User login
VA SHIELD: A Biorepository for Veterans and the Nation
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
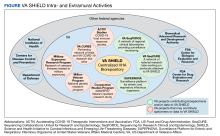
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
Nationwide Genomic Surveillance and Response to COVID-19: The VA SeqFORCE and SeqCURE Consortiums
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
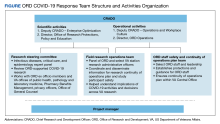
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
The VA Research Enterprise: A Platform for National Partnerships Toward Evidence Building and Scientific Innovation
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
Introduction
Bad times have a scientific value. These are occasions a good learner would not miss.
Ralph Waldo Emerson
Like the flip of a light switch, the world in March 2020 went into lockdown. Suddenly the novel coronavirus disease (COVID-19) was ever-present and everywhere. At a time when very little was certain, scientific inquiry—along with its related skills and disciplines—offered a much-needed pathway for navigating the virus’s myriad unknowns.
From the pandemic’s onset, the Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) made singular contributions to the advancement and acceleration of national and international research activity. This special issue of Federal Practitioner demonstrates how the VHA, through its Office of Research and Development (ORD), took advantage of its newly deployed enterprise strategy to meet the unprecedented demands of this public health emergency.
Launched in 2017, the ORD enterprise strategy enabled the VHA not only to capitalize on existing collaborations—both internal and external—but also move swiftly in forging new ones. Additionally, the strategy was key to leveraging unique VHA assets as the nation’s largest integrated health care system, including: (1) nationwide clinical trials infrastructure, including its longstanding Cooperative Studies Program; (2) a tightly integrated system of clinical care and research that serves as a ready platform for big data science, the world’s largest genomic database, and emergent capabilities; and (3) an established innovation ecosystem that worked with VA research to address rapidly changing circumstances.
In The VA Research Enterprise (p. S12), Garcia and colleagues demonstrate how the VHA pandemic response “arose from an enterprise strategy that was already in motion and aimed at identifying needs for supporting the clinical care mission, more rapidly leveraging resources, and coordinating research across the national VA health care system.” Thus, the VHA took a “model for a culture of cooperative research within the VA and with external groups” and translated it beyond the scope of clinical trials, which had been its foundation.
Led by Chief Research and Development Officer Rachel Ramoni, DMD, ScD, this strategy forged 121 VA medical centers conducting research into an integrated enterprise that could respond to needs for scientific evidence in a coordinated fashion, thereby translating research into practice for real impact on veterans. This approach built on relationships with not only scientific communities but also clinical and operational partners working within the VA to address the immediate pandemic-related needs.
In tandem with its physical infrastructure, the VA’s longstanding network of collaborators, physical infrastructure, and ability to develop new partnerships became drivers of success. Because of previous, ongoing, multisite clinical trials and observational studies, the VA had already partnered with numerous federal government agencies and industry groups and was able to quickly set up a VA COVID-19 clinical trial master protocol framework called the CURES (VA Coronavirus Research and Efficacy studies) network. The ORD enterprise strategy is noted by several other authors, including Caroff and colleagues, who show how the VA efforts to broaden partnerships prepandemic were critical to its participation in 7 large-scale COVID-19 therapeutic and vaccine trials (p. S18).
Similarly, in discussing the VA Million Veteran Program (MVP), Whitbourne and colleagues (p. S23) demonstrate how the VA research strategy and infrastructure were key to leveraging “unique MVP and VA electronic health record data to drive rapid scientific discovery and inform clinical operations.”
Launched in 2011, the MVP is one of the world’s largest genomic cohorts, with more than 985,000 veterans enrolled. MVP developers had the prescience to foresee how a robust genomic database could inform public health emergencies. Whitbourne and colleagues show the many ways the MVP facilitated the VHA COVID-19 response. By extending the MVP centralized recruitment and enrollment infrastructure, an ORD COVID-19 volunteer registry successfully registered 50,000 veterans interested in volunteering for clinical trials.
This tight integration between research and clinical care is one of the VHA’s greatest assets as a health care system. More than 60% of VA researchers are also clinicians who provide direct patient care. This enables VA physician-researchers to learn directly from veteran patients and quickly translate new findings into improved care. It also supported numerous capabilities that played a key role during the pandemic.
