User login
Engaging Veterans With Serious Mental Illness in Primary Care
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
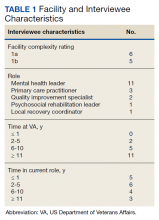
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
Incidentally Detected SARS-COV-2 Among Hospitalized Patients in Los Angeles County, August to October 2020
Many of the 85 hospitals in Los Angeles County (LAC) routinely test patients for SARS-CoV-2, the virus that causes COVID-19, upon admission to the hospital.1 However, not all SARS-CoV-2 detections represent acute COVID-19 for at least two reasons. First, the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) assay can report a false-positive result.2 Second, approximately 40% to 45% of persons with SARS-CoV-2 infection are asymptomatic, and RT-PCR tests can remain positive more than 2 months after an individual recovers from COVID-19; thus, SARS-CoV-2 detected on admission might represent shedding of nonviable virus from a prior unrecognized or undiagnosed infection.1,3
Public health policymakers closely monitor the rate of COVID-19 hospitalizations because it informs decisions to impose or relax COVID-19 control measures. However, the percentage of hospitalizations misclassified as COVID-19–associated because of incidentally detected SARS-CoV-2 (ie, COVID-19 was not a primary or contributing cause of hospitalization) is unknown. Therefore, we sought to determine the percentage of hospitalizations in LAC classified as having COVID-19 that might have had incidental SARS-CoV-2 detection.
METHODS
The state of California requires healthcare providers to report all COVID-19 cases and clinical laboratories to report all SARS-CoV-2 diagnostic test results. Hospitals in LAC are mandated to report daily lists of all persons hospitalized with suspected or confirmed COVID-19 to the LAC Department of Public Health (DPH) COVID-19 Hospital Electronic Surveillance System (CHESS).4 Hospitals provide daily data to CHESS containing information about patients in their facilities with COVID-19. We conducted a cross-sectional retrospective study by selecting a random set of medical records from CHESS for review.
We began regularly and systematically reviewing medical records of patients in CHESS discharged after August 1, 2020, as part of LAC DPH surveillance to characterize persons experiencing severe COVID-19, defined as illness requiring hospitalization. For severe COVID-19 surveillance, we randomly selected 45 discharged patients per week from CHESS in August 2020 and 50 discharged patients per week between September and October 2020. To ensure that the sample represented the overall age distribution of patients in CHESS, we ordered patients by birth date and selected every k record, where k represented the interval between patients needed to meet the target for the week. Before random sample selection, several free text fields from the CHESS dataset were queried to identify and remove patients who were not LAC residents, were seen in the emergency department but not admitted, were hospitalized for <1 day, were discharged from a non-acute care hospital, or if the hospital-reported patient did not have a positive SARS-CoV-2 test. We then requested full medical records for these patients from the respective hospitals. After we received the medical records, a team of four nurses independently reviewed the medical charts and excluded patients who did not meet the above listed exclusion criteria; patients were excluded at two points—during the automated query and again by manual review.
In addition, severe COVID-19 surveillance was intended to characterize primary admissions for COVID-19, defined as having a documented positive SARS-CoV-2 result within 10 days of symptom onset or hospital admission and no prior hospitalization for COVID-19. The date of the first positive result was validated by locating the positive SARS-COV-2 result in the patient’s medical record and/or the LAC COVID surveillance database; the patient was excluded from analysis if a positive SARS-CoV-2 result could not be found. Excluded discharges were not replaced by a new randomly selected patient. Instead, we oversampled the number of weekly charts to request with a goal of having 40 to 45 charts per week that met inclusion criteria for abstraction.
For this analysis, we examined medical records abstracted for discharges occurring between August 1 and October 31, 2020. We categorized hospitalizations into one of the following: (1) “likely COVID-19–associated” if the patient had
Descriptive statistics and all analyses were conducted using SAS version 9.4 (SAS Institute). Confidence limits (CL) were calculated using the proc freq CL option in SAS. Chi-square analysis was conducted to determine whether trends in hospitalization categories changed over time. Statistical significance was set at P < .05.
RESULTS
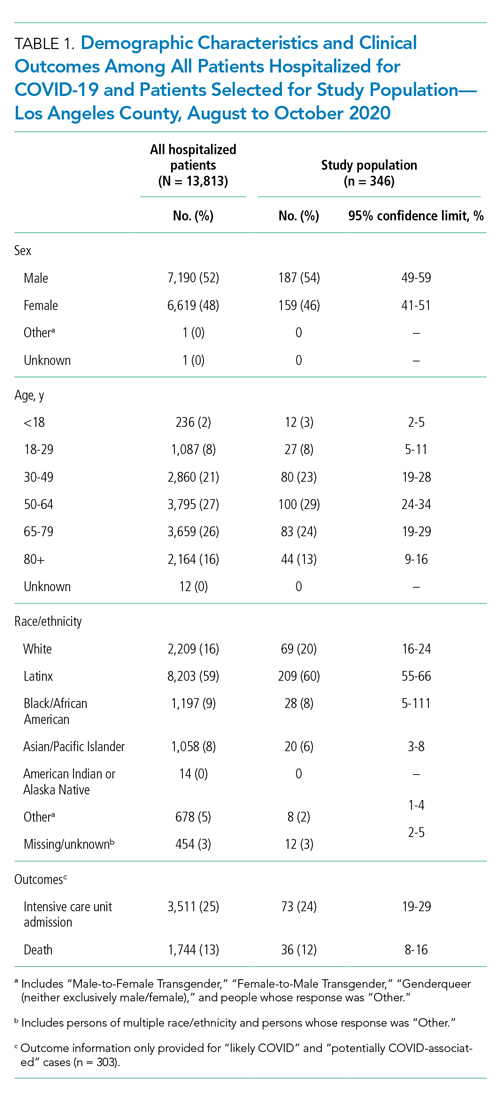
Of the 13,813 hospital discharges reported to CHESS from August to October 2020, 3,182 (23%) records were not eligible for inclusion in the random selection sample for the following reasons: 1,765 (13%) patients reported by hospitals did not have a positive COVID-19 test, 734 (5%) discharges were for non-LAC residents, 636 (5%) patients had a length of hospital stay <1 day, and 47 (<1%) discharges were from a non-acute care hospital. From the 10,631 discharges in CHESS meeting preliminary inclusion criteria from August 1 to October 31, 2020, we randomly selected 618 discharges for medical record review. Of the 618 discharges, 504 (85%) medical records were available for review as of November 30, 2020. After review of the 504 medical records, an additional 158 were excluded because 83 (13%) had a first documented positive SARS-CoV-2 test that was >10 days from hospital admission or symptom onset, 34 (6%) were previously hospitalized for COVID-19, 29 (5%) had an emergency department visit only, 6 (1%) were discharged from a non-acute care hospital, and 6 (1%) were non-LAC residents. We reviewed medical records for 346 (56%) of the 618 hospitalizations that met our inclusion criteria.
