled implementation of methodologically sound test-negative and case-control analysis. Analyses sufficiently powered to conclude mRNA vaccines were highly effective when used in real-world conditions among a diverse population from nearly every state and territory during a period in which multiple COVID-19 variants were already circulating.3 This is unique to the VA and would not be possible for any other US health care system. With planning, the VA system could produce product-specific, real-world evidence of effectiveness comparable to the timeliness and quality of the safety data currently produced to support regulatory benefit-risk assessments. For example, the VA conducted an effectiveness study of tixagevimab/cilgavimab for preventing COVID-19 during the initial Omicron surge, which is continually updated while Omicron circulates and repeatable for different subvariants.12
The FDA continues to collaborate with the VA on demonstration projects to evaluate the impact of available vaccines and treatment against COVID-19 variants. The VA has also initiated several large-scale sequencing programs for COVID-19 specimens that will support these efforts, including VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD), VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE).13,14 Successful proof-of-concept studies using these data could provide a template for VA and other medical systems/databases to report effectiveness in near real time.
Interagency Collaboration
The potential advantages of federal agencies working with the VA to build an infrastructure capable of generating real-world evidence effectiveness analyses in near real time is not limited to needs that will arise in the next pandemic. For example, generating randomized, placebo-controlled, clinical trial endpoint data on the effectiveness of new variant vaccines will be difficult from a feasibility and ethical standpoint. Combining the VA’s robust virus sequencing program with preexisting mechanisms, such as expanded access studies (allowed under FDA Investigational New Drug regulations), researchers could enable a large-scale effective evaluation program of vaccination with variant or universal COVID-19 vaccines, using rapidly accruing effectiveness data.
The pandemic created opportunities to advance innovative approaches to medical product development. Some have advocated these innovative approaches should proceed together toward a seamless convergence between the domains of medical research and clinical care. A shift toward expecting, as a matter of routine, effectiveness data to be generated in near real time and made available for benefit-risk assessment would be a useful step in that direction.
Expanding and sharing analytical platforms, including methodology and programming codes, will allow increased access to rapidly refreshed real-world data. A common adaptive platform of complete and continuously updated data will also enable a wider community of researchers to create multiple investigatory groups simultaneously accessing fully de-identified data for concurrent observational studies. In turn, researchers need to have programming, study design, and methodology ready in an open-source platform. An efficient platform would also require the adoption of artificial intelligence, natural language processing, imaging processing, and quantum computing for validation and improved data quality.
COVID-19 has demonstrated the need for open science data synchronization with universal access for faster action and improved outcomes able to gain public confidence. OpenSafely (UK), a software platform for analysis of EHR data that is shared automatically and openly for scientific review and efficient reuse, created a cohort of about 23.4 million records for observational review of monoclonal COVID-19 treatments. To keep pace with the UK, Israel, and other nationalized systems, the US would benefit from duplicating this example of coordination between federal agencies and their data repositories. For example, combining data between the DoD, which captures active military health care data through TRICARE, and VA, which follows postmilitary discharge, would create datasets encompassing complete life spans. Additionally, expanding the National COVID Cohort Collaborative (N3C) program—one of the largest collections of clinical data related to COVID-19 symptoms and patient outcomes in the US—to include EHR data from DoD, VA, Medicare, and Test to Treat initiative partners would further expand research capabilities. This could be accomplished through a framework of anonymized, readily available, harmonized data. EHRs with synchronized datasets from every health care practitioner—independent pharmacies, primary care physicians, and hospitals—could all work to create a de-identified, comprehensive, continuously updated, near real-time dataset accessible to all federal researchers.
Conclusions
The VA has been lauded for its rapid, effective response to the current pandemic. The successful management and prescription of vaccines and treatment to the largely high-risk veteran population was possible because of the existing data framework within the VA. VA Research continues to build and refine infrastructure to improve speed, quality, and value of data analytics. We can do more. Expanding partnerships to use existing VA data strategies in designing a cooperative national data alliance would deliver necessary progress to research and public health.
Acknowledgments
The authors thank Jeff Roberts, MD, for his insight on the US Food and Drug Administration, its responsibilities, and the potential benefit of real world data to its missions.
The COVID-19 pandemic emphasized the need for rapid response research in health care. The robust enterprise approach used by the US Department of Veterans Affairs (VA), termed VA Research, is meeting these needs by using existing outstanding data resources and interdisciplinary collaborations.1 In the first 7 months of 2021 alone, while many US health care systems struggled with limited data, VA Research published more than 300 unique and instrumental research papers addressing urgent questions about transmission, vaccination, therapeutics, and health impacts of COVID-19 on its high-risk population.1 The ability to leverage the VA electronic health record (EHR) and Corporate Data Warehouse (CDW)—a fully established data system bringing together test results, prescriptions, and complete patient health records, readily accessible and updated daily—was substantial.
With more than 9 million veterans enrolled in care at 171 medical centers and 1113 outpatient facilities across the US and its territories, the CDW provides an unprecedented opportunity to examine outcomes in real time. This allowed research groups such as the VA St Louis Health Care System Research and Education Service to build a cohort of 181,280 veterans with diabetes and positive COVID-19 test results within a 6-month period in 2021 to study the incidence of new diagnoses of diabetes after COVID-19 infection.2 Similarly, the Clinical Epidemiology Program (CEP) at VA White River Junction Health Care System built a cohort of 1,363,180 veterans who received at least 1 COVID-19 vaccine by March 7, 2021, to analyze coverage and effectiveness of those vaccines
The innovation and speed of COVID-19 vaccine development and distribution in the US were unprecedented. The rapid discovery and implementation of multiple preventives and therapeutics for COVID-19 could not have been possible without shared information within a competitive industry. VA studies added significantly to understanding the clinical performance of the messenger RNA (mRNA) COVID-19 vaccines, antivirals, and monoclonal treatments in a real-world setting. For example, a vaccine coverage study by VA Research illustrated how successful vaccination for COVID-19 at the VA has been in protecting a diverse community of patients from hospitalization and death, particularly the highly comorbid, racial and ethnic minorities, and other high-risk populations.3 The study demonstrated the power of the VA system to generate robust and compelling clinical endpoint effectiveness data across a broad range of high-risk groups.
This success is promising. However, the COVID-19 pandemic is not over, and the next could prove even more challenging. For example, through a recent partnership with the US Department of Defense (DoD), the VA was able to rapidly analyze the effectiveness of previous smallpox vaccination efforts in the military for preventing mpox infections.5 We should take this opportunity to think creatively about ways to improve our existing infrastructure based on what we have learned.
A Role for VA Research in Efficacy
The US Food and Drug Administration (FDA) Reauthorization Act of 2017 requires that manufacturers submit evidence establishing a product’s benefits (effectiveness) outweigh its risks (safety) before it can be promoted and distributed.6 As such, the FDA has been obligated by external stakeholders and Congress to be more explicit and transparent about benefit-risk profile supporting its decisions on licensure. This process led to requiring more phase 4 postmarketing observational studies for safety and effectiveness.7 Although the FDA postlicensure system remains vigilant toward safety, effectiveness information is limited due to insufficient reporting (with exceptions of manufacturer studies for new indications or to exhibit superior comparative effectiveness). The agency typically relies on a static set of efficacy data generated prelicensure with a dynamic and evolving set of safety data accrued postlicensure to support its assessment that benefits outweigh risks.
For example, operating in near real time, postauthorization safety monitoring systems, led by the Centers for Disease Control and Prevention and other federal systems, identified a safety signal for thrombosis following the Janssen COVID-19 vaccination. Distribution was quickly paused, the safety signal was investigated, the magnitude of the risk was characterized, new language describing the risk and providing guidance regarding clinical management was included in labeling, and distribution was resumed, all within a few weeks. This remarkable success demonstrated how timely the safety system can operate to evaluate risk.
In contrast, the duration and extent of protection against COVID-19 variants are largely limited to the assessment of immune biomarker surrogates. Such clinical effectiveness data are urgently needed for the FDA’s Center for Biologics Evaluation and Research and Center for Drug Evaluation and Research to make accurate benefit-risk assessments and continue to conclude the balance is favorable. As we prepare for the next pandemic, we must consider plans for monitoring postauthorization/postlicensure effectiveness as well as safety in real time. VA Research is ideally situated for this task.
Published studies on effectiveness at the VA serve as a prototype and could lead the way to initiating those preparations.4,8-11 One of the striking features of the VA system that became apparent in the preparation of the mRNA vaccine study was the speed at which an enormous volume of COVID-19 testing data were produced. This enabled implementation of methodologically sound test-negative and case-control analysis. Analyses sufficiently powered to conclude mRNA vaccines were highly effective when used in real-world conditions among a diverse population from nearly every state and territory during a period in which multiple COVID-19 variants were already circulating.3 This is unique to the VA and would not be possible for any other US health care system. With planning, the VA system could produce product-specific, real-world evidence of effectiveness comparable to the timeliness and quality of the safety data currently produced to support regulatory benefit-risk assessments. For example, the VA conducted an effectiveness study of tixagevimab/cilgavimab for preventing COVID-19 during the initial Omicron surge, which is continually updated while Omicron circulates and repeatable for different subvariants.12
The FDA continues to collaborate with the VA on demonstration projects to evaluate the impact of available vaccines and treatment against COVID-19 variants. The VA has also initiated several large-scale sequencing programs for COVID-19 specimens that will support these efforts, including VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD), VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE).13,14 Successful proof-of-concept studies using these data could provide a template for VA and other medical systems/databases to report effectiveness in near real time.
Interagency Collaboration
The potential advantages of federal agencies working with the VA to build an infrastructure capable of generating real-world evidence effectiveness analyses in near real time is not limited to needs that will arise in the next pandemic. For example, generating randomized, placebo-controlled, clinical trial endpoint data on the effectiveness of new variant vaccines will be difficult from a feasibility and ethical standpoint. Combining the VA’s robust virus sequencing program with preexisting mechanisms, such as expanded access studies (allowed under FDA Investigational New Drug regulations), researchers could enable a large-scale effective evaluation program of vaccination with variant or universal COVID-19 vaccines, using rapidly accruing effectiveness data.
The pandemic created opportunities to advance innovative approaches to medical product development. Some have advocated these innovative approaches should proceed together toward a seamless convergence between the domains of medical research and clinical care. A shift toward expecting, as a matter of routine, effectiveness data to be generated in near real time and made available for benefit-risk assessment would be a useful step in that direction.
Expanding and sharing analytical platforms, including methodology and programming codes, will allow increased access to rapidly refreshed real-world data. A common adaptive platform of complete and continuously updated data will also enable a wider community of researchers to create multiple investigatory groups simultaneously accessing fully de-identified data for concurrent observational studies. In turn, researchers need to have programming, study design, and methodology ready in an open-source platform. An efficient platform would also require the adoption of artificial intelligence, natural language processing, imaging processing, and quantum computing for validation and improved data quality.
COVID-19 has demonstrated the need for open science data synchronization with universal access for faster action and improved outcomes able to gain public confidence. OpenSafely (UK), a software platform for analysis of EHR data that is shared automatically and openly for scientific review and efficient reuse, created a cohort of about 23.4 million records for observational review of monoclonal COVID-19 treatments. To keep pace with the UK, Israel, and other nationalized systems, the US would benefit from duplicating this example of coordination between federal agencies and their data repositories. For example, combining data between the DoD, which captures active military health care data through TRICARE, and VA, which follows postmilitary discharge, would create datasets encompassing complete life spans. Additionally, expanding the National COVID Cohort Collaborative (N3C) program—one of the largest collections of clinical data related to COVID-19 symptoms and patient outcomes in the US—to include EHR data from DoD, VA, Medicare, and Test to Treat initiative partners would further expand research capabilities. This could be accomplished through a framework of anonymized, readily available, harmonized data. EHRs with synchronized datasets from every health care practitioner—independent pharmacies, primary care physicians, and hospitals—could all work to create a de-identified, comprehensive, continuously updated, near real-time dataset accessible to all federal researchers.
Conclusions
The VA has been lauded for its rapid, effective response to the current pandemic. The successful management and prescription of vaccines and treatment to the largely high-risk veteran population was possible because of the existing data framework within the VA. VA Research continues to build and refine infrastructure to improve speed, quality, and value of data analytics. We can do more. Expanding partnerships to use existing VA data strategies in designing a cooperative national data alliance would deliver necessary progress to research and public health.
Acknowledgments
The authors thank Jeff Roberts, MD, for his insight on the US Food and Drug Administration, its responsibilities, and the potential benefit of real world data to its missions.