For example, in the article VA Big Data Science (p. S39), Young-Xu and colleagues note that the VA use of health care data proved medical research could be performed “quickly and judiciously.” Foundational to this research was a data sharing framework, electronic health record, and VA Corporate Data Warehouse that were accessible to all VA researchers. Researchers had access to clinical data and patient health records that allowed them to perform targeted, time-sensitive research. By building a cohort of 1,363,180 veterans who received ≥ 1 vaccine dose by March 7, 2021, VA researchers added significantly to our understanding of the real-world COVID-19 vaccine clinical performance.
In addition to leveraging existing capabilities, VHA clinicians and researchers created new ones in response. Krishnan and colleagues discuss the launch of 2 clinical and research consortiums focused on COVID-19 genomic surveillance (p. S44). SeqFORCE positioned the VHA to rapidly detect emergent variants and better inform the care of patients with COVID-19. SeqCURE focused on the broader study and trends of variants through sequencing.
The tightly integrated nature of VA care also supported the creation of a large-scale biorepository of specimens with accompanying clinical data to advance research and improve diagnostic and therapeutic research. Epstein and colleagues share the developmental history of the VA SHIELD biorepository, its structure, and its current and future contributions to research science (p. S48).
Finally, the same forward-learning culture which gave rise to the ORD enterprise strategy also resulted in an innovation ecosystem that was well established prior to March 2020. Now a firmly established portfolio within the VHA Office of Healthcare Innovation and Learning (OHIL), the VHA Innovation Ecosystem engages frontline clinicians in reimagining veteran health care. Iaquinto and colleagues discuss how the ecosystem’s preexisting partnerships were critical to addressing shortages in personal protective equipment and other vital resources (p. S52). The OHIL provided the quality system and manufacturing oversight and delivery of swabs for testing, while the ORD furnished research infrastructure and human subjects oversight. Together, these offices not only addressed the shortage by producing swabs but also validated the swabs’ safety and efficacy in the clinical setting.
The articles in this special issue chronicle how the VA quickly mobilized its considerable enterprise-wide resources—especially during the pandemic’s acute phases—to contribute to timely veteran, national, and global evidence about what interventions were effective, what factors were associated with better care and outcomes, and how to flip the switch back to a nonemergency response. As Emerson might have observed, the scientific value of these recent “bad times” did not go unnoticed by VHA learners. In addition to catalyzing opportunities that accelerated the VHA enterprise strategy, the pandemic strengthened existing partnerships, led to new ones, and yielded lessons learned. With variants of the virus continuing to circulate, the VHA continues to harness the lessons learned from the emergency response perspective of the pandemic in order to effectively meet and exceed our mission to serve veterans.
The 35 authors whose work is featured in this issue—and their 3665 colleagues across the VHA research enterprise—offer testament not only to the power of scientific inquiry but of dedication to the mission by the individuals whose lives and families were also impacted by the pandemic.
VA Research continues working to unravel the ongoing impact of COVID-19. As the nation observes an increase in cases again, the VA is ready and well positioned to help lead and address needs for this and other public health crises.
Acknowledgments
This special issue is dedicated to Mitchell (Mitch) Mirkin and his enduring legacy at VA Research, helping to make the contributions of VA Research known as broadly as possible. A superb writer and “editor’s editor,” Mitch had an outstanding ability to translate complex scientific findings into layman’s terms. From the start of the pandemic to his unexpected passing in 2022, Mitch was Acting Director of VA Research Communications. He was a key member of the VA Office of Research and Development COVID-19 research response team. His contributions included his work leading to the generation of this Issue.
Bad times have a scientific value. These are occasions a good learner would not miss.
Ralph Waldo Emerson
Like the flip of a light switch, the world in March 2020 went into lockdown. Suddenly the novel coronavirus disease (COVID-19) was ever-present and everywhere. At a time when very little was certain, scientific inquiry—along with its related skills and disciplines—offered a much-needed pathway for navigating the virus’s myriad unknowns.
From the pandemic’s onset, the Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) made singular contributions to the advancement and acceleration of national and international research activity. This special issue of Federal Practitioner demonstrates how the VHA, through its Office of Research and Development (ORD), took advantage of its newly deployed enterprise strategy to meet the unprecedented demands of this public health emergency.
Launched in 2017, the ORD enterprise strategy enabled the VHA not only to capitalize on existing collaborations—both internal and external—but also move swiftly in forging new ones. Additionally, the strategy was key to leveraging unique VHA assets as the nation’s largest integrated health care system, including: (1) nationwide clinical trials infrastructure, including its longstanding Cooperative Studies Program; (2) a tightly integrated system of clinical care and research that serves as a ready platform for big data science, the world’s largest genomic database, and emergent capabilities; and (3) an established innovation ecosystem that worked with VA research to address rapidly changing circumstances.