The demographic characteristics of patients included in our sample were similar to those of the overall patient population in CHESS (Table 1). Most patients in our final study population were male (54%), older than 50 years (66%), and Hispanic (60%); the median length of hospital stay for survivors was 5 days (first quartile–third quartile: 3 to 8 days).
Our analysis indicates that 71% (95% CL, 66%-75%) of hospital discharges were “likely COVID-19-associated”; 12% (CL, 9%-16%) were “not COVID-19–associated” and, therefore, had incidentally detected SARS-CoV-2; and 17% were “potentially COVID-19–associated” (CL, 13%-21%). The percentage of hospitalizations classified as “likely,” “potentially,” and “not COVID-19–associated” did not change from month-to-month during the study period (P = .81). Full-term delivery was the most common reason for hospitalization among patients with incidentally detected SARS-CoV-2 (Table 2).
DISCUSSION
The primary public health objective of the COVID-19 pandemic response has been to prevent overwhelming the healthcare system by slowing disease transmission. LAC DPH closely monitors the daily number of hospitalized COVID-19 patients, defined as hospitalization of a person with an associated positive SARS-CoV-2 result. However, increasing community transmission of SARS-CoV-2 can complicate interpretation of hospitalization data because it is likely that some patients with incidentally detected, nonviable virus will be misclassified as having COVID-19. Overestimating the burden of COVID-19–associated hospitalizations may lead public health policymakers to impose more restrictive control measures or remove restrictions more slowly. Results from this study can inform policymakers about the potential magnitude of overestimating COVID-19–associated hospitalizations.
Our results indicate that SARS-CoV-2 detection might be incidental (ie, “not COVID-19–associated”) in approximately one of eight persons hospitalized with COVID-19 in LAC. We likely underestimated the percentage of hospitalizations with incidental SARS-CoV-2 detection because our definition of “not COVID-19–associated” hospitalizations was intended to be specific for identifying patients who had no clear reason for SARS-CoV-2 testing except a presumed hospital policy of testing on admission or preoperatively. In addition, several patients classified as having a “potentially COVID-19–associated” hospitalization also had a primary reason for admission that currently does not have a clear link to COVID-19 (eg, Bell’s palsy and pelvic inflammatory disease). Although our sample size was relatively small, it was representative of all potential COVID-19 hospitalizations in LAC over a 3-month period.
CONCLUSION
Detection of SARS-CoV-2 in a person with a clinical presentation that is not compatible with COVID-19 can complicate initial clinical management because it is unclear if the result represents presymptomatic or asymptomatic infection, prolonged shedding of nonviable virus, or a false-positive result. Considering the consequences of missing a true infection, such as transmission to other staff or patients, healthcare providers are obligated to treat the test result as a real infection. Therefore, our results are not applicable to patient-level clinical management decisions, but highlight the need for policymakers and emergency preparedness personnel to consider that hospital-reported data might overestimate the burden of COVID-19 hospitalizations when making decisions that rely on hospitalization data as a metric. Additional research is needed to develop methods for correcting hospitalization data to account for patients in whom incidentally detected SARS-CoV-2 was not a direct or contributing cause of hospitalization. Adjusting COVID-19–associated hospitalization rates to account for incidental SARS-CoV-2 detection could allow for optimal resource planning by public health policymakers.
1. Liotti, FM, Menchinelli, G, Marchetti, S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181(5):702-704. https://doi.org/10.1001/jamainternmed.2020.7570
2. Centers for Disease Control and Prevention and Infectious Disease Society of America. RT-PCR Testing. Accessed April 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing
3. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. https://doi.org/10.7326/M20-3012
4 Los Angeles County Department of Public Health. Daily reporting of hospitalized COVID-19 positive inpatients: updated data submission requirements and guide for acute care facilities in Los Angeles County. Accessed on December 10, 2020. http://publichealth.lacounty.gov/acd/docs/HospCOVIDReportingGuide.pdf
Many of the 85 hospitals in Los Angeles County (LAC) routinely test patients for SARS-CoV-2, the virus that causes COVID-19, upon admission to the hospital.1 However, not all SARS-CoV-2 detections represent acute COVID-19 for at least two reasons. First, the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) assay can report a false-positive result.2 Second, approximately 40% to 45% of persons with SARS-CoV-2 infection are asymptomatic, and RT-PCR tests can remain positive more than 2 months after an individual recovers from COVID-19; thus, SARS-CoV-2 detected on admission might represent shedding of nonviable virus from a prior unrecognized or undiagnosed infection.1,3
Public health policymakers closely monitor the rate of COVID-19 hospitalizations because it informs decisions to impose or relax COVID-19 control measures. However, the percentage of hospitalizations misclassified as COVID-19–associated because of incidentally detected SARS-CoV-2 (ie, COVID-19 was not a primary or contributing cause of hospitalization) is unknown. Therefore, we sought to determine the percentage of hospitalizations in LAC classified as having COVID-19 that might have had incidental SARS-CoV-2 detection.
METHODS
The state of California requires healthcare providers to report all COVID-19 cases and clinical laboratories to report all SARS-CoV-2 diagnostic test results. Hospitals in LAC are mandated to report daily lists of all persons hospitalized with suspected or confirmed COVID-19 to the LAC Department of Public Health (DPH) COVID-19 Hospital Electronic Surveillance System (CHESS).4 Hospitals provide daily data to CHESS containing information about patients in their facilities with COVID-19. We conducted a cross-sectional retrospective study by selecting a random set of medical records from CHESS for review.