1. US Department of Veterans Affairs, Veterans Health Administration. Third report details VA’s continued efforts addressing COVID-19 pandemic. Accessed August 15, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5748
2. Xie Y, Ziyad A. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi:10.1016/S2213-8587(22)00044-4
3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4(10):e2128391. doi:10.1001/jamanetworkopen.2021.28391
4. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi:10.1056/NEJMoa2115463
5. Titanji BK, Eick-Cost A, Partan ES, et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. N Engl J Med. 2023;389(12):1147-1148. doi:10.1056/NEJMc2300805
6. Sarata AK, Dabrowska A, Johnson JA, Thaul S. FDA Reauthorization Act of 2017. Accessed August 15, 2023. https://sgp.fas.org/crs/misc/R44961.pdf
7. US Food and Drug Administration. FDA’s sentinel initiative–background. February 2, 2022. Updated February 4, 2022. Accessed August 15, 2023. https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
8. Bajema KL, Dahl RM, Prill MM, et al; SUPERNOVA COVID-19; Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly. 2021;70(37):1294-1299. doi:10.15585/mmwr.mm7037e3
9. Sharma A, Oda G, Holodniy M. COVID-19 vaccine breakthrough infections in Veterans Health Administration. medRxiv. Posted September 26, 2021. doi:10.1101/2021.09.23.21263864
10. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of third doses of mRNA-based COVID-19 vaccines in US veterans. Nat Microbiol. 2023;8(1):55-63. doi:10.1038/s41564-022-01272-z
11. Tang F, Hammel IS, Andrew MK, Ruiz JG. Frailty reduces vaccine effectiveness against SARS-CoV-2 infection: a test-negative case control study using national VA data. J Nutr Health Aging. 2023;27(2):81-88. doi:10.1007/s12603-023-1885-1
12. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023;14(4):e0102423. doi:10.1128/mbio.01024-23
13. US Department of Veterans Affairs. VA science and health initiative to combat infectious and emerging life-threatening diseases. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac64
14. Bilal MY. Similarity index–probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia. 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
1. US Department of Veterans Affairs, Veterans Health Administration. Third report details VA’s continued efforts addressing COVID-19 pandemic. Accessed August 15, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5748
2. Xie Y, Ziyad A. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi:10.1016/S2213-8587(22)00044-4
3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open. 2021;4(10):e2128391. doi:10.1001/jamanetworkopen.2021.28391
4. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi:10.1056/NEJMoa2115463
5. Titanji BK, Eick-Cost A, Partan ES, et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. N Engl J Med. 2023;389(12):1147-1148. doi:10.1056/NEJMc2300805
6. Sarata AK, Dabrowska A, Johnson JA, Thaul S. FDA Reauthorization Act of 2017. Accessed August 15, 2023. https://sgp.fas.org/crs/misc/R44961.pdf
7. US Food and Drug Administration. FDA’s sentinel initiative–background. February 2, 2022. Updated February 4, 2022. Accessed August 15, 2023. https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
8. Bajema KL, Dahl RM, Prill MM, et al; SUPERNOVA COVID-19; Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly. 2021;70(37):1294-1299. doi:10.15585/mmwr.mm7037e3
9. Sharma A, Oda G, Holodniy M. COVID-19 vaccine breakthrough infections in Veterans Health Administration. medRxiv. Posted September 26, 2021. doi:10.1101/2021.09.23.21263864
10. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of third doses of mRNA-based COVID-19 vaccines in US veterans. Nat Microbiol. 2023;8(1):55-63. doi:10.1038/s41564-022-01272-z
11. Tang F, Hammel IS, Andrew MK, Ruiz JG. Frailty reduces vaccine effectiveness against SARS-CoV-2 infection: a test-negative case control study using national VA data. J Nutr Health Aging. 2023;27(2):81-88. doi:10.1007/s12603-023-1885-1
12. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023;14(4):e0102423. doi:10.1128/mbio.01024-23
13. US Department of Veterans Affairs. VA science and health initiative to combat infectious and emerging life-threatening diseases. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac64
14. Bilal MY. Similarity index–probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia. 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
Pneumonia Treatment Duration
Pneumonia is the leading inpatient infectious diagnosis for which antimicrobials are prescribed in the United States.[1] Supported by moderate‐ to high‐quality evidence, guidelines produced jointly by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) recommend treating pneumonia with the shortest appropriate duration of antimicrobial therapy to minimize risk for antimicrobial‐related adverse events.[2, 3, 4]
Evidence supports short duration of therapy for treatment of uncomplicated pneumonia.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] IDSA/ATS guidelines state, patients with CAP [community‐acquired pneumonia] should be treated for a minimum of 5 days (level 1 evidence), should be afebrile for 4872 hours, and should have no more than 1 CAP‐associated sign of clinical instabilitybefore discontinuation of therapy (level II evidence). (Moderate recommendation.) A longer duration of therapy may be warranted if initial therapy was not active against the identified pathogen or if it was complicated by [abscess, empyema, severe immunosuppression, or] extra‐pulmonary infection such as meningitis or endocarditis. (Weak recommendation; level III evidence).[3] Recommended therapy duration for patients with uncomplicated healthcare‐associated pneumonia (HCAP) who respond to initial therapy is 7 to 8 days unless gram‐negative nonfermenting rods or complications are identified (level I evidence).[4]
Within the Veterans Health Administration (VHA), the Antimicrobial Stewardship Taskforce (ASTF) was created to optimize care by developing, deploying, and monitoring a national‐level strategic plan for antimicrobial therapy management improvements.[13, 14] Although single‐center studies have found antimicrobial therapy for CAP being frequently prescribed for longer than recommended, the reproducibility of this finding across different facilities has not been assessed.[15, 16] The ASTF collaborated with the VHA Center for Medication Safety to assess total duration of antimicrobial therapy prescribed for veterans hospitalized with uncomplicated pneumonia.[17]
METHODS
This retrospective multicenter evaluation was conducted in 30 VHA facilities that volunteered to participate in this project. Inpatients discharged with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for pneumonia (or pneumonia diagnosis secondary to primary sepsis diagnosis) during 2013 were evaluated.[18] Diagnoses, admissions, and patient demographics were identified using Veterans Affairs (VA) integrated databases through the Austin Integrated Technology Center. Up to 200 admissions per facility were randomly selected for review. Clinical pharmacists at each facility performed manual record reviews utilizing a standardized protocol and collection form. Completed cases were uploaded to a central database for analysis. Standardized chart abstraction was facilitated by detailed instructions, a data dictionary, and monthly conference calls.
Inclusion criteria required patient admission to any medical ward including intensive care unit (ICU) wards for 48 hours, receipt of >24 hours inpatient antimicrobial therapy (eg, at least 2 doses of a once‐daily antibiotic), documentation of pneumonia discharge diagnosis, and survival until discharge. Exclusion criteria were: complicated pneumonia (lung abscess, necrotizing pneumonia, thoracentesis performed), significant immunosuppression (cancer chemotherapy or absolute neutrophil count <1500 cell/mm3 within 28 days, organ transplantation, human immunodeficiency virus infection); or extrapulmonary infection (eg, meningitis, endocarditis).[3] Patients were also excluded if directly transferred from another inpatient facility, pneumonia occurred >48 hours after admission, index hospitalization was >14 days, previously hospitalized within 28 days prior to index admission, or discharged without documentation of completing a full course of therapy. In addition, patients who received initial therapy discordant with culture and susceptibility findings, were not clinically stable by discharge, or had gram‐negative nonfermentative bacilli cultured were excluded from analysis because according to the guidelines, either data are lacking to support a short duration of therapy such as initial discordant therapy, or a longer duration of therapy may be warranted such as gram‐negative nonfermentative bacilli and clinical instability at discharge.[4] Our intent for these exclusions was to minimize bias against clinician decision making for cases where a longer duration of therapy may have been appropriate.
Patients meeting all criteria had the following abstracted: demographics; prior healthcare exposures, admitting location (ICU or non‐ICU ward), parameters for calculation of Pneumonia Severity Index (PSI), culture results obtained 48 hours of admission, duration of antimicrobials administered during hospitalization and prescribed upon discharge (or recommendations for outpatient duration in the discharge summary for patients receiving medications from non‐VA sources), daily clinical stability assessment, Clostridium difficile infection (CDI) test results, and readmission or death within 28 days of discharge.[19]
Guideline‐similar CAP therapy duration was defined as a minimum of 5 days of antimicrobials, up to a maximum of 3 additional days beginning the first day the patient was afebrile and exhibited 1 sign of clinical instability (heart rate > 100 beats/minute, respiratory rate >24 breaths/minute, systolic blood pressure <90, oxygen saturation <90% or partial pressure of oxygen <60 mm Hg on room air or baseline O2 requirements, or not returned to baseline mental status).[3] This definition was made by consensus decision of the investigators and was necessary to operationalize the relationship between clinical stability and appropriate duration of therapy. Guideline‐similar HCAP therapy duration was defined as 8 days.[4] CDI was defined in accordance with VA criteria for hospital onset and community‐onset healthcare‐facilityassociated CDI.[20] All‐cause hospital readmission and all‐cause death were defined as inpatient readmission or any death, respectively, within 28 days after discharge for the pneumonia admission.
Demographics, comorbidities, microbiology results, antimicrobial utilization, CDI, readmission, and death rates between guideline‐similar and guideline‐excessive duration of antimicrobial therapy groups were characterized with descriptive statistics, Mann‐Whitney U test, or 2 test as indicated (significance defined as P < 0.05). Multivariable logistic regression (SAS version 9.3 [SAS Institute, Cary, NC]) was used to assess association between duration of therapy exceeding recommended guidelines with all‐cause readmission and all‐cause death after adjustment for pertinent covariates. Odds ratios (OR) with 95% confidence intervals ( 95% CI) were reported. This medication utilization evaluation (MUE) was reviewed by the Hines VHA Institutional Review Board for Human Subjects Protection. Based on VHA Policy Handbook 1058.05, which defines operations activities that may constitute research, the board determined that the evaluation constituted quality improvement rather than research, and thus was exempt from VHA Human Subjects Research requirements.
RESULTS
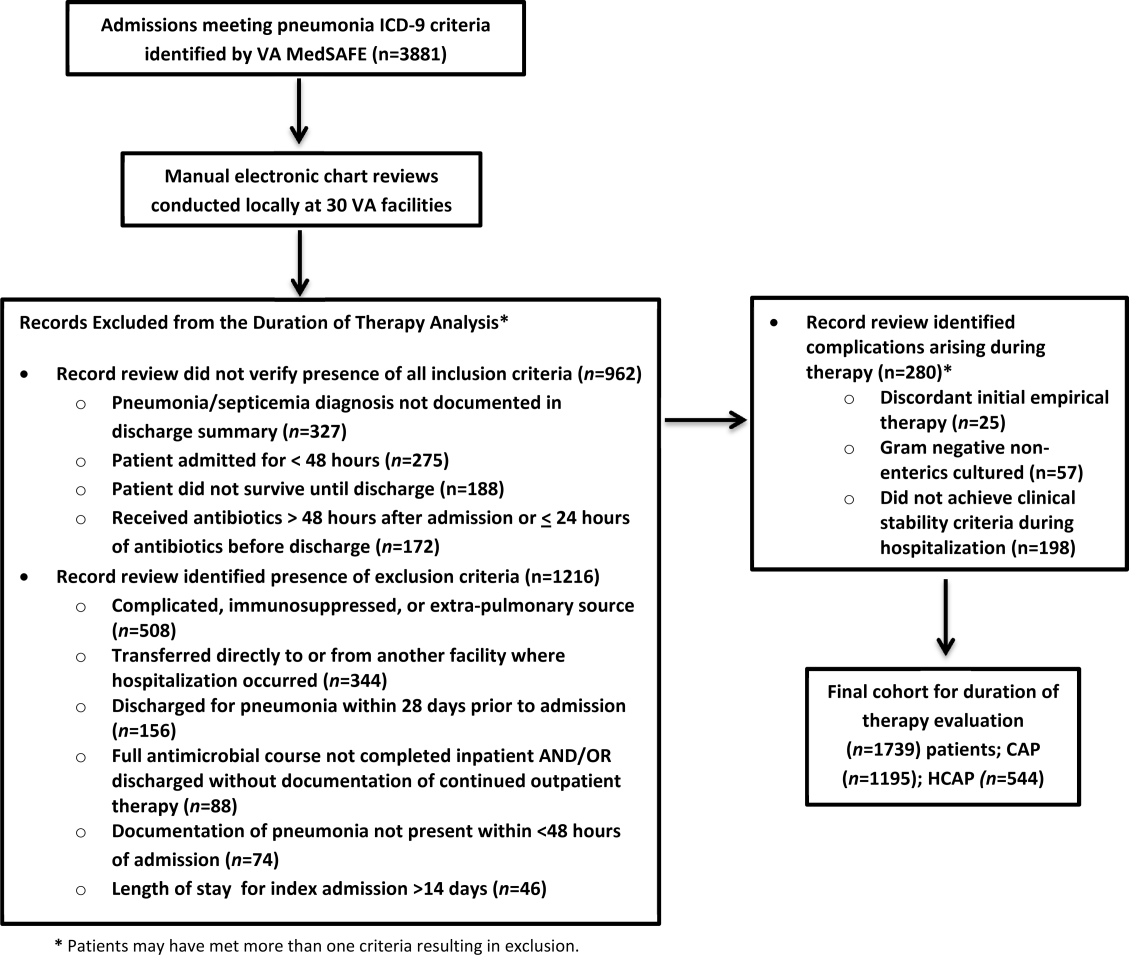
There were 3881 admissions eligible for chart review. After manual chart review of inclusion and exclusion criteria, 1739 (44.8%) patients were available for duration of therapy analysis. (Figure 1). Only 1 admission for each patient was analyzed.
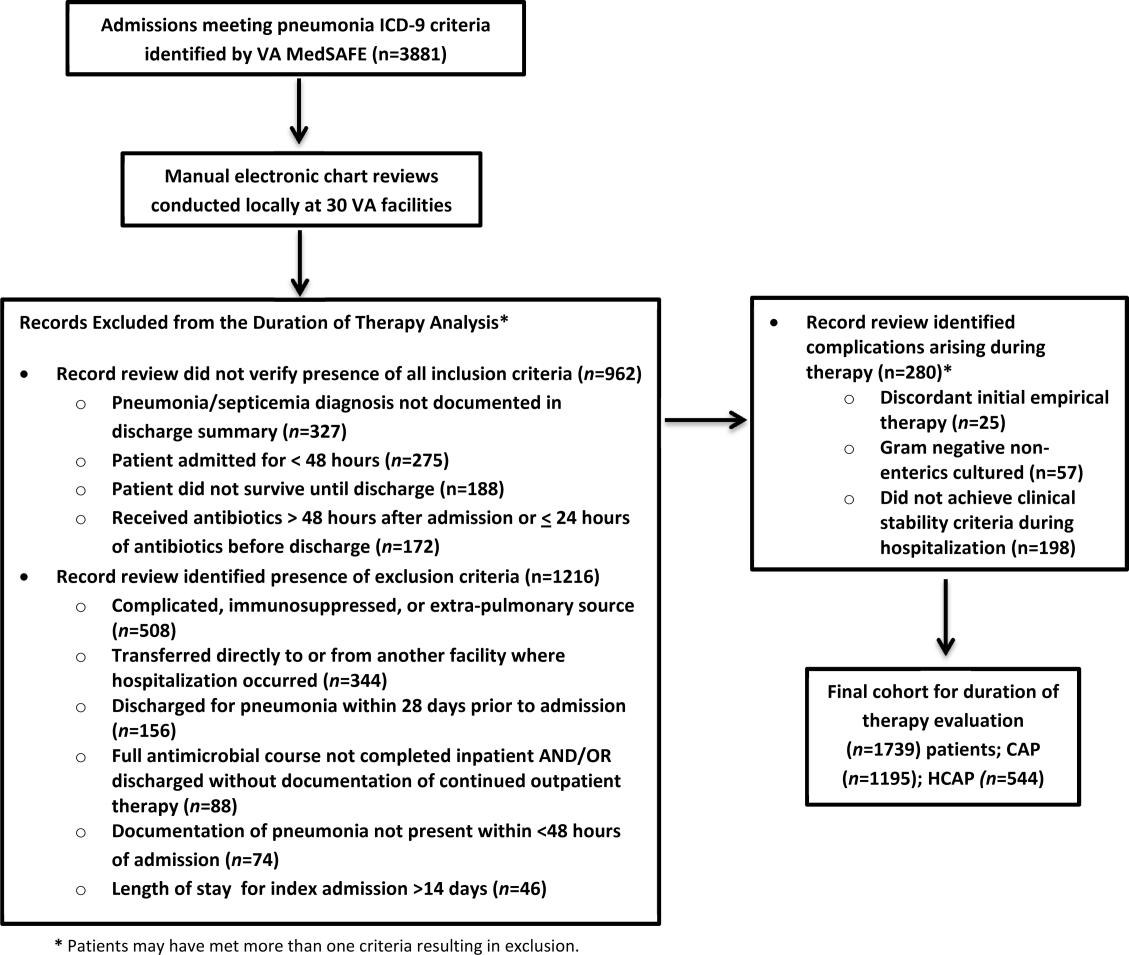
The cohort was comprised primarily of elderly male patients (96.6%) of whom more than two‐thirds were hospitalized for CAP (Table 1). Most patients had significant disease severity as indicated by PSI score; however, only 12% were directly admitted to the ICU. Blood cultures were collected in >95% of cases; lower respiratory cultures were obtained in 39.9% of cases.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean SD | 71.8 (12.7) |
| Gender, male, n (%) | 1,680 (96.6) |
| Living environment at time of index admission, n (%) | |
| Home | 1,416 (81.4) |
| VA community‐based living center | 88 (5.1) |
| Non‐VA long‐term skilled care facility | 95 (5.5) |
| Assisted living facility | 52 (2.9) |
| Not documented | 46 (2.7) |
| Other | 29 (1.7) |
| Prior healthcare exposures, n (%) | |
| Prior hospitalization within last 90 days | 310 (17.8) |
| Residence in a long‐term skilled care facility in last 90 days | 209 (12.0) |
| Chronic dialysis within last 28 days | 52 (3.0) |
| Intravenous antimicrobials within last 28 days | 76 (4.4) |
| Wound, tracheostomy, or ventilator care in last 28 days | 37 (2.1) |
| Community‐acquired pneumonia, n (%) | 1,195 (68.7) |
| Healthcare‐associated pneumonia, n (%) | 544 (31.3) |
| Comorbidities, n (%) | |
| Renal disease | 438 (25.2) |
| Liver disease | 39 (2.2) |
| Congestive heart failure | 436 (25.1) |
| Cerebrovascular disease | 356 (20.4) |
| Neoplastic disease (excluding skin) | 384 (22.1) |
| Severity of illness, n (%) | |
| Pneumonia Severity Index | |
| Class I | 30 (1.8) |
| Class II | 198 (11.4) |
| Class III | 349 (20.1) |
| Class IV | 759 (43.6) |
| Class V | 403 (23.2) |
| Intensive care upon admission | 212 (12.2) |
| Culture collection 48 hours of admission, n (%) | 1,687 (97.0) |
| Blood | 1,631 (96.7) |
| Lower respiratory tract (sputum) | 673 (39.9) |
| Bronchoalveolar lavage | 20 (1.2) |
| Urine | 632 (37.5) |
| Skin/wound | 3 (0.2) |
| Other | 158 (9.4) |
| Facility complexity, n (%) | |
| Level 1a‐c | 1,286 (74.0) |
| Level 2 | 437 (25.1) |
| Level 3 | 16 (0.9) |
Commonly administered antimicrobials during hospitalization and at discharge are summarized in Table 2. Anti‐pseudomonal ‐lactams and antimethicillin‐resistant Staphylococcus aureus antimicrobials were more frequently administered to patients with HCAP, whereas third‐generation cephalosporins and macrolides were more likely to be administered to patients with CAP. Fluoroquinolones were prescribed to 55.3% of patients upon discharge.