In The VA Research Enterprise (p. S12), Garcia and colleagues demonstrate how the VHA pandemic response “arose from an enterprise strategy that was already in motion and aimed at identifying needs for supporting the clinical care mission, more rapidly leveraging resources, and coordinating research across the national VA health care system.” Thus, the VHA took a “model for a culture of cooperative research within the VA and with external groups” and translated it beyond the scope of clinical trials, which had been its foundation.
Led by Chief Research and Development Officer Rachel Ramoni, DMD, ScD, this strategy forged 121 VA medical centers conducting research into an integrated enterprise that could respond to needs for scientific evidence in a coordinated fashion, thereby translating research into practice for real impact on veterans. This approach built on relationships with not only scientific communities but also clinical and operational partners working within the VA to address the immediate pandemic-related needs.
In tandem with its physical infrastructure, the VA’s longstanding network of collaborators, physical infrastructure, and ability to develop new partnerships became drivers of success. Because of previous, ongoing, multisite clinical trials and observational studies, the VA had already partnered with numerous federal government agencies and industry groups and was able to quickly set up a VA COVID-19 clinical trial master protocol framework called the CURES (VA Coronavirus Research and Efficacy studies) network. The ORD enterprise strategy is noted by several other authors, including Caroff and colleagues, who show how the VA efforts to broaden partnerships prepandemic were critical to its participation in 7 large-scale COVID-19 therapeutic and vaccine trials (p. S18).
Similarly, in discussing the VA Million Veteran Program (MVP), Whitbourne and colleagues (p. S23) demonstrate how the VA research strategy and infrastructure were key to leveraging “unique MVP and VA electronic health record data to drive rapid scientific discovery and inform clinical operations.”
Launched in 2011, the MVP is one of the world’s largest genomic cohorts, with more than 985,000 veterans enrolled. MVP developers had the prescience to foresee how a robust genomic database could inform public health emergencies. Whitbourne and colleagues show the many ways the MVP facilitated the VHA COVID-19 response. By extending the MVP centralized recruitment and enrollment infrastructure, an ORD COVID-19 volunteer registry successfully registered 50,000 veterans interested in volunteering for clinical trials.
This tight integration between research and clinical care is one of the VHA’s greatest assets as a health care system. More than 60% of VA researchers are also clinicians who provide direct patient care. This enables VA physician-researchers to learn directly from veteran patients and quickly translate new findings into improved care. It also supported numerous capabilities that played a key role during the pandemic.
For example, in the article VA Big Data Science (p. S39), Young-Xu and colleagues note that the VA use of health care data proved medical research could be performed “quickly and judiciously.” Foundational to this research was a data sharing framework, electronic health record, and VA Corporate Data Warehouse that were accessible to all VA researchers. Researchers had access to clinical data and patient health records that allowed them to perform targeted, time-sensitive research. By building a cohort of 1,363,180 veterans who received ≥ 1 vaccine dose by March 7, 2021, VA researchers added significantly to our understanding of the real-world COVID-19 vaccine clinical performance.
In addition to leveraging existing capabilities, VHA clinicians and researchers created new ones in response. Krishnan and colleagues discuss the launch of 2 clinical and research consortiums focused on COVID-19 genomic surveillance (p. S44). SeqFORCE positioned the VHA to rapidly detect emergent variants and better inform the care of patients with COVID-19. SeqCURE focused on the broader study and trends of variants through sequencing.
The tightly integrated nature of VA care also supported the creation of a large-scale biorepository of specimens with accompanying clinical data to advance research and improve diagnostic and therapeutic research. Epstein and colleagues share the developmental history of the VA SHIELD biorepository, its structure, and its current and future contributions to research science (p. S48).
Finally, the same forward-learning culture which gave rise to the ORD enterprise strategy also resulted in an innovation ecosystem that was well established prior to March 2020. Now a firmly established portfolio within the VHA Office of Healthcare Innovation and Learning (OHIL), the VHA Innovation Ecosystem engages frontline clinicians in reimagining veteran health care. Iaquinto and colleagues discuss how the ecosystem’s preexisting partnerships were critical to addressing shortages in personal protective equipment and other vital resources (p. S52). The OHIL provided the quality system and manufacturing oversight and delivery of swabs for testing, while the ORD furnished research infrastructure and human subjects oversight. Together, these offices not only addressed the shortage by producing swabs but also validated the swabs’ safety and efficacy in the clinical setting.