We began regularly and systematically reviewing medical records of patients in CHESS discharged after August 1, 2020, as part of LAC DPH surveillance to characterize persons experiencing severe COVID-19, defined as illness requiring hospitalization. For severe COVID-19 surveillance, we randomly selected 45 discharged patients per week from CHESS in August 2020 and 50 discharged patients per week between September and October 2020. To ensure that the sample represented the overall age distribution of patients in CHESS, we ordered patients by birth date and selected every k record, where k represented the interval between patients needed to meet the target for the week. Before random sample selection, several free text fields from the CHESS dataset were queried to identify and remove patients who were not LAC residents, were seen in the emergency department but not admitted, were hospitalized for <1 day, were discharged from a non-acute care hospital, or if the hospital-reported patient did not have a positive SARS-CoV-2 test. We then requested full medical records for these patients from the respective hospitals. After we received the medical records, a team of four nurses independently reviewed the medical charts and excluded patients who did not meet the above listed exclusion criteria; patients were excluded at two points—during the automated query and again by manual review.
In addition, severe COVID-19 surveillance was intended to characterize primary admissions for COVID-19, defined as having a documented positive SARS-CoV-2 result within 10 days of symptom onset or hospital admission and no prior hospitalization for COVID-19. The date of the first positive result was validated by locating the positive SARS-COV-2 result in the patient’s medical record and/or the LAC COVID surveillance database; the patient was excluded from analysis if a positive SARS-CoV-2 result could not be found. Excluded discharges were not replaced by a new randomly selected patient. Instead, we oversampled the number of weekly charts to request with a goal of having 40 to 45 charts per week that met inclusion criteria for abstraction.
For this analysis, we examined medical records abstracted for discharges occurring between August 1 and October 31, 2020. We categorized hospitalizations into one of the following: (1) “likely COVID-19–associated” if the patient had
Descriptive statistics and all analyses were conducted using SAS version 9.4 (SAS Institute). Confidence limits (CL) were calculated using the proc freq CL option in SAS. Chi-square analysis was conducted to determine whether trends in hospitalization categories changed over time. Statistical significance was set at P < .05.
RESULTS
Of the 13,813 hospital discharges reported to CHESS from August to October 2020, 3,182 (23%) records were not eligible for inclusion in the random selection sample for the following reasons: 1,765 (13%) patients reported by hospitals did not have a positive COVID-19 test, 734 (5%) discharges were for non-LAC residents, 636 (5%) patients had a length of hospital stay <1 day, and 47 (<1%) discharges were from a non-acute care hospital. From the 10,631 discharges in CHESS meeting preliminary inclusion criteria from August 1 to October 31, 2020, we randomly selected 618 discharges for medical record review. Of the 618 discharges, 504 (85%) medical records were available for review as of November 30, 2020. After review of the 504 medical records, an additional 158 were excluded because 83 (13%) had a first documented positive SARS-CoV-2 test that was >10 days from hospital admission or symptom onset, 34 (6%) were previously hospitalized for COVID-19, 29 (5%) had an emergency department visit only, 6 (1%) were discharged from a non-acute care hospital, and 6 (1%) were non-LAC residents. We reviewed medical records for 346 (56%) of the 618 hospitalizations that met our inclusion criteria.
The demographic characteristics of patients included in our sample were similar to those of the overall patient population in CHESS (Table 1). Most patients in our final study population were male (54%), older than 50 years (66%), and Hispanic (60%); the median length of hospital stay for survivors was 5 days (first quartile–third quartile: 3 to 8 days).
Our analysis indicates that 71% (95% CL, 66%-75%) of hospital discharges were “likely COVID-19-associated”; 12% (CL, 9%-16%) were “not COVID-19–associated” and, therefore, had incidentally detected SARS-CoV-2; and 17% were “potentially COVID-19–associated” (CL, 13%-21%). The percentage of hospitalizations classified as “likely,” “potentially,” and “not COVID-19–associated” did not change from month-to-month during the study period (P = .81). Full-term delivery was the most common reason for hospitalization among patients with incidentally detected SARS-CoV-2 (Table 2).
DISCUSSION
The primary public health objective of the COVID-19 pandemic response has been to prevent overwhelming the healthcare system by slowing disease transmission. LAC DPH closely monitors the daily number of hospitalized COVID-19 patients, defined as hospitalization of a person with an associated positive SARS-CoV-2 result. However, increasing community transmission of SARS-CoV-2 can complicate interpretation of hospitalization data because it is likely that some patients with incidentally detected, nonviable virus will be misclassified as having COVID-19. Overestimating the burden of COVID-19–associated hospitalizations may lead public health policymakers to impose more restrictive control measures or remove restrictions more slowly. Results from this study can inform policymakers about the potential magnitude of overestimating COVID-19–associated hospitalizations.
Our results indicate that SARS-CoV-2 detection might be incidental (ie, “not COVID-19–associated”) in approximately one of eight persons hospitalized with COVID-19 in LAC. We likely underestimated the percentage of hospitalizations with incidental SARS-CoV-2 detection because our definition of “not COVID-19–associated” hospitalizations was intended to be specific for identifying patients who had no clear reason for SARS-CoV-2 testing except a presumed hospital policy of testing on admission or preoperatively. In addition, several patients classified as having a “potentially COVID-19–associated” hospitalization also had a primary reason for admission that currently does not have a clear link to COVID-19 (eg, Bell’s palsy and pelvic inflammatory disease). Although our sample size was relatively small, it was representative of all potential COVID-19 hospitalizations in LAC over a 3-month period.
CONCLUSION
Detection of SARS-CoV-2 in a person with a clinical presentation that is not compatible with COVID-19 can complicate initial clinical management because it is unclear if the result represents presymptomatic or asymptomatic infection, prolonged shedding of nonviable virus, or a false-positive result. Considering the consequences of missing a true infection, such as transmission to other staff or patients, healthcare providers are obligated to treat the test result as a real infection. Therefore, our results are not applicable to patient-level clinical management decisions, but highlight the need for policymakers and emergency preparedness personnel to consider that hospital-reported data might overestimate the burden of COVID-19 hospitalizations when making decisions that rely on hospitalization data as a metric. Additional research is needed to develop methods for correcting hospitalization data to account for patients in whom incidentally detected SARS-CoV-2 was not a direct or contributing cause of hospitalization. Adjusting COVID-19–associated hospitalization rates to account for incidental SARS-CoV-2 detection could allow for optimal resource planning by public health policymakers.