| Inpatient Antimicrobials Administered* | ||||
|---|---|---|---|---|
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,739 |
Therapy Duration Similar With Guidelines, n (%), n = 241 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,498 | Significance | |
| Antimicrobials Dispensed or Recommended at Discharge | ||||
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,471 |
Therapy Duration Similar With Guidelines, n (%), n = 151 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,320 | Significance | |
| ||||
| Third‐generation cephalosporins | 809 (46.5) | 75 (31.1) | 734 (49.0) | <0.001 |
| Fluoroquinolones | 836 (48.1) | 114 (47.3) | 722 (48.2) | 0.80 |
| Macrolides | 788 (45.3) | 90 (37.3) | 698 (46.6) | <0.01 |
| Pseudomonal ‐lactams | 692 (39.8) | 138 (57.3) | 554 (37.0) | 0.01 |
| Anti‐MRSA antimicrobials | 663 (38.1) | 135 (56.0) | 528 (35.3) | <0.01 |
| Other ‐lactams | 139 (8.0) | 10 (4.2) | 129 (8.6) | 0.02 |
| Tetracyclines | 119 (6.8) | 14 (5.8) | 105 (7.0) | 0.49 |
| Other | 97 (5.6) | 15 (6.2) | 82 (5.5) | 0.64 |
| Third‐generation cephalosporins | 285 (19.4) | 27 (17.9) | 258 (19.6) | 0.62 |
| Fluoroquinolones | 813 (55.3) | 95 (62.9) | 718 (54.4) | 0.05 |
| Macrolides | 203 (13.8) | 20 (13.3) | 183 (13.9) | 0.83 |
| Pseudomonal ‐lactams | 31 (2.1) | 4 (2.7) | 27 (2.1) | 0.62 |
| Anti‐MRSA antimicrobials | 45 (3.1) | 6 (4.0) | 39 (3.0) | 0.49 |
| Other ‐lactams | 239 (16.3) | 13 (8.6) | 226 (17.1) | 0.01 |
| Tetracyclines | 95 (6.5) | 10 (6.6) | 85 (6.4) | 0.93 |
| Other | 44 (3.0) | 5 (3.3) | 39 (3.0) | 0.81 |
Overall, 13.9% of patients with uncomplicated pneumonia received guideline‐similar duration of therapy (Table 3). A greater proportion of HCAP patients (29.0%) received guideline‐similar therapy duration as compared to CAP patients (6.9%) (P < 0.01 (Table 3). Median duration of therapy was 7 days (interquartile range [IQR] = 78 days) for guideline‐similar therapy compared to 10 days (913 days) for therapy duration in excess of guideline recommendations. Overall, 97.1 % of patients met clinical stability criteria before day 4 of therapy, yet 50% received 4 days of intravenous (IV) therapy (median was 4 days, IQR = 36 days). Antimicrobial therapy was generally completed after discharge, as only 17.3% received their entire treatment course during hospitalization. Median duration of outpatient oral (PO) antimicrobial therapy was twice as long for guideline‐excessive therapy compared to guideline‐similar therapy (6 vs 3 days), whereas duration of inpatient IV and PO antimicrobial therapy was similar. Patients discharged on a fluoroquinolone were more likely to receive guideline‐similar duration of therapy. The VHA classifies facilities into 3 levels of complexity, with lower scores indicating more complex facilities.[21] Guideline‐similar therapy duration occurred in 10.4% of cases in lower complexity facilities (levels 2 and 3),and 15.1% in more complex facilities (level 1) (P = 0.01). The median duration of therapy was similar for more and less complex facilities, respectively (10 days, IQR = 812 days vs 10 days, IQR = 813 days).
| Outcome |
Therapy Duration Similar With IDSA/ATS Guidelines |
Therapy Duration in Excess of IDSA/ATS Guideline Recommendations | Significance |
|---|---|---|---|
| |||
| Antimicrobial duration consistent with guideline recommendations, n (%) | 241 (13.9) | 1,498 (86.1) | NR |
| CAP* | 83 (6.9) | 1,112 (93.1) | NR |
| HCAP* | 158 (29.0) | 386 (71.0) | NR |
| Total days of therapy for pneumonia, median (IQR) | 7 (78) | 10 (913) | NR |
| CAP | 6 (59) | 10 (812) | <0.01 |
| HCAP | 7 (78) | 11 (1014) | <0.01 |
| Days of IV therapy administered for pneumonia, median (IQR) | 4 (37) | 4 (36) | 0.50 |
| Days of PO inpatient therapy administered, median (IQR) | 1 (03) | 1 (03) | 0.78 |
| Days of PO outpatient therapy dispensed at discharge, median (IQR) | 3 (25) | 6 (47) | <0.01 |
| Days of PO outpatient therapy recommended in Discharge Summary for patients without a VA prescription, median (IQR) | 3 (24) | 5 (47) | <0.01 |
| Aggregate 28‐day hospital readmission, n (%) | 42 (17.4) | 183 (12.2) | 0.03 |
| CAP∥# | 7 (8.4) | 112 (10.1) | 0.58 |
| HCAP∥# | 35 (22.2) | 71 (18.4) | 0.28 |
| Aggregate 28‐day CDI rate, n (%) | 6 (2.5) | 9 (0.6) | 0.03 |
| CAP∥** | 1 (1.2) | 6 (0.5) | 0.44 |
| HCAP∥** | 5 (3.2) | 3 (0.8) | 0.04 |
| Aggregate 28‐day death after discharge, n (%) | 6 (2.5) | 52 (3.5) | 0.43 |
| CAP∥** | 1 (1.2) | 33 (3.0) | 0.35 |
| HCAP∥** | 5 (3.2) | 19 (4.9) | 0.37 |
The 28‐day postdischarge all‐cause readmission rate for patients who received guideline‐similar therapy duration was higher (17.4%) than for patients who received therapy duration in excess of guideline recommendations (12.2%) (P = 0.03). After adjustment for covariates associated with readmission (HCAP, age, prior skilled nursing facility residence, PSI score comorbidity elements), we found no evidence that patients who received guideline‐similar therapy duration were more likely to be readmitted than were patients who received guideline‐excessive duration (OR: 1.1 [95% CI: 0.8, 1.7]) (Table 3). Likewise, no difference in 28‐day all‐cause postdischarge mortality was identified between guideline‐similar and guideline‐excessive duration after adjustment for the same covariates (adjusted OR: 0.5 [95% CI: 0.2, 1.2]) (Table 4).
| Model Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Readmission model | |||
| Duration of antibiotics | 1.11 | 0.75, 1.64 | 0.62 |
| HCAP | 1.94 | 1.38, 2.72 | <0.01 |
| Age | 1.01 | 1.00, 1.03 | 0.04 |
| Prior skilled nursing facility residence | 0.91 | 0.59, 1.40 | 0.67 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.20 | 0.86, 1.67 | 0.29 |
| Liver disease | 1.55 | 0.66, 3.64 | 0.31 |
| CHF | 1.15 | 0.83, 1.59 | 0.41 |
| Cerebrovascular disease | 1.06 | 0.75, 1.50 | 0.75 |
| Renal disease | 1.51 | 1.09, 2.08 | 0.01 |
| Mortality model | |||
| Duration of antibiotics | 0.53 | 0.23, 1.22 | 0.14 |
| HCAP | 2.53 | 1.38, 4.65 | <0.01 |
| Age | 1.06 | 1.03, 1.09 | <0.01 |
| Prior skilled nursing facility residence | 0.79 | 0.38, 1.66 | 0.53 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.03 | 0.57, 1.87 | 0.91 |
| Liver disease | <0.001 | <0.001, >999.9 | 0.98 |
| CHF | 0.73 | 0.39, 1.38 | 0.34 |
| Cerebrovascular disease | 0.82 | 0.43, 1.56 | 0.55 |
| Renal disease | 0.72 | 0.39, 1.35 | 0.31 |
CDI cases (n = 15) were too sparse to adequately perform multivariable logistic regression analysis; however, a higher percentage of patients who received guideline‐similar duration of therapy developed CDI compared to patients who received guideline‐excessive duration of therapy (40.0% vs 13.6%, P < 0.01). The median duration of therapy for patients who did and did not develop CDI was similar (8 days, IQR = 714 days vs 10 days, IQR = 812 days, P = 0.85, respectively). Patients who developed CDI had a higher rate of HCAP diagnosis (1.5% vs 0.6%; P = 0.06), were more likely to have concomitant non‐pneumonia infection (40.0% vs 9.5%, P < 0.01), have chronic comorbidity (86.7% vs 59.1%, P = 0.03), and to have been admitted to the ICU (26.7% vs 12.1%, P = 0.09).
DISCUSSION
IDSA/ATS guidelines for pneumonia duration of therapy generally agree with other professional society guidelines including the British Thoracic Society and National Institute for Health and Care Excellence.[22, 23] In contrast to existing evidence and guideline recommendations, this multi‐centered evaluation identified prolonged durations of antimicrobial therapy prescribed in 93% and 71% of patients with uncomplicated CAP and HCAP (Table 3), respectively.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] Almost all (97.1%) uncomplicated CAP and HCAP patients met clinical stability criteria before day 4 of hospitalization, yet the median duration of IV therapy was 4 days. Because criteria for IV to PO conversion and the clinical stability definition utilized in this analysis were similar, many patients may have been eligible for PO therapy earlier, favorably impacting length of stay, cost, and adverse effects.[3, 12, 24, 25, 26] Although median days of inpatient PO therapy administered was 1 day (IQR = 03 days), inpatient observation after PO conversion may not be necessary. The duration of PO therapy was based on calendar days, where if a patient received 1 dose of a once daily antibiotic (ie, levofloxacin), they were considered to have received 1 day of inpatient PO antibiotics even if discharged the same day.
Approximately half of all days of therapy occurred after discharge. Although the median therapy duration for inpatients was similar, the median duration of antimicrobials administered upon hospital discharge was twice as long for patients receiving guideline‐excessive compared to guideline‐similar duration of therapy. The median excess in antibiotic duration is almost entirely accounted for by excess outpatient days of therapy. This is an important consideration for antimicrobial stewardship programs that tend to focus on inpatient antimicrobial use.
Noteworthy observations include the low rate of respiratory tract culture collection (41%) and frequent use of fluoroquinolones upon discharge. Collection of respiratory tract cultures is recommended for all patients with HCAP and patients with CAP who have risk factors for resistant pathogens, characteristics that were common in this cohort.[3, 4] Recently, we identified that respiratory culture collection is associated with increased de‐escalation rates in HCAP, and that culture‐negative patients frequently receive fluoroquinolones.[27] IDSA/ATS CAP guidelines discourage empirically switching to PO fluoroquinolone therapy based on bioavailability considerations alone.[3] Further, fluoroquinolones are considered to be associated with high risk of CDI.[28, 29] Prescription of fluoroquinolone upon discharge was associated with guideline‐similar duration of therapy and was not shown to be associated with CDI; however, power to detect differences between exposures to specific antimicrobials and CDI was low.
CDI was more common in patients with CAP (1.2% vs 0.5%) and HCAP (3.2% vs 0.8%) who received duration of therapy similar with guideline recommendations. This observation is confounded, as patients with CDI had significantly greater comorbidity as well as secondary infections and tended to more frequently receive ICU care. There were no differences in adjusted rates of readmission or death between patients receiving guideline‐similar and guideline‐excessive duration of therapy.
Evaluation strengths included exclusion of patients with complicating conditions possibly requiring prolonged antimicrobial treatment courses, which allowed the evaluation to focus on patients most likely to benefit from shorter course therapy. The definition of appropriate therapy duration was based upon daily assessment of clinical stability criteria that paralleled the CAP guidelines. The definition utilized objective parameters while accounting for patient variability in achieving clinical stability criteria. Finally, the analyses of clinical end points suggest that shorter duration of therapy may be as safe and effective as longer duration of therapy in uncomplicated pneumonia.
Limitations include those common to other analyses conducted within the VHA, including a predominantly elderly male cohort.[30] Only ICD‐9‐CM codes consistent with a discharge diagnosis of pneumonia were used to identify the cohort, and clinical impressions not documented in the medical record may have impacted the clinician's treatment duration decisions. The upper limit of appropriate duration of therapy for CAP was arbitrarily set at up to 3 days beyond meeting clinical stability criteria to provide a reasonable duration of appropriate therapy beyond clinical stability to operationalize the duration of therapy recommendations within the context of the IDSA/ATS guidelines. Additionally, CIs for the ORs of readmission and mortality were broad, and thus too imprecise to determine whether guideline‐similar durations increased or decreased readmission or mortality in comparison with therapy that exceeded guideline recommendations. We could not fully assess the potential for association between guideline‐excessive therapy duration and risk for CDI due to sparse cases. Finally, non‐VA prescription data were not available for all patients, and we relied on intended duration of therapy as recommended by the discharging provider in 4.1% of cases.