The articles in this special issue chronicle how the VA quickly mobilized its considerable enterprise-wide resources—especially during the pandemic’s acute phases—to contribute to timely veteran, national, and global evidence about what interventions were effective, what factors were associated with better care and outcomes, and how to flip the switch back to a nonemergency response. As Emerson might have observed, the scientific value of these recent “bad times” did not go unnoticed by VHA learners. In addition to catalyzing opportunities that accelerated the VHA enterprise strategy, the pandemic strengthened existing partnerships, led to new ones, and yielded lessons learned. With variants of the virus continuing to circulate, the VHA continues to harness the lessons learned from the emergency response perspective of the pandemic in order to effectively meet and exceed our mission to serve veterans.
The 35 authors whose work is featured in this issue—and their 3665 colleagues across the VHA research enterprise—offer testament not only to the power of scientific inquiry but of dedication to the mission by the individuals whose lives and families were also impacted by the pandemic.
VA Research continues working to unravel the ongoing impact of COVID-19. As the nation observes an increase in cases again, the VA is ready and well positioned to help lead and address needs for this and other public health crises.
Acknowledgments
This special issue is dedicated to Mitchell (Mitch) Mirkin and his enduring legacy at VA Research, helping to make the contributions of VA Research known as broadly as possible. A superb writer and “editor’s editor,” Mitch had an outstanding ability to translate complex scientific findings into layman’s terms. From the start of the pandemic to his unexpected passing in 2022, Mitch was Acting Director of VA Research Communications. He was a key member of the VA Office of Research and Development COVID-19 research response team. His contributions included his work leading to the generation of this Issue.
Bad times have a scientific value. These are occasions a good learner would not miss.
Ralph Waldo Emerson
Like the flip of a light switch, the world in March 2020 went into lockdown. Suddenly the novel coronavirus disease (COVID-19) was ever-present and everywhere. At a time when very little was certain, scientific inquiry—along with its related skills and disciplines—offered a much-needed pathway for navigating the virus’s myriad unknowns.
From the pandemic’s onset, the Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) made singular contributions to the advancement and acceleration of national and international research activity. This special issue of Federal Practitioner demonstrates how the VHA, through its Office of Research and Development (ORD), took advantage of its newly deployed enterprise strategy to meet the unprecedented demands of this public health emergency.
Launched in 2017, the ORD enterprise strategy enabled the VHA not only to capitalize on existing collaborations—both internal and external—but also move swiftly in forging new ones. Additionally, the strategy was key to leveraging unique VHA assets as the nation’s largest integrated health care system, including: (1) nationwide clinical trials infrastructure, including its longstanding Cooperative Studies Program; (2) a tightly integrated system of clinical care and research that serves as a ready platform for big data science, the world’s largest genomic database, and emergent capabilities; and (3) an established innovation ecosystem that worked with VA research to address rapidly changing circumstances.
In The VA Research Enterprise (p. S12), Garcia and colleagues demonstrate how the VHA pandemic response “arose from an enterprise strategy that was already in motion and aimed at identifying needs for supporting the clinical care mission, more rapidly leveraging resources, and coordinating research across the national VA health care system.” Thus, the VHA took a “model for a culture of cooperative research within the VA and with external groups” and translated it beyond the scope of clinical trials, which had been its foundation.
Led by Chief Research and Development Officer Rachel Ramoni, DMD, ScD, this strategy forged 121 VA medical centers conducting research into an integrated enterprise that could respond to needs for scientific evidence in a coordinated fashion, thereby translating research into practice for real impact on veterans. This approach built on relationships with not only scientific communities but also clinical and operational partners working within the VA to address the immediate pandemic-related needs.
In tandem with its physical infrastructure, the VA’s longstanding network of collaborators, physical infrastructure, and ability to develop new partnerships became drivers of success. Because of previous, ongoing, multisite clinical trials and observational studies, the VA had already partnered with numerous federal government agencies and industry groups and was able to quickly set up a VA COVID-19 clinical trial master protocol framework called the CURES (VA Coronavirus Research and Efficacy studies) network. The ORD enterprise strategy is noted by several other authors, including Caroff and colleagues, who show how the VA efforts to broaden partnerships prepandemic were critical to its participation in 7 large-scale COVID-19 therapeutic and vaccine trials (p. S18).