Many of the 85 hospitals in Los Angeles County (LAC) routinely test patients for SARS-CoV-2, the virus that causes COVID-19, upon admission to the hospital.1 However, not all SARS-CoV-2 detections represent acute COVID-19 for at least two reasons. First, the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) assay can report a false-positive result.2 Second, approximately 40% to 45% of persons with SARS-CoV-2 infection are asymptomatic, and RT-PCR tests can remain positive more than 2 months after an individual recovers from COVID-19; thus, SARS-CoV-2 detected on admission might represent shedding of nonviable virus from a prior unrecognized or undiagnosed infection.1,3
Public health policymakers closely monitor the rate of COVID-19 hospitalizations because it informs decisions to impose or relax COVID-19 control measures. However, the percentage of hospitalizations misclassified as COVID-19–associated because of incidentally detected SARS-CoV-2 (ie, COVID-19 was not a primary or contributing cause of hospitalization) is unknown. Therefore, we sought to determine the percentage of hospitalizations in LAC classified as having COVID-19 that might have had incidental SARS-CoV-2 detection.
METHODS
The state of California requires healthcare providers to report all COVID-19 cases and clinical laboratories to report all SARS-CoV-2 diagnostic test results. Hospitals in LAC are mandated to report daily lists of all persons hospitalized with suspected or confirmed COVID-19 to the LAC Department of Public Health (DPH) COVID-19 Hospital Electronic Surveillance System (CHESS).4 Hospitals provide daily data to CHESS containing information about patients in their facilities with COVID-19. We conducted a cross-sectional retrospective study by selecting a random set of medical records from CHESS for review.
We began regularly and systematically reviewing medical records of patients in CHESS discharged after August 1, 2020, as part of LAC DPH surveillance to characterize persons experiencing severe COVID-19, defined as illness requiring hospitalization. For severe COVID-19 surveillance, we randomly selected 45 discharged patients per week from CHESS in August 2020 and 50 discharged patients per week between September and October 2020. To ensure that the sample represented the overall age distribution of patients in CHESS, we ordered patients by birth date and selected every k record, where k represented the interval between patients needed to meet the target for the week. Before random sample selection, several free text fields from the CHESS dataset were queried to identify and remove patients who were not LAC residents, were seen in the emergency department but not admitted, were hospitalized for <1 day, were discharged from a non-acute care hospital, or if the hospital-reported patient did not have a positive SARS-CoV-2 test. We then requested full medical records for these patients from the respective hospitals. After we received the medical records, a team of four nurses independently reviewed the medical charts and excluded patients who did not meet the above listed exclusion criteria; patients were excluded at two points—during the automated query and again by manual review.
In addition, severe COVID-19 surveillance was intended to characterize primary admissions for COVID-19, defined as having a documented positive SARS-CoV-2 result within 10 days of symptom onset or hospital admission and no prior hospitalization for COVID-19. The date of the first positive result was validated by locating the positive SARS-COV-2 result in the patient’s medical record and/or the LAC COVID surveillance database; the patient was excluded from analysis if a positive SARS-CoV-2 result could not be found. Excluded discharges were not replaced by a new randomly selected patient. Instead, we oversampled the number of weekly charts to request with a goal of having 40 to 45 charts per week that met inclusion criteria for abstraction.
For this analysis, we examined medical records abstracted for discharges occurring between August 1 and October 31, 2020. We categorized hospitalizations into one of the following: (1) “likely COVID-19–associated” if the patient had
Descriptive statistics and all analyses were conducted using SAS version 9.4 (SAS Institute). Confidence limits (CL) were calculated using the proc freq CL option in SAS. Chi-square analysis was conducted to determine whether trends in hospitalization categories changed over time. Statistical significance was set at P < .05.
RESULTS
Of the 13,813 hospital discharges reported to CHESS from August to October 2020, 3,182 (23%) records were not eligible for inclusion in the random selection sample for the following reasons: 1,765 (13%) patients reported by hospitals did not have a positive COVID-19 test, 734 (5%) discharges were for non-LAC residents, 636 (5%) patients had a length of hospital stay <1 day, and 47 (<1%) discharges were from a non-acute care hospital. From the 10,631 discharges in CHESS meeting preliminary inclusion criteria from August 1 to October 31, 2020, we randomly selected 618 discharges for medical record review. Of the 618 discharges, 504 (85%) medical records were available for review as of November 30, 2020. After review of the 504 medical records, an additional 158 were excluded because 83 (13%) had a first documented positive SARS-CoV-2 test that was >10 days from hospital admission or symptom onset, 34 (6%) were previously hospitalized for COVID-19, 29 (5%) had an emergency department visit only, 6 (1%) were discharged from a non-acute care hospital, and 6 (1%) were non-LAC residents. We reviewed medical records for 346 (56%) of the 618 hospitalizations that met our inclusion criteria.
The demographic characteristics of patients included in our sample were similar to those of the overall patient population in CHESS (Table 1). Most patients in our final study population were male (54%), older than 50 years (66%), and Hispanic (60%); the median length of hospital stay for survivors was 5 days (first quartile–third quartile: 3 to 8 days).
Our analysis indicates that 71% (95% CL, 66%-75%) of hospital discharges were “likely COVID-19-associated”; 12% (CL, 9%-16%) were “not COVID-19–associated” and, therefore, had incidentally detected SARS-CoV-2; and 17% were “potentially COVID-19–associated” (CL, 13%-21%). The percentage of hospitalizations classified as “likely,” “potentially,” and “not COVID-19–associated” did not change from month-to-month during the study period (P = .81). Full-term delivery was the most common reason for hospitalization among patients with incidentally detected SARS-CoV-2 (Table 2).
DISCUSSION
The primary public health objective of the COVID-19 pandemic response has been to prevent overwhelming the healthcare system by slowing disease transmission. LAC DPH closely monitors the daily number of hospitalized COVID-19 patients, defined as hospitalization of a person with an associated positive SARS-CoV-2 result. However, increasing community transmission of SARS-CoV-2 can complicate interpretation of hospitalization data because it is likely that some patients with incidentally detected, nonviable virus will be misclassified as having COVID-19. Overestimating the burden of COVID-19–associated hospitalizations may lead public health policymakers to impose more restrictive control measures or remove restrictions more slowly. Results from this study can inform policymakers about the potential magnitude of overestimating COVID-19–associated hospitalizations.