Most quality assessments of pneumonia treatment have focused on antimicrobial selection and timely administration or conversion from IV to PO therapy.[31, 32] This evaluation identified potential opportunities for expansion of antimicrobial stewardship activities during the transition of care setting. The efficacy of short‐course ‐lactam, macrolide, or fluoroquinolone therapy for CAP appears equivalent to longer treatment regimens with no difference in adverse event rates, suggesting that optimal duration of therapy may be a rational target for quality improvement.[5, 6, 7, 8, 9, 10, 11, 12, 15, 31] Recommendations for HCAP duration of therapy are extrapolated from a prospective multicentered study, which randomized patients with hospital‐acquired pneumonia to receive 8 versus 15 days of therapy, that identified similar outcomes to ours.[4, 12]
Single‐center studies have identified that antimicrobial therapy for pneumonia is frequently prescribed for longer than recommended by guidelines, which found a similar median duration of therapy as our evaluation.[15, 16] Similar to Jenkins et al., we observed a high rate of fluoroquinolone prescriptions upon discharge.[16]
There are few published examples of interventions designed to limit excessive duration of therapy, particularly for antimicrobials prescribed upon hospital discharge.[15, 33, 34] Serial procalcitonin measurements have been used to guide duration of therapy for pneumonia; however, the costbenefit ratio of procalcitonin measurement is unclear.[35, 36] Procalcitonin use was uncommon, and none of the participating facilities in our evaluation utilized a specific algorithm to guide therapy duration. Limited data suggest that patient‐level prospective audit with feedback may be effective. Advic et al. evaluated management of presumed CAP before and after education and prospective feedback to medical teams concerning antimicrobial selection and duration of therapy.[15] The intervention led to a decrease in median duration of therapy from 10 days (IQR = 813 days) to 7 days (IQR = 78 days) without increasing clinical failure or readmission rates. We recently reported a single‐center evaluation in which pharmacists utilizing a decision support tool while performing discharge medication reconciliation were able to reduce excessive mean duration of therapy from 9.5 days ( 2.4 days) to 8.3 days ( 2.9 days) in patients without complicated pneumonia, with a 19.2% reduction in duration of therapy prescribed at discharge.[37] A similar approach utilizing pharmacists performing discharge review has recently been reported in a community hospital.[38]
Future work should recognize that few patients complete their entire course of therapy as inpatients, and the majority of treatment is prescribed upon discharge. Pivotal time points for antimicrobial stewardship intervention include day 2 to 3 of hospitalization when conveying suggestions for antimicrobial de‐escalation and/or IV to PO conversion, and toward the end of hospitalization during discharge planning. Although it may not be feasible for antimicrobial stewards to review all uncomplicated cases of pneumonia during hospitalization, most facilities have a systematic process for reviewing medications during transitions of care. We believe that interventions intended to assess and recommend shortened courses of therapy are appropriate. These interventions should include a mechanism for support by stewardship personnel or other infectious diseases specialists. Based on our evaluation, the ASTF produced and disseminated clinical guidance documents and tools to triage pneumonia case severity and assess response to therapy. Qualified personnel are encouraged to use this information to make recommendations to providers regarding excessive duration of therapy for uncomplicated cases where appropriate. Other work should include an in‐depth assessment of clinical outcomes related to treatment duration, investigation of provider rationale for prolonged treatment, and duration of antimicrobial therapy prescribed upon discharge for other common disease states. Finally, manual chart review to classify uncomplicated cases and related outcomes was laborious, and automated case identification is technologically plausible and should be explored.[39]
In conclusion, this national VHA MUE found that patients with uncomplicated pneumonia were commonly prescribed antimicrobials for the duration of therapy in excess of guideline recommendations. Patients with uncomplicated pneumonia who received therapy duration consistent with guideline recommendations did not have significantly different all‐cause readmission and death rates compared to patients receiving prolonged treatment. Approximately half of all therapy was prescribed upon hospital discharge, and clinicians as well as antimicrobial stewardship programs should consider these findings to address excessive duration of antimicrobial therapy upon hospital discharge.
Acknowledgements
The authors acknowledge Dr. Michael Fine for his assistance with utilization of the Pneumonia Severity Index, Kenneth Bukowski for assisting with development of data collection tools and data management, and members of the Antimicrobial Stewardship Taskforce Implementation Sub‐Committee. Collaborators in the Pneumonia Duration of Therapy Medication Utilization Evaluation Group include: Biloxi VA (VA Gulf Coast): Cheryl Hankins, PharmD, BCPS; Central Alabama VAMC: Lauren Rass, PharmD, BCPS, Kelly Mooney, PharmD, BCPS; Central Arkansas: Nicholas Tinsley, MS, PharmD; Chillicothe VA: Stephen Hanson, PharmD, BCPS, Beth Gallaugher, BSN, RN, Elizabeth Baltenberger, PharmD; Cincinnati VA: Jason Hiett, PharmD, BCPS, Victoria Tate, PharmD, BCPS, Brian Salzman, PharmD; Dorn Medical Center: MaryAnne Maurer, PharmD, BCPS, BCACP, Rebekah Sipes, PharmD, BCACP, Ginger Ervin, PharmD; Dwight D. Eisenhower VAMC: Emily Potter, PharmD; Hudson Valley: Rita Lee Bodine, PharmD, Clement Chen, PharmD, Cristina Fantino, PharmD; James H. Quillen VAMC: Marty Vannoy, PharmD, BCPS, Erin Harshbarger, PharmD, Kristen Nelsen, PharmD; Jesse Brown VAMC: Lisa Young, PharmD, BCPS, AQ‐ID, Andrea Bidlencik, PharmD, BCPS; Kansas City VA: Jamie Guyear, PharmD, AQ‐ID, Ann Ungerman, PharmD, BCPS, Lauri Witt, PharmD, BCACP; Louis Stokes Cleveland VAMC: Amy Hirsch, PharmD, BCPS, Steven Adoryan, PharmD, BCP‐CC, Amanda Miller, PharmD, BCPS; Maine VAMC: Joel Coon, PharmD, Rachel Naida, PharmD, Kelly Grossman, PharmD; Martinsburg VAMC: Kelly Li, PharmD, Sarah Mickanis, PharmD, BCPS; Miami VA Medical Center: Mara Carrasquillo, BS, PharmD, Maribel Toro, PharmD; North Florida/South Georgia Veterans Health System: Nora Morgan, PharmD, Hugh Frank, PharmD, BCPS, BCPP, Sarah Onofrio, PharmD, BCPS; North Texas HCS: Susan Duquaine, PharmD, BCPS, AQ‐ID, Ruben Villaneuva, PharmD, BCPS, Jaela Dahl, PharmD, BCPS; Ozarks: Andrew Siler, PharmD, BCPS, Michele Walker, PharmD, CGP, Jennifer Cole, PharmD, BCPS, BCCCP; Providence VAMC: Kerry LaPlante, PharmD, FCCP, Lindsey Williamson, PharmD; Richmond VA: Daniel Tassone, PharmD, BCPS; Salisbury VAMC: Brett Norem, PharmD, Marrisa Ragonesi, PharmD; San Juan VA: Monica Sanabria‐Seda, PharmD, BCPS, Jaime Velez‐Fores, PharmD, BCPS, AQ‐ID, Norma Ayala‐Burgos, PharmD; Sioux Falls VA: Andrea Aylward, PharmD, BCPS; South Texas HCS: Kelly Echevarria, PharmD, BCPS, AQ‐ID, Manuel Escobar, PharmD; Tennessee Valley HCS: Casey Ryals, PharmD, BCACP, Molly Hurst, PharmD, Jonathan Hale, PharmD; VA Central Iowa Health Care System: Jenny Phabmixay, PharmD, BCPS, Mackenzie Brown, PharmD, BCPS, Cynthia Muthusi, PharmD, BCPS; VA Loma Linda: Tony Chau, PharmD; VA Sierra Nevada: Scott Mambourg, PharmD, BCPS, AAHIVP, Matthew Han, PharmD, Nathan Mihoch, PharmD; VA WNY Healthcare System: Kari Mergenhagen, PharmD, BCPS, AQ‐ID, Christine Ruh, PharmD, BCPS; Veterans Affairs Salt Lake City Health System: Emily Spivak, MD, MHS, Patricia Orlando, PharmD
Disclosures: Karl Madaras‐Kelly is employed full time by Idaho State University and has a without compensation appointment as a clinical pharmacist at the Boise VA Medical Center. He receives grant support unrelated to this work through the Department of Veterans Affairs subcontracted to Idaho State University. Muriel Burk is employed full time through the Department of Veterans Affairs as clinical pharmacy specialist in outcomes and medication safety evaluation. Christina Caplinger was employed by the Department of Veterans Affairs as an infectious diseases fellow at the time this work was completed. She is currently employed by Micromedex. Jefferson Bohan is employed full time by the Department of Veterans Affairs as an infectious diseases fellow. Melinda Neuhauser is employed full time through the Department of Veterans Affairs as a clinical pharmacy specialistinfectious diseases. Matthew Goetz is employed full time through the Department of Veterans Affairs as an infectious diseases physician. Rhongping Zhang is employed full time through the Department of Veterans Affairs as a data analyst. Francesca Cunningham is employed full time through the Department of Veterans Affairs as the director of the VA Center for Medication Safety. This work was supported with resources and use of the Department of Veterans Affairs healthcare system. The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- Centers for Disease Control and Prevention. National hospital discharge survey 2010. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed December 1, 2014.
- , , , et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- , , , et al. Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68(13):1841–1854.
- , , , et al. Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis. Am J Med. 2007;120:783–790.
- , , , et al. High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760.
- , , , et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective. Am J Ther. 1999;6(4):217–222.
- , , , et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind trial. BMJ. 2006;332(7554):1355.
- , , , et al. Efficacy of a three day course of azithromycin in moderately severe community‐acquired pneumonia. Eur Respir J. 1995;8(3):398–402.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333(7580):1193.
- , , , , . Unnecessary antimicrobial use in the context of Clostridium difficile infection: a call to arms for the Veterans Affairs Antimicrobial Stewardship Task Force. Infect Control Hosp Epidemiol. 2013;34(6):651–653.
- VHA Directive 1031. Antimicrobial stewardship programs. Available at: https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Accessed December 1, 2014.
- , , , et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community‐acquired pneumonia. Clin Infect Dis. 2012;54:1581–1587.
- , , , et al. Targets for antibiotic and healthcare resource stewardship in inpatient community‐acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–144.
- , , , , . Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104–112.
- , , , . Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , , . Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35(8):1037–1042.
- , , , , . Organization complexity and primary care providers' perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31(2):139–146.
- , , , et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55.
- National Institute for Health and Care Excellence. Pneumonia in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/cg191. Published December 2014. Accessed May 9, 2016.
- , , , , , . A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia: the optimal duration of therapy. Chest. 1996;110(4):965–971.
- , , , et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community‐acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- , , , . Correlates and economic and clinical outcomes of an adult IV to PO antimicrobial conversion program at an academic medical center in Midwest United States. J Pharm Pract. 2015;28(3):238–248.
- , , , et al. Antimicrobial De‐escalation of treatment for healthcare‐associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71(2):539–546.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951.
- , , , . Meta‐analysis of antibiotics and the risk of community‐associated Clostridium difficle infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332.
- , , , et al. Evaluating diagnosis‐based case‐mix measures: how well do they apply to the VA population? Med Care. 2001;39:692–704.
- , , . What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211–228.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , , , et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high‐risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67(12):2988–2996.
- , , , et al.; Centers for Disease Control and Prevention. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157–1164.
- , , , , , . Interim evaluation of a Protocol to Optimize the Duration of Pneumonia Therapy at Hospital Discharge. Open Forum Infect Dis. 2015;2(suppl 1):S379.
- , , , et al. Intervention to improve antibiotic selection and shorten treatment durations at the time of hospital discharge. Open Forum Infect Dis. 2015;2(suppl 1):S1.
- , , , et al. Using the electronic medical record to identify community‐acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013;8(8):e70944.
Pneumonia is the leading inpatient infectious diagnosis for which antimicrobials are prescribed in the United States.[1] Supported by moderate‐ to high‐quality evidence, guidelines produced jointly by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) recommend treating pneumonia with the shortest appropriate duration of antimicrobial therapy to minimize risk for antimicrobial‐related adverse events.[2, 3, 4]
Evidence supports short duration of therapy for treatment of uncomplicated pneumonia.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] IDSA/ATS guidelines state, patients with CAP [community‐acquired pneumonia] should be treated for a minimum of 5 days (level 1 evidence), should be afebrile for 4872 hours, and should have no more than 1 CAP‐associated sign of clinical instabilitybefore discontinuation of therapy (level II evidence). (Moderate recommendation.) A longer duration of therapy may be warranted if initial therapy was not active against the identified pathogen or if it was complicated by [abscess, empyema, severe immunosuppression, or] extra‐pulmonary infection such as meningitis or endocarditis. (Weak recommendation; level III evidence).[3] Recommended therapy duration for patients with uncomplicated healthcare‐associated pneumonia (HCAP) who respond to initial therapy is 7 to 8 days unless gram‐negative nonfermenting rods or complications are identified (level I evidence).[4]
Within the Veterans Health Administration (VHA), the Antimicrobial Stewardship Taskforce (ASTF) was created to optimize care by developing, deploying, and monitoring a national‐level strategic plan for antimicrobial therapy management improvements.[13, 14] Although single‐center studies have found antimicrobial therapy for CAP being frequently prescribed for longer than recommended, the reproducibility of this finding across different facilities has not been assessed.[15, 16] The ASTF collaborated with the VHA Center for Medication Safety to assess total duration of antimicrobial therapy prescribed for veterans hospitalized with uncomplicated pneumonia.[17]
METHODS
This retrospective multicenter evaluation was conducted in 30 VHA facilities that volunteered to participate in this project. Inpatients discharged with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for pneumonia (or pneumonia diagnosis secondary to primary sepsis diagnosis) during 2013 were evaluated.[18] Diagnoses, admissions, and patient demographics were identified using Veterans Affairs (VA) integrated databases through the Austin Integrated Technology Center. Up to 200 admissions per facility were randomly selected for review. Clinical pharmacists at each facility performed manual record reviews utilizing a standardized protocol and collection form. Completed cases were uploaded to a central database for analysis. Standardized chart abstraction was facilitated by detailed instructions, a data dictionary, and monthly conference calls.
Inclusion criteria required patient admission to any medical ward including intensive care unit (ICU) wards for 48 hours, receipt of >24 hours inpatient antimicrobial therapy (eg, at least 2 doses of a once‐daily antibiotic), documentation of pneumonia discharge diagnosis, and survival until discharge. Exclusion criteria were: complicated pneumonia (lung abscess, necrotizing pneumonia, thoracentesis performed), significant immunosuppression (cancer chemotherapy or absolute neutrophil count <1500 cell/mm3 within 28 days, organ transplantation, human immunodeficiency virus infection); or extrapulmonary infection (eg, meningitis, endocarditis).[3] Patients were also excluded if directly transferred from another inpatient facility, pneumonia occurred >48 hours after admission, index hospitalization was >14 days, previously hospitalized within 28 days prior to index admission, or discharged without documentation of completing a full course of therapy. In addition, patients who received initial therapy discordant with culture and susceptibility findings, were not clinically stable by discharge, or had gram‐negative nonfermentative bacilli cultured were excluded from analysis because according to the guidelines, either data are lacking to support a short duration of therapy such as initial discordant therapy, or a longer duration of therapy may be warranted such as gram‐negative nonfermentative bacilli and clinical instability at discharge.[4] Our intent for these exclusions was to minimize bias against clinician decision making for cases where a longer duration of therapy may have been appropriate.
Patients meeting all criteria had the following abstracted: demographics; prior healthcare exposures, admitting location (ICU or non‐ICU ward), parameters for calculation of Pneumonia Severity Index (PSI), culture results obtained 48 hours of admission, duration of antimicrobials administered during hospitalization and prescribed upon discharge (or recommendations for outpatient duration in the discharge summary for patients receiving medications from non‐VA sources), daily clinical stability assessment, Clostridium difficile infection (CDI) test results, and readmission or death within 28 days of discharge.[19]
Guideline‐similar CAP therapy duration was defined as a minimum of 5 days of antimicrobials, up to a maximum of 3 additional days beginning the first day the patient was afebrile and exhibited 1 sign of clinical instability (heart rate > 100 beats/minute, respiratory rate >24 breaths/minute, systolic blood pressure <90, oxygen saturation <90% or partial pressure of oxygen <60 mm Hg on room air or baseline O2 requirements, or not returned to baseline mental status).[3] This definition was made by consensus decision of the investigators and was necessary to operationalize the relationship between clinical stability and appropriate duration of therapy. Guideline‐similar HCAP therapy duration was defined as 8 days.[4] CDI was defined in accordance with VA criteria for hospital onset and community‐onset healthcare‐facilityassociated CDI.[20] All‐cause hospital readmission and all‐cause death were defined as inpatient readmission or any death, respectively, within 28 days after discharge for the pneumonia admission.