Similarly, in discussing the VA Million Veteran Program (MVP), Whitbourne and colleagues (p. S23) demonstrate how the VA research strategy and infrastructure were key to leveraging “unique MVP and VA electronic health record data to drive rapid scientific discovery and inform clinical operations.”
Launched in 2011, the MVP is one of the world’s largest genomic cohorts, with more than 985,000 veterans enrolled. MVP developers had the prescience to foresee how a robust genomic database could inform public health emergencies. Whitbourne and colleagues show the many ways the MVP facilitated the VHA COVID-19 response. By extending the MVP centralized recruitment and enrollment infrastructure, an ORD COVID-19 volunteer registry successfully registered 50,000 veterans interested in volunteering for clinical trials.
This tight integration between research and clinical care is one of the VHA’s greatest assets as a health care system. More than 60% of VA researchers are also clinicians who provide direct patient care. This enables VA physician-researchers to learn directly from veteran patients and quickly translate new findings into improved care. It also supported numerous capabilities that played a key role during the pandemic.
For example, in the article VA Big Data Science (p. S39), Young-Xu and colleagues note that the VA use of health care data proved medical research could be performed “quickly and judiciously.” Foundational to this research was a data sharing framework, electronic health record, and VA Corporate Data Warehouse that were accessible to all VA researchers. Researchers had access to clinical data and patient health records that allowed them to perform targeted, time-sensitive research. By building a cohort of 1,363,180 veterans who received ≥ 1 vaccine dose by March 7, 2021, VA researchers added significantly to our understanding of the real-world COVID-19 vaccine clinical performance.
In addition to leveraging existing capabilities, VHA clinicians and researchers created new ones in response. Krishnan and colleagues discuss the launch of 2 clinical and research consortiums focused on COVID-19 genomic surveillance (p. S44). SeqFORCE positioned the VHA to rapidly detect emergent variants and better inform the care of patients with COVID-19. SeqCURE focused on the broader study and trends of variants through sequencing.
The tightly integrated nature of VA care also supported the creation of a large-scale biorepository of specimens with accompanying clinical data to advance research and improve diagnostic and therapeutic research. Epstein and colleagues share the developmental history of the VA SHIELD biorepository, its structure, and its current and future contributions to research science (p. S48).
Finally, the same forward-learning culture which gave rise to the ORD enterprise strategy also resulted in an innovation ecosystem that was well established prior to March 2020. Now a firmly established portfolio within the VHA Office of Healthcare Innovation and Learning (OHIL), the VHA Innovation Ecosystem engages frontline clinicians in reimagining veteran health care. Iaquinto and colleagues discuss how the ecosystem’s preexisting partnerships were critical to addressing shortages in personal protective equipment and other vital resources (p. S52). The OHIL provided the quality system and manufacturing oversight and delivery of swabs for testing, while the ORD furnished research infrastructure and human subjects oversight. Together, these offices not only addressed the shortage by producing swabs but also validated the swabs’ safety and efficacy in the clinical setting.
The articles in this special issue chronicle how the VA quickly mobilized its considerable enterprise-wide resources—especially during the pandemic’s acute phases—to contribute to timely veteran, national, and global evidence about what interventions were effective, what factors were associated with better care and outcomes, and how to flip the switch back to a nonemergency response. As Emerson might have observed, the scientific value of these recent “bad times” did not go unnoticed by VHA learners. In addition to catalyzing opportunities that accelerated the VHA enterprise strategy, the pandemic strengthened existing partnerships, led to new ones, and yielded lessons learned. With variants of the virus continuing to circulate, the VHA continues to harness the lessons learned from the emergency response perspective of the pandemic in order to effectively meet and exceed our mission to serve veterans.
The 35 authors whose work is featured in this issue—and their 3665 colleagues across the VHA research enterprise—offer testament not only to the power of scientific inquiry but of dedication to the mission by the individuals whose lives and families were also impacted by the pandemic.
VA Research continues working to unravel the ongoing impact of COVID-19. As the nation observes an increase in cases again, the VA is ready and well positioned to help lead and address needs for this and other public health crises.
Acknowledgments
This special issue is dedicated to Mitchell (Mitch) Mirkin and his enduring legacy at VA Research, helping to make the contributions of VA Research known as broadly as possible. A superb writer and “editor’s editor,” Mitch had an outstanding ability to translate complex scientific findings into layman’s terms. From the start of the pandemic to his unexpected passing in 2022, Mitch was Acting Director of VA Research Communications. He was a key member of the VA Office of Research and Development COVID-19 research response team. His contributions included his work leading to the generation of this Issue.