Our results indicate that SARS-CoV-2 detection might be incidental (ie, “not COVID-19–associated”) in approximately one of eight persons hospitalized with COVID-19 in LAC. We likely underestimated the percentage of hospitalizations with incidental SARS-CoV-2 detection because our definition of “not COVID-19–associated” hospitalizations was intended to be specific for identifying patients who had no clear reason for SARS-CoV-2 testing except a presumed hospital policy of testing on admission or preoperatively. In addition, several patients classified as having a “potentially COVID-19–associated” hospitalization also had a primary reason for admission that currently does not have a clear link to COVID-19 (eg, Bell’s palsy and pelvic inflammatory disease). Although our sample size was relatively small, it was representative of all potential COVID-19 hospitalizations in LAC over a 3-month period.
CONCLUSION
Detection of SARS-CoV-2 in a person with a clinical presentation that is not compatible with COVID-19 can complicate initial clinical management because it is unclear if the result represents presymptomatic or asymptomatic infection, prolonged shedding of nonviable virus, or a false-positive result. Considering the consequences of missing a true infection, such as transmission to other staff or patients, healthcare providers are obligated to treat the test result as a real infection. Therefore, our results are not applicable to patient-level clinical management decisions, but highlight the need for policymakers and emergency preparedness personnel to consider that hospital-reported data might overestimate the burden of COVID-19 hospitalizations when making decisions that rely on hospitalization data as a metric. Additional research is needed to develop methods for correcting hospitalization data to account for patients in whom incidentally detected SARS-CoV-2 was not a direct or contributing cause of hospitalization. Adjusting COVID-19–associated hospitalization rates to account for incidental SARS-CoV-2 detection could allow for optimal resource planning by public health policymakers.
1. Liotti, FM, Menchinelli, G, Marchetti, S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181(5):702-704. https://doi.org/10.1001/jamainternmed.2020.7570
2. Centers for Disease Control and Prevention and Infectious Disease Society of America. RT-PCR Testing. Accessed April 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing
3. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. https://doi.org/10.7326/M20-3012
4 Los Angeles County Department of Public Health. Daily reporting of hospitalized COVID-19 positive inpatients: updated data submission requirements and guide for acute care facilities in Los Angeles County. Accessed on December 10, 2020. http://publichealth.lacounty.gov/acd/docs/HospCOVIDReportingGuide.pdf
1. Liotti, FM, Menchinelli, G, Marchetti, S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181(5):702-704. https://doi.org/10.1001/jamainternmed.2020.7570
2. Centers for Disease Control and Prevention and Infectious Disease Society of America. RT-PCR Testing. Accessed April 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing
3. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. https://doi.org/10.7326/M20-3012
4 Los Angeles County Department of Public Health. Daily reporting of hospitalized COVID-19 positive inpatients: updated data submission requirements and guide for acute care facilities in Los Angeles County. Accessed on December 10, 2020. http://publichealth.lacounty.gov/acd/docs/HospCOVIDReportingGuide.pdf
© 2021 Society of Hospital Medicine
Serious Choices: A Systematic Environmental Scan of Decision Aids and Their Use for Seriously Ill People Near Death
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
© 2019 Society of Hospital Medicine
Association of Weekend Admission and Weekend Discharge with Length of Stay and 30-Day Readmission in Children’s Hospitals
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
© 2018 Society of Hospital Medicine
Treatment and Management of Patients With Non-Small Cell Lung Cancer (FULL)
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Improving Veteran Access to Treatment for Hepatitis C Virus Infection (FULL)
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
Journal of Hospital Medicine – Dec. 2017
BACKGROUND: Identifying hospitals that are both early and consistent adopters of high-value care can help shed light on the culture and practices at those institutions that are necessary to promote high-value care nationwide. The use of troponin testing to diagnose acute myocardial infarction (AMI), and not testing for myoglobin or creatine kinase-MB (CK-MB), is a high-value recommendation of the Choosing Wisely® campaign.
OBJECTIVE: To examine the variation in cardiac biomarker testing and the effect of the Choosing Wisely® troponin-only testing recommendation for the diagnosis of AMI.
DESIGN: A retrospective, observational study using administrative ordering data from Vizient’s Clinical Database/Resource Manager.
PATIENTS: Hospitalized patients with a principal discharge diagnosis of AMI.
INTERVENTION: The Choosing Wisely® recommendation to order troponin-only testing to diagnose AMI was released during the first quarter of 2015.
RESULTS: In 19 hospitals, troponin-only testing was consistently ordered to diagnose AMI before the Choosing Wisely® recommendation and throughout the study period. In 34 hospitals, both troponin testing and myoglobin/CK-MB testing were ordered to diagnose AMI even after the Choosing Wisely® recommendation. In 26 hospitals with low rates of troponin-only testing before the Choosing Wisely® recommendation, the release of the recommendation was associated with a statistically significant increase in the rate of troponin-only testing to diagnose AMI.
CONCLUSION: In institutions with low rates of troponin-only testing prior to the Choosing Wisely® recommendation, the recommendation was associated with a significant increase in the rate of troponin-only testing.
Read the entire article in the Dec. 2017 issue of the Journal of Hospital Medicine.
Also in JHM this month
Hospital perceptions of Medicare’s Sepsis Quality Reporting Initiative
AUTHORS: Ian J. Barbash, MD, MS; Kimberly J. Rak, PhD; Courtney C. Kuza, MPH; and Jeremy M. Kahn, MD, MS
Health literacy and hospital length of stay: An inpatient cohort study
AUTHORS: Ethan G. Jaffee, MD; Vineet M. Arora, MD, MAPP; Madeleine I. Matthiesen, MD; David O. Meltzer, MD, PhD, MHM; and Valerie G. Press, MD, FAAP, FACP, MPH
How exemplary teaching physicians interact with hospitalized patients
AUTHORS: Sanjay Saint, MD, MPH, FHM; Molly Harrod, PhD; Karen E. Fowler, MPH; and Nathan Houchens, MD, FACP, FHM
A randomized cohort controlled trial to compare intern sign-out training interventions
AUTHORS: Soo-Hoon Lee, PhD; Christopher Terndrup, MD; Phillip H. Phan, PhD; Sandra E. Zaeh, MD; Kwame Atsina, MD; Nicole Minkove, MD; Alexander Billioux, MD; DPhil, Souvik Chatterjee, MD; Idoreyin Montague, MD; Bennett Clark, MD; Andrew Hughes, MD; and Sanjay V. Desai, MD
BACKGROUND: Identifying hospitals that are both early and consistent adopters of high-value care can help shed light on the culture and practices at those institutions that are necessary to promote high-value care nationwide. The use of troponin testing to diagnose acute myocardial infarction (AMI), and not testing for myoglobin or creatine kinase-MB (CK-MB), is a high-value recommendation of the Choosing Wisely® campaign.