Demographics, comorbidities, microbiology results, antimicrobial utilization, CDI, readmission, and death rates between guideline‐similar and guideline‐excessive duration of antimicrobial therapy groups were characterized with descriptive statistics, Mann‐Whitney U test, or 2 test as indicated (significance defined as P < 0.05). Multivariable logistic regression (SAS version 9.3 [SAS Institute, Cary, NC]) was used to assess association between duration of therapy exceeding recommended guidelines with all‐cause readmission and all‐cause death after adjustment for pertinent covariates. Odds ratios (OR) with 95% confidence intervals ( 95% CI) were reported. This medication utilization evaluation (MUE) was reviewed by the Hines VHA Institutional Review Board for Human Subjects Protection. Based on VHA Policy Handbook 1058.05, which defines operations activities that may constitute research, the board determined that the evaluation constituted quality improvement rather than research, and thus was exempt from VHA Human Subjects Research requirements.
RESULTS
There were 3881 admissions eligible for chart review. After manual chart review of inclusion and exclusion criteria, 1739 (44.8%) patients were available for duration of therapy analysis. (Figure 1). Only 1 admission for each patient was analyzed.

The cohort was comprised primarily of elderly male patients (96.6%) of whom more than two‐thirds were hospitalized for CAP (Table 1). Most patients had significant disease severity as indicated by PSI score; however, only 12% were directly admitted to the ICU. Blood cultures were collected in >95% of cases; lower respiratory cultures were obtained in 39.9% of cases.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean SD | 71.8 (12.7) |
| Gender, male, n (%) | 1,680 (96.6) |
| Living environment at time of index admission, n (%) | |
| Home | 1,416 (81.4) |
| VA community‐based living center | 88 (5.1) |
| Non‐VA long‐term skilled care facility | 95 (5.5) |
| Assisted living facility | 52 (2.9) |
| Not documented | 46 (2.7) |
| Other | 29 (1.7) |
| Prior healthcare exposures, n (%) | |
| Prior hospitalization within last 90 days | 310 (17.8) |
| Residence in a long‐term skilled care facility in last 90 days | 209 (12.0) |
| Chronic dialysis within last 28 days | 52 (3.0) |
| Intravenous antimicrobials within last 28 days | 76 (4.4) |
| Wound, tracheostomy, or ventilator care in last 28 days | 37 (2.1) |
| Community‐acquired pneumonia, n (%) | 1,195 (68.7) |
| Healthcare‐associated pneumonia, n (%) | 544 (31.3) |
| Comorbidities, n (%) | |
| Renal disease | 438 (25.2) |
| Liver disease | 39 (2.2) |
| Congestive heart failure | 436 (25.1) |
| Cerebrovascular disease | 356 (20.4) |
| Neoplastic disease (excluding skin) | 384 (22.1) |
| Severity of illness, n (%) | |
| Pneumonia Severity Index | |
| Class I | 30 (1.8) |
| Class II | 198 (11.4) |
| Class III | 349 (20.1) |
| Class IV | 759 (43.6) |
| Class V | 403 (23.2) |
| Intensive care upon admission | 212 (12.2) |
| Culture collection 48 hours of admission, n (%) | 1,687 (97.0) |
| Blood | 1,631 (96.7) |
| Lower respiratory tract (sputum) | 673 (39.9) |
| Bronchoalveolar lavage | 20 (1.2) |
| Urine | 632 (37.5) |
| Skin/wound | 3 (0.2) |
| Other | 158 (9.4) |
| Facility complexity, n (%) | |
| Level 1a‐c | 1,286 (74.0) |
| Level 2 | 437 (25.1) |
| Level 3 | 16 (0.9) |
Commonly administered antimicrobials during hospitalization and at discharge are summarized in Table 2. Anti‐pseudomonal ‐lactams and antimethicillin‐resistant Staphylococcus aureus antimicrobials were more frequently administered to patients with HCAP, whereas third‐generation cephalosporins and macrolides were more likely to be administered to patients with CAP. Fluoroquinolones were prescribed to 55.3% of patients upon discharge.
| Inpatient Antimicrobials Administered* | ||||
|---|---|---|---|---|
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,739 |
Therapy Duration Similar With Guidelines, n (%), n = 241 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,498 | Significance | |
| Antimicrobials Dispensed or Recommended at Discharge | ||||
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,471 |
Therapy Duration Similar With Guidelines, n (%), n = 151 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,320 | Significance | |
| ||||
| Third‐generation cephalosporins | 809 (46.5) | 75 (31.1) | 734 (49.0) | <0.001 |
| Fluoroquinolones | 836 (48.1) | 114 (47.3) | 722 (48.2) | 0.80 |
| Macrolides | 788 (45.3) | 90 (37.3) | 698 (46.6) | <0.01 |
| Pseudomonal ‐lactams | 692 (39.8) | 138 (57.3) | 554 (37.0) | 0.01 |
| Anti‐MRSA antimicrobials | 663 (38.1) | 135 (56.0) | 528 (35.3) | <0.01 |
| Other ‐lactams | 139 (8.0) | 10 (4.2) | 129 (8.6) | 0.02 |
| Tetracyclines | 119 (6.8) | 14 (5.8) | 105 (7.0) | 0.49 |
| Other | 97 (5.6) | 15 (6.2) | 82 (5.5) | 0.64 |
| Third‐generation cephalosporins | 285 (19.4) | 27 (17.9) | 258 (19.6) | 0.62 |
| Fluoroquinolones | 813 (55.3) | 95 (62.9) | 718 (54.4) | 0.05 |
| Macrolides | 203 (13.8) | 20 (13.3) | 183 (13.9) | 0.83 |
| Pseudomonal ‐lactams | 31 (2.1) | 4 (2.7) | 27 (2.1) | 0.62 |
| Anti‐MRSA antimicrobials | 45 (3.1) | 6 (4.0) | 39 (3.0) | 0.49 |
| Other ‐lactams | 239 (16.3) | 13 (8.6) | 226 (17.1) | 0.01 |
| Tetracyclines | 95 (6.5) | 10 (6.6) | 85 (6.4) | 0.93 |
| Other | 44 (3.0) | 5 (3.3) | 39 (3.0) | 0.81 |
Overall, 13.9% of patients with uncomplicated pneumonia received guideline‐similar duration of therapy (Table 3). A greater proportion of HCAP patients (29.0%) received guideline‐similar therapy duration as compared to CAP patients (6.9%) (P < 0.01 (Table 3). Median duration of therapy was 7 days (interquartile range [IQR] = 78 days) for guideline‐similar therapy compared to 10 days (913 days) for therapy duration in excess of guideline recommendations. Overall, 97.1 % of patients met clinical stability criteria before day 4 of therapy, yet 50% received 4 days of intravenous (IV) therapy (median was 4 days, IQR = 36 days). Antimicrobial therapy was generally completed after discharge, as only 17.3% received their entire treatment course during hospitalization. Median duration of outpatient oral (PO) antimicrobial therapy was twice as long for guideline‐excessive therapy compared to guideline‐similar therapy (6 vs 3 days), whereas duration of inpatient IV and PO antimicrobial therapy was similar. Patients discharged on a fluoroquinolone were more likely to receive guideline‐similar duration of therapy. The VHA classifies facilities into 3 levels of complexity, with lower scores indicating more complex facilities.[21] Guideline‐similar therapy duration occurred in 10.4% of cases in lower complexity facilities (levels 2 and 3),and 15.1% in more complex facilities (level 1) (P = 0.01). The median duration of therapy was similar for more and less complex facilities, respectively (10 days, IQR = 812 days vs 10 days, IQR = 813 days).
| Outcome |
Therapy Duration Similar With IDSA/ATS Guidelines |
Therapy Duration in Excess of IDSA/ATS Guideline Recommendations | Significance |
|---|---|---|---|
| |||
| Antimicrobial duration consistent with guideline recommendations, n (%) | 241 (13.9) | 1,498 (86.1) | NR |
| CAP* | 83 (6.9) | 1,112 (93.1) | NR |
| HCAP* | 158 (29.0) | 386 (71.0) | NR |
| Total days of therapy for pneumonia, median (IQR) | 7 (78) | 10 (913) | NR |
| CAP | 6 (59) | 10 (812) | <0.01 |
| HCAP | 7 (78) | 11 (1014) | <0.01 |
| Days of IV therapy administered for pneumonia, median (IQR) | 4 (37) | 4 (36) | 0.50 |
| Days of PO inpatient therapy administered, median (IQR) | 1 (03) | 1 (03) | 0.78 |
| Days of PO outpatient therapy dispensed at discharge, median (IQR) | 3 (25) | 6 (47) | <0.01 |
| Days of PO outpatient therapy recommended in Discharge Summary for patients without a VA prescription, median (IQR) | 3 (24) | 5 (47) | <0.01 |
| Aggregate 28‐day hospital readmission, n (%) | 42 (17.4) | 183 (12.2) | 0.03 |
| CAP∥# | 7 (8.4) | 112 (10.1) | 0.58 |
| HCAP∥# | 35 (22.2) | 71 (18.4) | 0.28 |
| Aggregate 28‐day CDI rate, n (%) | 6 (2.5) | 9 (0.6) | 0.03 |
| CAP∥** | 1 (1.2) | 6 (0.5) | 0.44 |
| HCAP∥** | 5 (3.2) | 3 (0.8) | 0.04 |
| Aggregate 28‐day death after discharge, n (%) | 6 (2.5) | 52 (3.5) | 0.43 |
| CAP∥** | 1 (1.2) | 33 (3.0) | 0.35 |
| HCAP∥** | 5 (3.2) | 19 (4.9) | 0.37 |
The 28‐day postdischarge all‐cause readmission rate for patients who received guideline‐similar therapy duration was higher (17.4%) than for patients who received therapy duration in excess of guideline recommendations (12.2%) (P = 0.03). After adjustment for covariates associated with readmission (HCAP, age, prior skilled nursing facility residence, PSI score comorbidity elements), we found no evidence that patients who received guideline‐similar therapy duration were more likely to be readmitted than were patients who received guideline‐excessive duration (OR: 1.1 [95% CI: 0.8, 1.7]) (Table 3). Likewise, no difference in 28‐day all‐cause postdischarge mortality was identified between guideline‐similar and guideline‐excessive duration after adjustment for the same covariates (adjusted OR: 0.5 [95% CI: 0.2, 1.2]) (Table 4).
| Model Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Readmission model | |||
| Duration of antibiotics | 1.11 | 0.75, 1.64 | 0.62 |
| HCAP | 1.94 | 1.38, 2.72 | <0.01 |
| Age | 1.01 | 1.00, 1.03 | 0.04 |
| Prior skilled nursing facility residence | 0.91 | 0.59, 1.40 | 0.67 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.20 | 0.86, 1.67 | 0.29 |
| Liver disease | 1.55 | 0.66, 3.64 | 0.31 |
| CHF | 1.15 | 0.83, 1.59 | 0.41 |
| Cerebrovascular disease | 1.06 | 0.75, 1.50 | 0.75 |
| Renal disease | 1.51 | 1.09, 2.08 | 0.01 |
| Mortality model | |||
| Duration of antibiotics | 0.53 | 0.23, 1.22 | 0.14 |
| HCAP | 2.53 | 1.38, 4.65 | <0.01 |
| Age | 1.06 | 1.03, 1.09 | <0.01 |
| Prior skilled nursing facility residence | 0.79 | 0.38, 1.66 | 0.53 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.03 | 0.57, 1.87 | 0.91 |
| Liver disease | <0.001 | <0.001, >999.9 | 0.98 |
| CHF | 0.73 | 0.39, 1.38 | 0.34 |
| Cerebrovascular disease | 0.82 | 0.43, 1.56 | 0.55 |
| Renal disease | 0.72 | 0.39, 1.35 | 0.31 |
CDI cases (n = 15) were too sparse to adequately perform multivariable logistic regression analysis; however, a higher percentage of patients who received guideline‐similar duration of therapy developed CDI compared to patients who received guideline‐excessive duration of therapy (40.0% vs 13.6%, P < 0.01). The median duration of therapy for patients who did and did not develop CDI was similar (8 days, IQR = 714 days vs 10 days, IQR = 812 days, P = 0.85, respectively). Patients who developed CDI had a higher rate of HCAP diagnosis (1.5% vs 0.6%; P = 0.06), were more likely to have concomitant non‐pneumonia infection (40.0% vs 9.5%, P < 0.01), have chronic comorbidity (86.7% vs 59.1%, P = 0.03), and to have been admitted to the ICU (26.7% vs 12.1%, P = 0.09).
DISCUSSION
IDSA/ATS guidelines for pneumonia duration of therapy generally agree with other professional society guidelines including the British Thoracic Society and National Institute for Health and Care Excellence.[22, 23] In contrast to existing evidence and guideline recommendations, this multi‐centered evaluation identified prolonged durations of antimicrobial therapy prescribed in 93% and 71% of patients with uncomplicated CAP and HCAP (Table 3), respectively.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] Almost all (97.1%) uncomplicated CAP and HCAP patients met clinical stability criteria before day 4 of hospitalization, yet the median duration of IV therapy was 4 days. Because criteria for IV to PO conversion and the clinical stability definition utilized in this analysis were similar, many patients may have been eligible for PO therapy earlier, favorably impacting length of stay, cost, and adverse effects.[3, 12, 24, 25, 26] Although median days of inpatient PO therapy administered was 1 day (IQR = 03 days), inpatient observation after PO conversion may not be necessary. The duration of PO therapy was based on calendar days, where if a patient received 1 dose of a once daily antibiotic (ie, levofloxacin), they were considered to have received 1 day of inpatient PO antibiotics even if discharged the same day.
Approximately half of all days of therapy occurred after discharge. Although the median therapy duration for inpatients was similar, the median duration of antimicrobials administered upon hospital discharge was twice as long for patients receiving guideline‐excessive compared to guideline‐similar duration of therapy. The median excess in antibiotic duration is almost entirely accounted for by excess outpatient days of therapy. This is an important consideration for antimicrobial stewardship programs that tend to focus on inpatient antimicrobial use.
Noteworthy observations include the low rate of respiratory tract culture collection (41%) and frequent use of fluoroquinolones upon discharge. Collection of respiratory tract cultures is recommended for all patients with HCAP and patients with CAP who have risk factors for resistant pathogens, characteristics that were common in this cohort.[3, 4] Recently, we identified that respiratory culture collection is associated with increased de‐escalation rates in HCAP, and that culture‐negative patients frequently receive fluoroquinolones.[27] IDSA/ATS CAP guidelines discourage empirically switching to PO fluoroquinolone therapy based on bioavailability considerations alone.[3] Further, fluoroquinolones are considered to be associated with high risk of CDI.[28, 29] Prescription of fluoroquinolone upon discharge was associated with guideline‐similar duration of therapy and was not shown to be associated with CDI; however, power to detect differences between exposures to specific antimicrobials and CDI was low.
CDI was more common in patients with CAP (1.2% vs 0.5%) and HCAP (3.2% vs 0.8%) who received duration of therapy similar with guideline recommendations. This observation is confounded, as patients with CDI had significantly greater comorbidity as well as secondary infections and tended to more frequently receive ICU care. There were no differences in adjusted rates of readmission or death between patients receiving guideline‐similar and guideline‐excessive duration of therapy.
Evaluation strengths included exclusion of patients with complicating conditions possibly requiring prolonged antimicrobial treatment courses, which allowed the evaluation to focus on patients most likely to benefit from shorter course therapy. The definition of appropriate therapy duration was based upon daily assessment of clinical stability criteria that paralleled the CAP guidelines. The definition utilized objective parameters while accounting for patient variability in achieving clinical stability criteria. Finally, the analyses of clinical end points suggest that shorter duration of therapy may be as safe and effective as longer duration of therapy in uncomplicated pneumonia.