OBJECTIVE: To examine the variation in cardiac biomarker testing and the effect of the Choosing Wisely® troponin-only testing recommendation for the diagnosis of AMI.
DESIGN: A retrospective, observational study using administrative ordering data from Vizient’s Clinical Database/Resource Manager.
PATIENTS: Hospitalized patients with a principal discharge diagnosis of AMI.
INTERVENTION: The Choosing Wisely® recommendation to order troponin-only testing to diagnose AMI was released during the first quarter of 2015.
RESULTS: In 19 hospitals, troponin-only testing was consistently ordered to diagnose AMI before the Choosing Wisely® recommendation and throughout the study period. In 34 hospitals, both troponin testing and myoglobin/CK-MB testing were ordered to diagnose AMI even after the Choosing Wisely® recommendation. In 26 hospitals with low rates of troponin-only testing before the Choosing Wisely® recommendation, the release of the recommendation was associated with a statistically significant increase in the rate of troponin-only testing to diagnose AMI.
CONCLUSION: In institutions with low rates of troponin-only testing prior to the Choosing Wisely® recommendation, the recommendation was associated with a significant increase in the rate of troponin-only testing.
Read the entire article in the Dec. 2017 issue of the Journal of Hospital Medicine.
Also in JHM this month
Hospital perceptions of Medicare’s Sepsis Quality Reporting Initiative
AUTHORS: Ian J. Barbash, MD, MS; Kimberly J. Rak, PhD; Courtney C. Kuza, MPH; and Jeremy M. Kahn, MD, MS
Health literacy and hospital length of stay: An inpatient cohort study
AUTHORS: Ethan G. Jaffee, MD; Vineet M. Arora, MD, MAPP; Madeleine I. Matthiesen, MD; David O. Meltzer, MD, PhD, MHM; and Valerie G. Press, MD, FAAP, FACP, MPH
How exemplary teaching physicians interact with hospitalized patients
AUTHORS: Sanjay Saint, MD, MPH, FHM; Molly Harrod, PhD; Karen E. Fowler, MPH; and Nathan Houchens, MD, FACP, FHM
A randomized cohort controlled trial to compare intern sign-out training interventions
AUTHORS: Soo-Hoon Lee, PhD; Christopher Terndrup, MD; Phillip H. Phan, PhD; Sandra E. Zaeh, MD; Kwame Atsina, MD; Nicole Minkove, MD; Alexander Billioux, MD; DPhil, Souvik Chatterjee, MD; Idoreyin Montague, MD; Bennett Clark, MD; Andrew Hughes, MD; and Sanjay V. Desai, MD
BACKGROUND: Identifying hospitals that are both early and consistent adopters of high-value care can help shed light on the culture and practices at those institutions that are necessary to promote high-value care nationwide. The use of troponin testing to diagnose acute myocardial infarction (AMI), and not testing for myoglobin or creatine kinase-MB (CK-MB), is a high-value recommendation of the Choosing Wisely® campaign.
OBJECTIVE: To examine the variation in cardiac biomarker testing and the effect of the Choosing Wisely® troponin-only testing recommendation for the diagnosis of AMI.
DESIGN: A retrospective, observational study using administrative ordering data from Vizient’s Clinical Database/Resource Manager.
PATIENTS: Hospitalized patients with a principal discharge diagnosis of AMI.
INTERVENTION: The Choosing Wisely® recommendation to order troponin-only testing to diagnose AMI was released during the first quarter of 2015.
RESULTS: In 19 hospitals, troponin-only testing was consistently ordered to diagnose AMI before the Choosing Wisely® recommendation and throughout the study period. In 34 hospitals, both troponin testing and myoglobin/CK-MB testing were ordered to diagnose AMI even after the Choosing Wisely® recommendation. In 26 hospitals with low rates of troponin-only testing before the Choosing Wisely® recommendation, the release of the recommendation was associated with a statistically significant increase in the rate of troponin-only testing to diagnose AMI.
CONCLUSION: In institutions with low rates of troponin-only testing prior to the Choosing Wisely® recommendation, the recommendation was associated with a significant increase in the rate of troponin-only testing.
Read the entire article in the Dec. 2017 issue of the Journal of Hospital Medicine.
Also in JHM this month
Hospital perceptions of Medicare’s Sepsis Quality Reporting Initiative
AUTHORS: Ian J. Barbash, MD, MS; Kimberly J. Rak, PhD; Courtney C. Kuza, MPH; and Jeremy M. Kahn, MD, MS
Health literacy and hospital length of stay: An inpatient cohort study
AUTHORS: Ethan G. Jaffee, MD; Vineet M. Arora, MD, MAPP; Madeleine I. Matthiesen, MD; David O. Meltzer, MD, PhD, MHM; and Valerie G. Press, MD, FAAP, FACP, MPH
How exemplary teaching physicians interact with hospitalized patients
AUTHORS: Sanjay Saint, MD, MPH, FHM; Molly Harrod, PhD; Karen E. Fowler, MPH; and Nathan Houchens, MD, FACP, FHM
A randomized cohort controlled trial to compare intern sign-out training interventions
AUTHORS: Soo-Hoon Lee, PhD; Christopher Terndrup, MD; Phillip H. Phan, PhD; Sandra E. Zaeh, MD; Kwame Atsina, MD; Nicole Minkove, MD; Alexander Billioux, MD; DPhil, Souvik Chatterjee, MD; Idoreyin Montague, MD; Bennett Clark, MD; Andrew Hughes, MD; and Sanjay V. Desai, MD
Multiple Myeloma: Updates on Diagnosis and Management
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level. There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy,
hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly
myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the
bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic
SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma.For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of
lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these
criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall
survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics,
which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require
therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to 3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients
with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS
and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either
be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared
with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that
could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib,
doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is
often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials
have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and
safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and
OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide
plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are
integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered
to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit
not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective
and well tolerated. There has been a general shift to using these agents upfront in transplant-ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered
in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay
before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as
neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refreactory/Relapsed Disease Treatments
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined
significance (MGUS) consistently precedes multiple myeloma: a prospective
study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal
Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney
injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop
Consensus Panel 3. Consensus recommendations for standard investigative workup:
report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working
Group consensus statement for the management, treatment, and supportive care
of patients with myeloma not eligible for standard autologous stem-cell transplantation.
J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.
2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more
entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation
of measured myeloma cell mass with presenting clinical features, response to
treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple
myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival
in multiple myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis
in monoclonal gammopathy of undetermined significance. N Engl J Med.
2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering
(asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering
multiple myeloma: biological insights and early treatment strategies. Hematology
Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone
for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple
myeloma limited to high-risk subgroup identified by gene expression profiling.
Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes.
Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in
oncology: multiple myeloma. National Comprehensive Cancer Network Website.
http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated
March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study
(EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide,
and lenalidomide in previously untreated multiple myeloma. Blood.
2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus
reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed multiple
myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous
administration of bortezomib in patients with relapsed multiple myeloma: a randomized,
phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in
multiple myeloma linked to bortezomib in total therapy 3: comparison with total
therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem
cell mobilization and engraftment post-peripheral blood stem cell transplantation
in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group.
Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell
collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining
regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis
for patients with newly diagnosed multiple myeloma patients treated with
lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in
combination with lenalidomide and low dose dexamethasone as a frontline treatment
for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of
carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended
dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed
multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide
plus dexamethasone in newly diagnosed multiple myeloma: a comparative
analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous
bone marrow transplantation and chemotherapy in multiple myeloma.
Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous
stem cell transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood.
1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second
on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol.
2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the
FIRST (frontline investigation of lenalidomide + dexamethasone versus standard
thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma
(NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood.
2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan
and prednisone for initial treatment of multiple myeloma. N Engl J Med.
2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan
and prednisone plus thalidomide versus melphalan and prednisone
alone or reduced-intensity autologous stem cell transplantation in
elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet.
2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide
in patients older than 75 years with newly diagnosed multiple myeloma.
IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stemcell
transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome
(IFM). Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose
thalidomide and prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol.
2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and
maintenance treatment in patients with newly diagnosed multiple myeloma:
results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol.
2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome
Inhibition for Extending Remissions (APEX) Investigators. Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med.
2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated
liposomal doxorubicin plus bortezomib compared with bortezomib alone
in relapsed or refractory multiple myeloma: combination therapy improves time
to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group.
Risk of progression and survival in multiple myeloma relapsing after therapy
with IMiDs and bortezomib: a multicenter international myeloma working group
study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus lowdose
dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol.
2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib
(PX-171-003-A1) in patients with relapsed and refractory multiple myeloma.
Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib
and dexamethasone versus placebo plus bortezomib and dexamethasone
in patients with relapsed or relapsed and refractory multiple
myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol.
2014;15(11):1195-1206.
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level. There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy,
hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly
myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the
bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic
SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma.For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of
lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these
criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall
survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics,
which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require
therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to 3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients
with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS
and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either
be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared
with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that
could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib,
doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is
often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials
have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and
safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and
OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide
plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are
integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered
to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit
not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective
and well tolerated. There has been a general shift to using these agents upfront in transplant-ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered
in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay
before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as
neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refreactory/Relapsed Disease Treatments
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level. There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy,
hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly
myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the
bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic
SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma.For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of
lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these
criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall
survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics,
which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require
therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to 3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients
with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS
and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either
be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared
with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that
could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib,
doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is
often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials
have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and
safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and
OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide
plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are
integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered
to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit
not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective
and well tolerated. There has been a general shift to using these agents upfront in transplant-ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered
in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay
before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as
neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refreactory/Relapsed Disease Treatments
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined
significance (MGUS) consistently precedes multiple myeloma: a prospective
study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal
Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney
injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop
Consensus Panel 3. Consensus recommendations for standard investigative workup:
report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working
Group consensus statement for the management, treatment, and supportive care
of patients with myeloma not eligible for standard autologous stem-cell transplantation.
J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.
2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more
entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation
of measured myeloma cell mass with presenting clinical features, response to
treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple
myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival
in multiple myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis
in monoclonal gammopathy of undetermined significance. N Engl J Med.
2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering
(asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering
multiple myeloma: biological insights and early treatment strategies. Hematology
Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone
for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple
myeloma limited to high-risk subgroup identified by gene expression profiling.
Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes.
Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in
oncology: multiple myeloma. National Comprehensive Cancer Network Website.
http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated
March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study
(EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide,
and lenalidomide in previously untreated multiple myeloma. Blood.
2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus
reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed multiple
myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous
administration of bortezomib in patients with relapsed multiple myeloma: a randomized,
phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in
multiple myeloma linked to bortezomib in total therapy 3: comparison with total
therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem
cell mobilization and engraftment post-peripheral blood stem cell transplantation
in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group.
Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell
collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining
regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis
for patients with newly diagnosed multiple myeloma patients treated with
lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in
combination with lenalidomide and low dose dexamethasone as a frontline treatment
for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of
carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended
dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed
multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide
plus dexamethasone in newly diagnosed multiple myeloma: a comparative
analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous
bone marrow transplantation and chemotherapy in multiple myeloma.
Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous
stem cell transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood.
1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second
on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol.
2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the
FIRST (frontline investigation of lenalidomide + dexamethasone versus standard
thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma
(NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood.
2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan
and prednisone for initial treatment of multiple myeloma. N Engl J Med.
2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan
and prednisone plus thalidomide versus melphalan and prednisone
alone or reduced-intensity autologous stem cell transplantation in
elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet.
2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide
in patients older than 75 years with newly diagnosed multiple myeloma.
IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stemcell
transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome
(IFM). Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose
thalidomide and prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol.
2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and
maintenance treatment in patients with newly diagnosed multiple myeloma:
results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol.
2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome
Inhibition for Extending Remissions (APEX) Investigators. Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med.
2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated
liposomal doxorubicin plus bortezomib compared with bortezomib alone
in relapsed or refractory multiple myeloma: combination therapy improves time
to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group.
Risk of progression and survival in multiple myeloma relapsing after therapy
with IMiDs and bortezomib: a multicenter international myeloma working group
study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus lowdose
dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol.