Limitations include those common to other analyses conducted within the VHA, including a predominantly elderly male cohort.[30] Only ICD‐9‐CM codes consistent with a discharge diagnosis of pneumonia were used to identify the cohort, and clinical impressions not documented in the medical record may have impacted the clinician's treatment duration decisions. The upper limit of appropriate duration of therapy for CAP was arbitrarily set at up to 3 days beyond meeting clinical stability criteria to provide a reasonable duration of appropriate therapy beyond clinical stability to operationalize the duration of therapy recommendations within the context of the IDSA/ATS guidelines. Additionally, CIs for the ORs of readmission and mortality were broad, and thus too imprecise to determine whether guideline‐similar durations increased or decreased readmission or mortality in comparison with therapy that exceeded guideline recommendations. We could not fully assess the potential for association between guideline‐excessive therapy duration and risk for CDI due to sparse cases. Finally, non‐VA prescription data were not available for all patients, and we relied on intended duration of therapy as recommended by the discharging provider in 4.1% of cases.
Most quality assessments of pneumonia treatment have focused on antimicrobial selection and timely administration or conversion from IV to PO therapy.[31, 32] This evaluation identified potential opportunities for expansion of antimicrobial stewardship activities during the transition of care setting. The efficacy of short‐course ‐lactam, macrolide, or fluoroquinolone therapy for CAP appears equivalent to longer treatment regimens with no difference in adverse event rates, suggesting that optimal duration of therapy may be a rational target for quality improvement.[5, 6, 7, 8, 9, 10, 11, 12, 15, 31] Recommendations for HCAP duration of therapy are extrapolated from a prospective multicentered study, which randomized patients with hospital‐acquired pneumonia to receive 8 versus 15 days of therapy, that identified similar outcomes to ours.[4, 12]
Single‐center studies have identified that antimicrobial therapy for pneumonia is frequently prescribed for longer than recommended by guidelines, which found a similar median duration of therapy as our evaluation.[15, 16] Similar to Jenkins et al., we observed a high rate of fluoroquinolone prescriptions upon discharge.[16]
There are few published examples of interventions designed to limit excessive duration of therapy, particularly for antimicrobials prescribed upon hospital discharge.[15, 33, 34] Serial procalcitonin measurements have been used to guide duration of therapy for pneumonia; however, the costbenefit ratio of procalcitonin measurement is unclear.[35, 36] Procalcitonin use was uncommon, and none of the participating facilities in our evaluation utilized a specific algorithm to guide therapy duration. Limited data suggest that patient‐level prospective audit with feedback may be effective. Advic et al. evaluated management of presumed CAP before and after education and prospective feedback to medical teams concerning antimicrobial selection and duration of therapy.[15] The intervention led to a decrease in median duration of therapy from 10 days (IQR = 813 days) to 7 days (IQR = 78 days) without increasing clinical failure or readmission rates. We recently reported a single‐center evaluation in which pharmacists utilizing a decision support tool while performing discharge medication reconciliation were able to reduce excessive mean duration of therapy from 9.5 days ( 2.4 days) to 8.3 days ( 2.9 days) in patients without complicated pneumonia, with a 19.2% reduction in duration of therapy prescribed at discharge.[37] A similar approach utilizing pharmacists performing discharge review has recently been reported in a community hospital.[38]
Future work should recognize that few patients complete their entire course of therapy as inpatients, and the majority of treatment is prescribed upon discharge. Pivotal time points for antimicrobial stewardship intervention include day 2 to 3 of hospitalization when conveying suggestions for antimicrobial de‐escalation and/or IV to PO conversion, and toward the end of hospitalization during discharge planning. Although it may not be feasible for antimicrobial stewards to review all uncomplicated cases of pneumonia during hospitalization, most facilities have a systematic process for reviewing medications during transitions of care. We believe that interventions intended to assess and recommend shortened courses of therapy are appropriate. These interventions should include a mechanism for support by stewardship personnel or other infectious diseases specialists. Based on our evaluation, the ASTF produced and disseminated clinical guidance documents and tools to triage pneumonia case severity and assess response to therapy. Qualified personnel are encouraged to use this information to make recommendations to providers regarding excessive duration of therapy for uncomplicated cases where appropriate. Other work should include an in‐depth assessment of clinical outcomes related to treatment duration, investigation of provider rationale for prolonged treatment, and duration of antimicrobial therapy prescribed upon discharge for other common disease states. Finally, manual chart review to classify uncomplicated cases and related outcomes was laborious, and automated case identification is technologically plausible and should be explored.[39]
In conclusion, this national VHA MUE found that patients with uncomplicated pneumonia were commonly prescribed antimicrobials for the duration of therapy in excess of guideline recommendations. Patients with uncomplicated pneumonia who received therapy duration consistent with guideline recommendations did not have significantly different all‐cause readmission and death rates compared to patients receiving prolonged treatment. Approximately half of all therapy was prescribed upon hospital discharge, and clinicians as well as antimicrobial stewardship programs should consider these findings to address excessive duration of antimicrobial therapy upon hospital discharge.
Acknowledgements
The authors acknowledge Dr. Michael Fine for his assistance with utilization of the Pneumonia Severity Index, Kenneth Bukowski for assisting with development of data collection tools and data management, and members of the Antimicrobial Stewardship Taskforce Implementation Sub‐Committee. Collaborators in the Pneumonia Duration of Therapy Medication Utilization Evaluation Group include: Biloxi VA (VA Gulf Coast): Cheryl Hankins, PharmD, BCPS; Central Alabama VAMC: Lauren Rass, PharmD, BCPS, Kelly Mooney, PharmD, BCPS; Central Arkansas: Nicholas Tinsley, MS, PharmD; Chillicothe VA: Stephen Hanson, PharmD, BCPS, Beth Gallaugher, BSN, RN, Elizabeth Baltenberger, PharmD; Cincinnati VA: Jason Hiett, PharmD, BCPS, Victoria Tate, PharmD, BCPS, Brian Salzman, PharmD; Dorn Medical Center: MaryAnne Maurer, PharmD, BCPS, BCACP, Rebekah Sipes, PharmD, BCACP, Ginger Ervin, PharmD; Dwight D. Eisenhower VAMC: Emily Potter, PharmD; Hudson Valley: Rita Lee Bodine, PharmD, Clement Chen, PharmD, Cristina Fantino, PharmD; James H. Quillen VAMC: Marty Vannoy, PharmD, BCPS, Erin Harshbarger, PharmD, Kristen Nelsen, PharmD; Jesse Brown VAMC: Lisa Young, PharmD, BCPS, AQ‐ID, Andrea Bidlencik, PharmD, BCPS; Kansas City VA: Jamie Guyear, PharmD, AQ‐ID, Ann Ungerman, PharmD, BCPS, Lauri Witt, PharmD, BCACP; Louis Stokes Cleveland VAMC: Amy Hirsch, PharmD, BCPS, Steven Adoryan, PharmD, BCP‐CC, Amanda Miller, PharmD, BCPS; Maine VAMC: Joel Coon, PharmD, Rachel Naida, PharmD, Kelly Grossman, PharmD; Martinsburg VAMC: Kelly Li, PharmD, Sarah Mickanis, PharmD, BCPS; Miami VA Medical Center: Mara Carrasquillo, BS, PharmD, Maribel Toro, PharmD; North Florida/South Georgia Veterans Health System: Nora Morgan, PharmD, Hugh Frank, PharmD, BCPS, BCPP, Sarah Onofrio, PharmD, BCPS; North Texas HCS: Susan Duquaine, PharmD, BCPS, AQ‐ID, Ruben Villaneuva, PharmD, BCPS, Jaela Dahl, PharmD, BCPS; Ozarks: Andrew Siler, PharmD, BCPS, Michele Walker, PharmD, CGP, Jennifer Cole, PharmD, BCPS, BCCCP; Providence VAMC: Kerry LaPlante, PharmD, FCCP, Lindsey Williamson, PharmD; Richmond VA: Daniel Tassone, PharmD, BCPS; Salisbury VAMC: Brett Norem, PharmD, Marrisa Ragonesi, PharmD; San Juan VA: Monica Sanabria‐Seda, PharmD, BCPS, Jaime Velez‐Fores, PharmD, BCPS, AQ‐ID, Norma Ayala‐Burgos, PharmD; Sioux Falls VA: Andrea Aylward, PharmD, BCPS; South Texas HCS: Kelly Echevarria, PharmD, BCPS, AQ‐ID, Manuel Escobar, PharmD; Tennessee Valley HCS: Casey Ryals, PharmD, BCACP, Molly Hurst, PharmD, Jonathan Hale, PharmD; VA Central Iowa Health Care System: Jenny Phabmixay, PharmD, BCPS, Mackenzie Brown, PharmD, BCPS, Cynthia Muthusi, PharmD, BCPS; VA Loma Linda: Tony Chau, PharmD; VA Sierra Nevada: Scott Mambourg, PharmD, BCPS, AAHIVP, Matthew Han, PharmD, Nathan Mihoch, PharmD; VA WNY Healthcare System: Kari Mergenhagen, PharmD, BCPS, AQ‐ID, Christine Ruh, PharmD, BCPS; Veterans Affairs Salt Lake City Health System: Emily Spivak, MD, MHS, Patricia Orlando, PharmD
Disclosures: Karl Madaras‐Kelly is employed full time by Idaho State University and has a without compensation appointment as a clinical pharmacist at the Boise VA Medical Center. He receives grant support unrelated to this work through the Department of Veterans Affairs subcontracted to Idaho State University. Muriel Burk is employed full time through the Department of Veterans Affairs as clinical pharmacy specialist in outcomes and medication safety evaluation. Christina Caplinger was employed by the Department of Veterans Affairs as an infectious diseases fellow at the time this work was completed. She is currently employed by Micromedex. Jefferson Bohan is employed full time by the Department of Veterans Affairs as an infectious diseases fellow. Melinda Neuhauser is employed full time through the Department of Veterans Affairs as a clinical pharmacy specialistinfectious diseases. Matthew Goetz is employed full time through the Department of Veterans Affairs as an infectious diseases physician. Rhongping Zhang is employed full time through the Department of Veterans Affairs as a data analyst. Francesca Cunningham is employed full time through the Department of Veterans Affairs as the director of the VA Center for Medication Safety. This work was supported with resources and use of the Department of Veterans Affairs healthcare system. The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
Pneumonia is the leading inpatient infectious diagnosis for which antimicrobials are prescribed in the United States.[1] Supported by moderate‐ to high‐quality evidence, guidelines produced jointly by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) recommend treating pneumonia with the shortest appropriate duration of antimicrobial therapy to minimize risk for antimicrobial‐related adverse events.[2, 3, 4]
Evidence supports short duration of therapy for treatment of uncomplicated pneumonia.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] IDSA/ATS guidelines state, patients with CAP [community‐acquired pneumonia] should be treated for a minimum of 5 days (level 1 evidence), should be afebrile for 4872 hours, and should have no more than 1 CAP‐associated sign of clinical instabilitybefore discontinuation of therapy (level II evidence). (Moderate recommendation.) A longer duration of therapy may be warranted if initial therapy was not active against the identified pathogen or if it was complicated by [abscess, empyema, severe immunosuppression, or] extra‐pulmonary infection such as meningitis or endocarditis. (Weak recommendation; level III evidence).[3] Recommended therapy duration for patients with uncomplicated healthcare‐associated pneumonia (HCAP) who respond to initial therapy is 7 to 8 days unless gram‐negative nonfermenting rods or complications are identified (level I evidence).[4]
Within the Veterans Health Administration (VHA), the Antimicrobial Stewardship Taskforce (ASTF) was created to optimize care by developing, deploying, and monitoring a national‐level strategic plan for antimicrobial therapy management improvements.[13, 14] Although single‐center studies have found antimicrobial therapy for CAP being frequently prescribed for longer than recommended, the reproducibility of this finding across different facilities has not been assessed.[15, 16] The ASTF collaborated with the VHA Center for Medication Safety to assess total duration of antimicrobial therapy prescribed for veterans hospitalized with uncomplicated pneumonia.[17]
METHODS
This retrospective multicenter evaluation was conducted in 30 VHA facilities that volunteered to participate in this project. Inpatients discharged with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for pneumonia (or pneumonia diagnosis secondary to primary sepsis diagnosis) during 2013 were evaluated.[18] Diagnoses, admissions, and patient demographics were identified using Veterans Affairs (VA) integrated databases through the Austin Integrated Technology Center. Up to 200 admissions per facility were randomly selected for review. Clinical pharmacists at each facility performed manual record reviews utilizing a standardized protocol and collection form. Completed cases were uploaded to a central database for analysis. Standardized chart abstraction was facilitated by detailed instructions, a data dictionary, and monthly conference calls.
Inclusion criteria required patient admission to any medical ward including intensive care unit (ICU) wards for 48 hours, receipt of >24 hours inpatient antimicrobial therapy (eg, at least 2 doses of a once‐daily antibiotic), documentation of pneumonia discharge diagnosis, and survival until discharge. Exclusion criteria were: complicated pneumonia (lung abscess, necrotizing pneumonia, thoracentesis performed), significant immunosuppression (cancer chemotherapy or absolute neutrophil count <1500 cell/mm3 within 28 days, organ transplantation, human immunodeficiency virus infection); or extrapulmonary infection (eg, meningitis, endocarditis).[3] Patients were also excluded if directly transferred from another inpatient facility, pneumonia occurred >48 hours after admission, index hospitalization was >14 days, previously hospitalized within 28 days prior to index admission, or discharged without documentation of completing a full course of therapy. In addition, patients who received initial therapy discordant with culture and susceptibility findings, were not clinically stable by discharge, or had gram‐negative nonfermentative bacilli cultured were excluded from analysis because according to the guidelines, either data are lacking to support a short duration of therapy such as initial discordant therapy, or a longer duration of therapy may be warranted such as gram‐negative nonfermentative bacilli and clinical instability at discharge.[4] Our intent for these exclusions was to minimize bias against clinician decision making for cases where a longer duration of therapy may have been appropriate.
Patients meeting all criteria had the following abstracted: demographics; prior healthcare exposures, admitting location (ICU or non‐ICU ward), parameters for calculation of Pneumonia Severity Index (PSI), culture results obtained 48 hours of admission, duration of antimicrobials administered during hospitalization and prescribed upon discharge (or recommendations for outpatient duration in the discharge summary for patients receiving medications from non‐VA sources), daily clinical stability assessment, Clostridium difficile infection (CDI) test results, and readmission or death within 28 days of discharge.[19]
Guideline‐similar CAP therapy duration was defined as a minimum of 5 days of antimicrobials, up to a maximum of 3 additional days beginning the first day the patient was afebrile and exhibited 1 sign of clinical instability (heart rate > 100 beats/minute, respiratory rate >24 breaths/minute, systolic blood pressure <90, oxygen saturation <90% or partial pressure of oxygen <60 mm Hg on room air or baseline O2 requirements, or not returned to baseline mental status).[3] This definition was made by consensus decision of the investigators and was necessary to operationalize the relationship between clinical stability and appropriate duration of therapy. Guideline‐similar HCAP therapy duration was defined as 8 days.[4] CDI was defined in accordance with VA criteria for hospital onset and community‐onset healthcare‐facilityassociated CDI.[20] All‐cause hospital readmission and all‐cause death were defined as inpatient readmission or any death, respectively, within 28 days after discharge for the pneumonia admission.
Demographics, comorbidities, microbiology results, antimicrobial utilization, CDI, readmission, and death rates between guideline‐similar and guideline‐excessive duration of antimicrobial therapy groups were characterized with descriptive statistics, Mann‐Whitney U test, or 2 test as indicated (significance defined as P < 0.05). Multivariable logistic regression (SAS version 9.3 [SAS Institute, Cary, NC]) was used to assess association between duration of therapy exceeding recommended guidelines with all‐cause readmission and all‐cause death after adjustment for pertinent covariates. Odds ratios (OR) with 95% confidence intervals ( 95% CI) were reported. This medication utilization evaluation (MUE) was reviewed by the Hines VHA Institutional Review Board for Human Subjects Protection. Based on VHA Policy Handbook 1058.05, which defines operations activities that may constitute research, the board determined that the evaluation constituted quality improvement rather than research, and thus was exempt from VHA Human Subjects Research requirements.
RESULTS
There were 3881 admissions eligible for chart review. After manual chart review of inclusion and exclusion criteria, 1739 (44.8%) patients were available for duration of therapy analysis. (Figure 1). Only 1 admission for each patient was analyzed.

The cohort was comprised primarily of elderly male patients (96.6%) of whom more than two‐thirds were hospitalized for CAP (Table 1). Most patients had significant disease severity as indicated by PSI score; however, only 12% were directly admitted to the ICU. Blood cultures were collected in >95% of cases; lower respiratory cultures were obtained in 39.9% of cases.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean SD | 71.8 (12.7) |
| Gender, male, n (%) | 1,680 (96.6) |
| Living environment at time of index admission, n (%) | |
| Home | 1,416 (81.4) |
| VA community‐based living center | 88 (5.1) |
| Non‐VA long‐term skilled care facility | 95 (5.5) |
| Assisted living facility | 52 (2.9) |
| Not documented | 46 (2.7) |
| Other | 29 (1.7) |
| Prior healthcare exposures, n (%) | |
| Prior hospitalization within last 90 days | 310 (17.8) |
| Residence in a long‐term skilled care facility in last 90 days | 209 (12.0) |
| Chronic dialysis within last 28 days | 52 (3.0) |
| Intravenous antimicrobials within last 28 days | 76 (4.4) |
| Wound, tracheostomy, or ventilator care in last 28 days | 37 (2.1) |
| Community‐acquired pneumonia, n (%) | 1,195 (68.7) |
| Healthcare‐associated pneumonia, n (%) | 544 (31.3) |
| Comorbidities, n (%) | |
| Renal disease | 438 (25.2) |
| Liver disease | 39 (2.2) |
| Congestive heart failure | 436 (25.1) |
| Cerebrovascular disease | 356 (20.4) |
| Neoplastic disease (excluding skin) | 384 (22.1) |
| Severity of illness, n (%) | |
| Pneumonia Severity Index | |
| Class I | 30 (1.8) |
| Class II | 198 (11.4) |
| Class III | 349 (20.1) |
| Class IV | 759 (43.6) |
| Class V | 403 (23.2) |
| Intensive care upon admission | 212 (12.2) |
| Culture collection 48 hours of admission, n (%) | 1,687 (97.0) |
| Blood | 1,631 (96.7) |
| Lower respiratory tract (sputum) | 673 (39.9) |
| Bronchoalveolar lavage | 20 (1.2) |
| Urine | 632 (37.5) |
| Skin/wound | 3 (0.2) |
| Other | 158 (9.4) |
| Facility complexity, n (%) | |
| Level 1a‐c | 1,286 (74.0) |
| Level 2 | 437 (25.1) |
| Level 3 | 16 (0.9) |
Commonly administered antimicrobials during hospitalization and at discharge are summarized in Table 2. Anti‐pseudomonal ‐lactams and antimethicillin‐resistant Staphylococcus aureus antimicrobials were more frequently administered to patients with HCAP, whereas third‐generation cephalosporins and macrolides were more likely to be administered to patients with CAP. Fluoroquinolones were prescribed to 55.3% of patients upon discharge.
| Inpatient Antimicrobials Administered* | ||||
|---|---|---|---|---|
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,739 |
Therapy Duration Similar With Guidelines, n (%), n = 241 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,498 | Significance | |
| Antimicrobials Dispensed or Recommended at Discharge | ||||
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,471 |
Therapy Duration Similar With Guidelines, n (%), n = 151 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,320 | Significance | |
| ||||
| Third‐generation cephalosporins | 809 (46.5) | 75 (31.1) | 734 (49.0) | <0.001 |
| Fluoroquinolones | 836 (48.1) | 114 (47.3) | 722 (48.2) | 0.80 |
| Macrolides | 788 (45.3) | 90 (37.3) | 698 (46.6) | <0.01 |
| Pseudomonal ‐lactams | 692 (39.8) | 138 (57.3) | 554 (37.0) | 0.01 |
| Anti‐MRSA antimicrobials | 663 (38.1) | 135 (56.0) | 528 (35.3) | <0.01 |
| Other ‐lactams | 139 (8.0) | 10 (4.2) | 129 (8.6) | 0.02 |
| Tetracyclines | 119 (6.8) | 14 (5.8) | 105 (7.0) | 0.49 |
| Other | 97 (5.6) | 15 (6.2) | 82 (5.5) | 0.64 |
| Third‐generation cephalosporins | 285 (19.4) | 27 (17.9) | 258 (19.6) | 0.62 |
| Fluoroquinolones | 813 (55.3) | 95 (62.9) | 718 (54.4) | 0.05 |
| Macrolides | 203 (13.8) | 20 (13.3) | 183 (13.9) | 0.83 |
| Pseudomonal ‐lactams | 31 (2.1) | 4 (2.7) | 27 (2.1) | 0.62 |
| Anti‐MRSA antimicrobials | 45 (3.1) | 6 (4.0) | 39 (3.0) | 0.49 |
| Other ‐lactams | 239 (16.3) | 13 (8.6) | 226 (17.1) | 0.01 |
| Tetracyclines | 95 (6.5) | 10 (6.6) | 85 (6.4) | 0.93 |
| Other | 44 (3.0) | 5 (3.3) | 39 (3.0) | 0.81 |
Overall, 13.9% of patients with uncomplicated pneumonia received guideline‐similar duration of therapy (Table 3). A greater proportion of HCAP patients (29.0%) received guideline‐similar therapy duration as compared to CAP patients (6.9%) (P < 0.01 (Table 3). Median duration of therapy was 7 days (interquartile range [IQR] = 78 days) for guideline‐similar therapy compared to 10 days (913 days) for therapy duration in excess of guideline recommendations. Overall, 97.1 % of patients met clinical stability criteria before day 4 of therapy, yet 50% received 4 days of intravenous (IV) therapy (median was 4 days, IQR = 36 days). Antimicrobial therapy was generally completed after discharge, as only 17.3% received their entire treatment course during hospitalization. Median duration of outpatient oral (PO) antimicrobial therapy was twice as long for guideline‐excessive therapy compared to guideline‐similar therapy (6 vs 3 days), whereas duration of inpatient IV and PO antimicrobial therapy was similar. Patients discharged on a fluoroquinolone were more likely to receive guideline‐similar duration of therapy. The VHA classifies facilities into 3 levels of complexity, with lower scores indicating more complex facilities.[21] Guideline‐similar therapy duration occurred in 10.4% of cases in lower complexity facilities (levels 2 and 3),and 15.1% in more complex facilities (level 1) (P = 0.01). The median duration of therapy was similar for more and less complex facilities, respectively (10 days, IQR = 812 days vs 10 days, IQR = 813 days).
| Outcome |
Therapy Duration Similar With IDSA/ATS Guidelines |
Therapy Duration in Excess of IDSA/ATS Guideline Recommendations | Significance |
|---|---|---|---|
| |||
| Antimicrobial duration consistent with guideline recommendations, n (%) | 241 (13.9) | 1,498 (86.1) | NR |
| CAP* | 83 (6.9) | 1,112 (93.1) | NR |
| HCAP* | 158 (29.0) | 386 (71.0) | NR |
| Total days of therapy for pneumonia, median (IQR) | 7 (78) | 10 (913) | NR |
| CAP | 6 (59) | 10 (812) | <0.01 |
| HCAP | 7 (78) | 11 (1014) | <0.01 |
| Days of IV therapy administered for pneumonia, median (IQR) | 4 (37) | 4 (36) | 0.50 |
| Days of PO inpatient therapy administered, median (IQR) | 1 (03) | 1 (03) | 0.78 |
| Days of PO outpatient therapy dispensed at discharge, median (IQR) | 3 (25) | 6 (47) | <0.01 |
| Days of PO outpatient therapy recommended in Discharge Summary for patients without a VA prescription, median (IQR) | 3 (24) | 5 (47) | <0.01 |
| Aggregate 28‐day hospital readmission, n (%) | 42 (17.4) | 183 (12.2) | 0.03 |
| CAP∥# | 7 (8.4) | 112 (10.1) | 0.58 |
| HCAP∥# | 35 (22.2) | 71 (18.4) | 0.28 |
| Aggregate 28‐day CDI rate, n (%) | 6 (2.5) | 9 (0.6) | 0.03 |
| CAP∥** | 1 (1.2) | 6 (0.5) | 0.44 |
| HCAP∥** | 5 (3.2) | 3 (0.8) | 0.04 |
| Aggregate 28‐day death after discharge, n (%) | 6 (2.5) | 52 (3.5) | 0.43 |
| CAP∥** | 1 (1.2) | 33 (3.0) | 0.35 |
| HCAP∥** | 5 (3.2) | 19 (4.9) | 0.37 |
The 28‐day postdischarge all‐cause readmission rate for patients who received guideline‐similar therapy duration was higher (17.4%) than for patients who received therapy duration in excess of guideline recommendations (12.2%) (P = 0.03). After adjustment for covariates associated with readmission (HCAP, age, prior skilled nursing facility residence, PSI score comorbidity elements), we found no evidence that patients who received guideline‐similar therapy duration were more likely to be readmitted than were patients who received guideline‐excessive duration (OR: 1.1 [95% CI: 0.8, 1.7]) (Table 3). Likewise, no difference in 28‐day all‐cause postdischarge mortality was identified between guideline‐similar and guideline‐excessive duration after adjustment for the same covariates (adjusted OR: 0.5 [95% CI: 0.2, 1.2]) (Table 4).
| Model Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Readmission model | |||
| Duration of antibiotics | 1.11 | 0.75, 1.64 | 0.62 |
| HCAP | 1.94 | 1.38, 2.72 | <0.01 |
| Age | 1.01 | 1.00, 1.03 | 0.04 |
| Prior skilled nursing facility residence | 0.91 | 0.59, 1.40 | 0.67 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.20 | 0.86, 1.67 | 0.29 |
| Liver disease | 1.55 | 0.66, 3.64 | 0.31 |
| CHF | 1.15 | 0.83, 1.59 | 0.41 |
| Cerebrovascular disease | 1.06 | 0.75, 1.50 | 0.75 |
| Renal disease | 1.51 | 1.09, 2.08 | 0.01 |
| Mortality model | |||
| Duration of antibiotics | 0.53 | 0.23, 1.22 | 0.14 |
| HCAP | 2.53 | 1.38, 4.65 | <0.01 |
| Age | 1.06 | 1.03, 1.09 | <0.01 |
| Prior skilled nursing facility residence | 0.79 | 0.38, 1.66 | 0.53 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.03 | 0.57, 1.87 | 0.91 |
| Liver disease | <0.001 | <0.001, >999.9 | 0.98 |
| CHF | 0.73 | 0.39, 1.38 | 0.34 |
| Cerebrovascular disease | 0.82 | 0.43, 1.56 | 0.55 |
| Renal disease | 0.72 | 0.39, 1.35 | 0.31 |
CDI cases (n = 15) were too sparse to adequately perform multivariable logistic regression analysis; however, a higher percentage of patients who received guideline‐similar duration of therapy developed CDI compared to patients who received guideline‐excessive duration of therapy (40.0% vs 13.6%, P < 0.01). The median duration of therapy for patients who did and did not develop CDI was similar (8 days, IQR = 714 days vs 10 days, IQR = 812 days, P = 0.85, respectively). Patients who developed CDI had a higher rate of HCAP diagnosis (1.5% vs 0.6%; P = 0.06), were more likely to have concomitant non‐pneumonia infection (40.0% vs 9.5%, P < 0.01), have chronic comorbidity (86.7% vs 59.1%, P = 0.03), and to have been admitted to the ICU (26.7% vs 12.1%, P = 0.09).
DISCUSSION
IDSA/ATS guidelines for pneumonia duration of therapy generally agree with other professional society guidelines including the British Thoracic Society and National Institute for Health and Care Excellence.[22, 23] In contrast to existing evidence and guideline recommendations, this multi‐centered evaluation identified prolonged durations of antimicrobial therapy prescribed in 93% and 71% of patients with uncomplicated CAP and HCAP (Table 3), respectively.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] Almost all (97.1%) uncomplicated CAP and HCAP patients met clinical stability criteria before day 4 of hospitalization, yet the median duration of IV therapy was 4 days. Because criteria for IV to PO conversion and the clinical stability definition utilized in this analysis were similar, many patients may have been eligible for PO therapy earlier, favorably impacting length of stay, cost, and adverse effects.[3, 12, 24, 25, 26] Although median days of inpatient PO therapy administered was 1 day (IQR = 03 days), inpatient observation after PO conversion may not be necessary. The duration of PO therapy was based on calendar days, where if a patient received 1 dose of a once daily antibiotic (ie, levofloxacin), they were considered to have received 1 day of inpatient PO antibiotics even if discharged the same day.
Approximately half of all days of therapy occurred after discharge. Although the median therapy duration for inpatients was similar, the median duration of antimicrobials administered upon hospital discharge was twice as long for patients receiving guideline‐excessive compared to guideline‐similar duration of therapy. The median excess in antibiotic duration is almost entirely accounted for by excess outpatient days of therapy. This is an important consideration for antimicrobial stewardship programs that tend to focus on inpatient antimicrobial use.
Noteworthy observations include the low rate of respiratory tract culture collection (41%) and frequent use of fluoroquinolones upon discharge. Collection of respiratory tract cultures is recommended for all patients with HCAP and patients with CAP who have risk factors for resistant pathogens, characteristics that were common in this cohort.[3, 4] Recently, we identified that respiratory culture collection is associated with increased de‐escalation rates in HCAP, and that culture‐negative patients frequently receive fluoroquinolones.[27] IDSA/ATS CAP guidelines discourage empirically switching to PO fluoroquinolone therapy based on bioavailability considerations alone.[3] Further, fluoroquinolones are considered to be associated with high risk of CDI.[28, 29] Prescription of fluoroquinolone upon discharge was associated with guideline‐similar duration of therapy and was not shown to be associated with CDI; however, power to detect differences between exposures to specific antimicrobials and CDI was low.
CDI was more common in patients with CAP (1.2% vs 0.5%) and HCAP (3.2% vs 0.8%) who received duration of therapy similar with guideline recommendations. This observation is confounded, as patients with CDI had significantly greater comorbidity as well as secondary infections and tended to more frequently receive ICU care. There were no differences in adjusted rates of readmission or death between patients receiving guideline‐similar and guideline‐excessive duration of therapy.
Evaluation strengths included exclusion of patients with complicating conditions possibly requiring prolonged antimicrobial treatment courses, which allowed the evaluation to focus on patients most likely to benefit from shorter course therapy. The definition of appropriate therapy duration was based upon daily assessment of clinical stability criteria that paralleled the CAP guidelines. The definition utilized objective parameters while accounting for patient variability in achieving clinical stability criteria. Finally, the analyses of clinical end points suggest that shorter duration of therapy may be as safe and effective as longer duration of therapy in uncomplicated pneumonia.
Limitations include those common to other analyses conducted within the VHA, including a predominantly elderly male cohort.[30] Only ICD‐9‐CM codes consistent with a discharge diagnosis of pneumonia were used to identify the cohort, and clinical impressions not documented in the medical record may have impacted the clinician's treatment duration decisions. The upper limit of appropriate duration of therapy for CAP was arbitrarily set at up to 3 days beyond meeting clinical stability criteria to provide a reasonable duration of appropriate therapy beyond clinical stability to operationalize the duration of therapy recommendations within the context of the IDSA/ATS guidelines. Additionally, CIs for the ORs of readmission and mortality were broad, and thus too imprecise to determine whether guideline‐similar durations increased or decreased readmission or mortality in comparison with therapy that exceeded guideline recommendations. We could not fully assess the potential for association between guideline‐excessive therapy duration and risk for CDI due to sparse cases. Finally, non‐VA prescription data were not available for all patients, and we relied on intended duration of therapy as recommended by the discharging provider in 4.1% of cases.
Most quality assessments of pneumonia treatment have focused on antimicrobial selection and timely administration or conversion from IV to PO therapy.[31, 32] This evaluation identified potential opportunities for expansion of antimicrobial stewardship activities during the transition of care setting. The efficacy of short‐course ‐lactam, macrolide, or fluoroquinolone therapy for CAP appears equivalent to longer treatment regimens with no difference in adverse event rates, suggesting that optimal duration of therapy may be a rational target for quality improvement.[5, 6, 7, 8, 9, 10, 11, 12, 15, 31] Recommendations for HCAP duration of therapy are extrapolated from a prospective multicentered study, which randomized patients with hospital‐acquired pneumonia to receive 8 versus 15 days of therapy, that identified similar outcomes to ours.[4, 12]
Single‐center studies have identified that antimicrobial therapy for pneumonia is frequently prescribed for longer than recommended by guidelines, which found a similar median duration of therapy as our evaluation.[15, 16] Similar to Jenkins et al., we observed a high rate of fluoroquinolone prescriptions upon discharge.[16]
There are few published examples of interventions designed to limit excessive duration of therapy, particularly for antimicrobials prescribed upon hospital discharge.[15, 33, 34] Serial procalcitonin measurements have been used to guide duration of therapy for pneumonia; however, the costbenefit ratio of procalcitonin measurement is unclear.[35, 36] Procalcitonin use was uncommon, and none of the participating facilities in our evaluation utilized a specific algorithm to guide therapy duration. Limited data suggest that patient‐level prospective audit with feedback may be effective. Advic et al. evaluated management of presumed CAP before and after education and prospective feedback to medical teams concerning antimicrobial selection and duration of therapy.[15] The intervention led to a decrease in median duration of therapy from 10 days (IQR = 813 days) to 7 days (IQR = 78 days) without increasing clinical failure or readmission rates. We recently reported a single‐center evaluation in which pharmacists utilizing a decision support tool while performing discharge medication reconciliation were able to reduce excessive mean duration of therapy from 9.5 days ( 2.4 days) to 8.3 days ( 2.9 days) in patients without complicated pneumonia, with a 19.2% reduction in duration of therapy prescribed at discharge.[37] A similar approach utilizing pharmacists performing discharge review has recently been reported in a community hospital.[38]
Future work should recognize that few patients complete their entire course of therapy as inpatients, and the majority of treatment is prescribed upon discharge. Pivotal time points for antimicrobial stewardship intervention include day 2 to 3 of hospitalization when conveying suggestions for antimicrobial de‐escalation and/or IV to PO conversion, and toward the end of hospitalization during discharge planning. Although it may not be feasible for antimicrobial stewards to review all uncomplicated cases of pneumonia during hospitalization, most facilities have a systematic process for reviewing medications during transitions of care. We believe that interventions intended to assess and recommend shortened courses of therapy are appropriate. These interventions should include a mechanism for support by stewardship personnel or other infectious diseases specialists. Based on our evaluation, the ASTF produced and disseminated clinical guidance documents and tools to triage pneumonia case severity and assess response to therapy. Qualified personnel are encouraged to use this information to make recommendations to providers regarding excessive duration of therapy for uncomplicated cases where appropriate. Other work should include an in‐depth assessment of clinical outcomes related to treatment duration, investigation of provider rationale for prolonged treatment, and duration of antimicrobial therapy prescribed upon discharge for other common disease states. Finally, manual chart review to classify uncomplicated cases and related outcomes was laborious, and automated case identification is technologically plausible and should be explored.[39]
In conclusion, this national VHA MUE found that patients with uncomplicated pneumonia were commonly prescribed antimicrobials for the duration of therapy in excess of guideline recommendations. Patients with uncomplicated pneumonia who received therapy duration consistent with guideline recommendations did not have significantly different all‐cause readmission and death rates compared to patients receiving prolonged treatment. Approximately half of all therapy was prescribed upon hospital discharge, and clinicians as well as antimicrobial stewardship programs should consider these findings to address excessive duration of antimicrobial therapy upon hospital discharge.
Acknowledgements
The authors acknowledge Dr. Michael Fine for his assistance with utilization of the Pneumonia Severity Index, Kenneth Bukowski for assisting with development of data collection tools and data management, and members of the Antimicrobial Stewardship Taskforce Implementation Sub‐Committee. Collaborators in the Pneumonia Duration of Therapy Medication Utilization Evaluation Group include: Biloxi VA (VA Gulf Coast): Cheryl Hankins, PharmD, BCPS; Central Alabama VAMC: Lauren Rass, PharmD, BCPS, Kelly Mooney, PharmD, BCPS; Central Arkansas: Nicholas Tinsley, MS, PharmD; Chillicothe VA: Stephen Hanson, PharmD, BCPS, Beth Gallaugher, BSN, RN, Elizabeth Baltenberger, PharmD; Cincinnati VA: Jason Hiett, PharmD, BCPS, Victoria Tate, PharmD, BCPS, Brian Salzman, PharmD; Dorn Medical Center: MaryAnne Maurer, PharmD, BCPS, BCACP, Rebekah Sipes, PharmD, BCACP, Ginger Ervin, PharmD; Dwight D. Eisenhower VAMC: Emily Potter, PharmD; Hudson Valley: Rita Lee Bodine, PharmD, Clement Chen, PharmD, Cristina Fantino, PharmD; James H. Quillen VAMC: Marty Vannoy, PharmD, BCPS, Erin Harshbarger, PharmD, Kristen Nelsen, PharmD; Jesse Brown VAMC: Lisa Young, PharmD, BCPS, AQ‐ID, Andrea Bidlencik, PharmD, BCPS; Kansas City VA: Jamie Guyear, PharmD, AQ‐ID, Ann Ungerman, PharmD, BCPS, Lauri Witt, PharmD, BCACP; Louis Stokes Cleveland VAMC: Amy Hirsch, PharmD, BCPS, Steven Adoryan, PharmD, BCP‐CC, Amanda Miller, PharmD, BCPS; Maine VAMC: Joel Coon, PharmD, Rachel Naida, PharmD, Kelly Grossman, PharmD; Martinsburg VAMC: Kelly Li, PharmD, Sarah Mickanis, PharmD, BCPS; Miami VA Medical Center: Mara Carrasquillo, BS, PharmD, Maribel Toro, PharmD; North Florida/South Georgia Veterans Health System: Nora Morgan, PharmD, Hugh Frank, PharmD, BCPS, BCPP, Sarah Onofrio, PharmD, BCPS; North Texas HCS: Susan Duquaine, PharmD, BCPS, AQ‐ID, Ruben Villaneuva, PharmD, BCPS, Jaela Dahl, PharmD, BCPS; Ozarks: Andrew Siler, PharmD, BCPS, Michele Walker, PharmD, CGP, Jennifer Cole, PharmD, BCPS, BCCCP; Providence VAMC: Kerry LaPlante, PharmD, FCCP, Lindsey Williamson, PharmD; Richmond VA: Daniel Tassone, PharmD, BCPS; Salisbury VAMC: Brett Norem, PharmD, Marrisa Ragonesi, PharmD; San Juan VA: Monica Sanabria‐Seda, PharmD, BCPS, Jaime Velez‐Fores, PharmD, BCPS, AQ‐ID, Norma Ayala‐Burgos, PharmD; Sioux Falls VA: Andrea Aylward, PharmD, BCPS; South Texas HCS: Kelly Echevarria, PharmD, BCPS, AQ‐ID, Manuel Escobar, PharmD; Tennessee Valley HCS: Casey Ryals, PharmD, BCACP, Molly Hurst, PharmD, Jonathan Hale, PharmD; VA Central Iowa Health Care System: Jenny Phabmixay, PharmD, BCPS, Mackenzie Brown, PharmD, BCPS, Cynthia Muthusi, PharmD, BCPS; VA Loma Linda: Tony Chau, PharmD; VA Sierra Nevada: Scott Mambourg, PharmD, BCPS, AAHIVP, Matthew Han, PharmD, Nathan Mihoch, PharmD; VA WNY Healthcare System: Kari Mergenhagen, PharmD, BCPS, AQ‐ID, Christine Ruh, PharmD, BCPS; Veterans Affairs Salt Lake City Health System: Emily Spivak, MD, MHS, Patricia Orlando, PharmD
Disclosures: Karl Madaras‐Kelly is employed full time by Idaho State University and has a without compensation appointment as a clinical pharmacist at the Boise VA Medical Center. He receives grant support unrelated to this work through the Department of Veterans Affairs subcontracted to Idaho State University. Muriel Burk is employed full time through the Department of Veterans Affairs as clinical pharmacy specialist in outcomes and medication safety evaluation. Christina Caplinger was employed by the Department of Veterans Affairs as an infectious diseases fellow at the time this work was completed. She is currently employed by Micromedex. Jefferson Bohan is employed full time by the Department of Veterans Affairs as an infectious diseases fellow. Melinda Neuhauser is employed full time through the Department of Veterans Affairs as a clinical pharmacy specialistinfectious diseases. Matthew Goetz is employed full time through the Department of Veterans Affairs as an infectious diseases physician. Rhongping Zhang is employed full time through the Department of Veterans Affairs as a data analyst. Francesca Cunningham is employed full time through the Department of Veterans Affairs as the director of the VA Center for Medication Safety. This work was supported with resources and use of the Department of Veterans Affairs healthcare system. The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- Centers for Disease Control and Prevention. National hospital discharge survey 2010. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed December 1, 2014.
- , , , et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- , , , et al. Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68(13):1841–1854.
- , , , et al. Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis. Am J Med. 2007;120:783–790.
- , , , et al. High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760.
- , , , et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective. Am J Ther. 1999;6(4):217–222.
- , , , et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind trial. BMJ. 2006;332(7554):1355.
- , , , et al. Efficacy of a three day course of azithromycin in moderately severe community‐acquired pneumonia. Eur Respir J. 1995;8(3):398–402.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333(7580):1193.
- , , , , . Unnecessary antimicrobial use in the context of Clostridium difficile infection: a call to arms for the Veterans Affairs Antimicrobial Stewardship Task Force. Infect Control Hosp Epidemiol. 2013;34(6):651–653.
- VHA Directive 1031. Antimicrobial stewardship programs. Available at: https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Accessed December 1, 2014.
- , , , et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community‐acquired pneumonia. Clin Infect Dis. 2012;54:1581–1587.
- , , , et al. Targets for antibiotic and healthcare resource stewardship in inpatient community‐acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–144.
- , , , , . Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104–112.
- , , , . Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , , . Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35(8):1037–1042.
- , , , , . Organization complexity and primary care providers' perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31(2):139–146.
- , , , et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55.
- National Institute for Health and Care Excellence. Pneumonia in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/cg191. Published December 2014. Accessed May 9, 2016.
- , , , , , . A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia: the optimal duration of therapy. Chest. 1996;110(4):965–971.
- , , , et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community‐acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- , , , . Correlates and economic and clinical outcomes of an adult IV to PO antimicrobial conversion program at an academic medical center in Midwest United States. J Pharm Pract. 2015;28(3):238–248.
- , , , et al. Antimicrobial De‐escalation of treatment for healthcare‐associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71(2):539–546.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951.
- , , , . Meta‐analysis of antibiotics and the risk of community‐associated Clostridium difficle infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332.
- , , , et al. Evaluating diagnosis‐based case‐mix measures: how well do they apply to the VA population? Med Care. 2001;39:692–704.
- , , . What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211–228.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , , , et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high‐risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67(12):2988–2996.
- , , , et al.; Centers for Disease Control and Prevention. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157–1164.
- , , , , , . Interim evaluation of a Protocol to Optimize the Duration of Pneumonia Therapy at Hospital Discharge. Open Forum Infect Dis. 2015;2(suppl 1):S379.
- , , , et al. Intervention to improve antibiotic selection and shorten treatment durations at the time of hospital discharge. Open Forum Infect Dis. 2015;2(suppl 1):S1.
- , , , et al. Using the electronic medical record to identify community‐acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013;8(8):e70944.
- Centers for Disease Control and Prevention. National hospital discharge survey 2010. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed December 1, 2014.
- , , , et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- , , , et al. Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68(13):1841–1854.
- , , , et al. Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis. Am J Med. 2007;120:783–790.
- , , , et al. High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760.
- , , , et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective. Am J Ther. 1999;6(4):217–222.
- , , , et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind trial. BMJ. 2006;332(7554):1355.
- , , , et al. Efficacy of a three day course of azithromycin in moderately severe community‐acquired pneumonia. Eur Respir J. 1995;8(3):398–402.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333(7580):1193.
- , , , , . Unnecessary antimicrobial use in the context of Clostridium difficile infection: a call to arms for the Veterans Affairs Antimicrobial Stewardship Task Force. Infect Control Hosp Epidemiol. 2013;34(6):651–653.
- VHA Directive 1031. Antimicrobial stewardship programs. Available at: https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Accessed December 1, 2014.
- , , , et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community‐acquired pneumonia. Clin Infect Dis. 2012;54:1581–1587.
- , , , et al. Targets for antibiotic and healthcare resource stewardship in inpatient community‐acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–144.
- , , , , . Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104–112.
- , , , . Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , , . Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35(8):1037–1042.
- , , , , . Organization complexity and primary care providers' perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31(2):139–146.
- , , , et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55.
- National Institute for Health and Care Excellence. Pneumonia in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/cg191. Published December 2014. Accessed May 9, 2016.
- , , , , , . A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia: the optimal duration of therapy. Chest. 1996;110(4):965–971.
- , , , et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community‐acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- , , , . Correlates and economic and clinical outcomes of an adult IV to PO antimicrobial conversion program at an academic medical center in Midwest United States. J Pharm Pract. 2015;28(3):238–248.
- , , , et al. Antimicrobial De‐escalation of treatment for healthcare‐associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71(2):539–546.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951.
- , , , . Meta‐analysis of antibiotics and the risk of community‐associated Clostridium difficle infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332.
- , , , et al. Evaluating diagnosis‐based case‐mix measures: how well do they apply to the VA population? Med Care. 2001;39:692–704.
- , , . What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211–228.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , , , et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high‐risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67(12):2988–2996.
- , , , et al.; Centers for Disease Control and Prevention. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157–1164.
- , , , , , . Interim evaluation of a Protocol to Optimize the Duration of Pneumonia Therapy at Hospital Discharge. Open Forum Infect Dis. 2015;2(suppl 1):S379.
- , , , et al. Intervention to improve antibiotic selection and shorten treatment durations at the time of hospital discharge. Open Forum Infect Dis. 2015;2(suppl 1):S1.
- , , , et al. Using the electronic medical record to identify community‐acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013;8(8):e70944.