2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib
(PX-171-003-A1) in patients with relapsed and refractory multiple myeloma.
Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib
and dexamethasone versus placebo plus bortezomib and dexamethasone
in patients with relapsed or relapsed and refractory multiple
myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol.
2014;15(11):1195-1206.
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined
significance (MGUS) consistently precedes multiple myeloma: a prospective
study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal
Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney
injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop
Consensus Panel 3. Consensus recommendations for standard investigative workup:
report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working
Group consensus statement for the management, treatment, and supportive care
of patients with myeloma not eligible for standard autologous stem-cell transplantation.
J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.
2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more
entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation
of measured myeloma cell mass with presenting clinical features, response to
treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple
myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival
in multiple myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis
in monoclonal gammopathy of undetermined significance. N Engl J Med.
2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering
(asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering
multiple myeloma: biological insights and early treatment strategies. Hematology
Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone
for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple
myeloma limited to high-risk subgroup identified by gene expression profiling.
Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes.
Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in
oncology: multiple myeloma. National Comprehensive Cancer Network Website.
http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated
March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study
(EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide,
and lenalidomide in previously untreated multiple myeloma. Blood.
2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus
reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed multiple
myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous
administration of bortezomib in patients with relapsed multiple myeloma: a randomized,
phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in
multiple myeloma linked to bortezomib in total therapy 3: comparison with total
therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem
cell mobilization and engraftment post-peripheral blood stem cell transplantation
in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group.
Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell
collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining
regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis
for patients with newly diagnosed multiple myeloma patients treated with
lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in
combination with lenalidomide and low dose dexamethasone as a frontline treatment
for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of
carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended
dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed
multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide
plus dexamethasone in newly diagnosed multiple myeloma: a comparative
analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous
bone marrow transplantation and chemotherapy in multiple myeloma.
Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous
stem cell transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood.
1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second
on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol.
2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the
FIRST (frontline investigation of lenalidomide + dexamethasone versus standard
thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma
(NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood.
2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan
and prednisone for initial treatment of multiple myeloma. N Engl J Med.
2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan
and prednisone plus thalidomide versus melphalan and prednisone
alone or reduced-intensity autologous stem cell transplantation in
elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet.
2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide
in patients older than 75 years with newly diagnosed multiple myeloma.
IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stemcell
transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome
(IFM). Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose
thalidomide and prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol.
2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and
maintenance treatment in patients with newly diagnosed multiple myeloma:
results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol.
2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome
Inhibition for Extending Remissions (APEX) Investigators. Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med.
2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated
liposomal doxorubicin plus bortezomib compared with bortezomib alone
in relapsed or refractory multiple myeloma: combination therapy improves time
to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group.
Risk of progression and survival in multiple myeloma relapsing after therapy
with IMiDs and bortezomib: a multicenter international myeloma working group
study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus lowdose
dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol.
2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib
(PX-171-003-A1) in patients with relapsed and refractory multiple myeloma.
Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib
and dexamethasone versus placebo plus bortezomib and dexamethasone
in patients with relapsed or relapsed and refractory multiple
myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol.
2014;15(11):1195-1206.
The Burden of COPD
Case Scenario
A 62-year-old man who regularly presented to the ED for exacerbations of chronic obstructive pulmonary disease (COPD) after running out of his medications presented again for evaluation and treatment. His outpatient care had been poorly coordinated, and he relied on the ED to provide him with the support he needed. This presentation represented his fifth visit to the ED over the past 3 months.
The patient’s medical history was positive for asthma since childhood, tobacco use, hypertension, and a recent diagnosis of congestive heart failure (CHF). Over the past year, he had four hospital admissions, and was currently unable to walk from his bedroom to another room without becoming short of breath. He also had recently experienced a 20-lb weight loss.
At this visit, the patient complained of chest pain and lightheadedness, which he described as suffocating. Prior to these recent symptoms, he enjoyed walking in his neighborhood and talking with friends. He was an avid reader and sports fan, but admitted that he now had trouble focusing on reading and following games on television. He lived alone, and his family lived across the country. The patient further admitted that although he had attempted to quit cigarette smoking, he was unable to give up his 50-pack per year habit. He had no completed advance health care directive and had significant challenges tending to his basic needs.
The Trajectory of COPD
Chronic obstructive pulmonary disease is a common chronic illness that causes significant morbidity and mortality. A 2016 National Health Services report cited respiratory illness, primarily from COPD, as the third leading cause of death in the United States in 2014.1The trajectory of this disease is marked by frequent exacerbations with partial recovery to baseline function. The burden of those living with COPD is significant and marked by a poor overall health-related quality of life (QOL). The ED has become a staging area for patients seeking care for exacerbations of COPD.2
The World Health Organization (WHO) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have defined COPD as a spectrum of diseases including emphysema, chronic bronchitis, and chronic obstructive asthma characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs.3 Exacerbations and comorbidities contribute to the overall severity of COPD in individual patients.4
The case presented in this article illustrates the common scenario of a patient whose COPD has become severe and highly symptomatic with declining function to the point where he requires home support. His physical decline had been rapid and resulted in many unmet needs. When a patient such as this presents for emergent care, he must first be stabilized; then a care plan will need to be developed prior to discharge.
Management Goals
The overall goals of treating COPD are based on preserving function and are not curative in nature. Chronic obstructive pulmonary disease is a progressive illness that will intensify over time.5 As such, palliative care services are warranted. However, many patients with COPD do not receive palliative care services compared to patients with such other serious and life-limiting disease as cancer and heart disease.
Acute Exacerbations of COPD
Incidence
The frequency of acute exacerbations of COPD (AECOPD) increases with age, productive cough, long-standing COPD, previous hospitalizations related to COPD, eosinophilia, and comorbidities (eg, CHF). Patients with moderate to severe COPD and a history of prior exacerbations were found to have a higher likelihood of future exacerbations. From a quality and cost perspective, it may be useful to identify high-risk patients and strengthen their outpatient program to lessen the need for ED care and more intensive support.6,7
In our case scenario, the patient could have been stabilized at home with a well-controlled plan and home support, which would have resulted in an improved QOL and more time free from his high symptom burden.
Causes
Bacterial and viral respiratory infections are the most likely cause of AECOPD. Environmental pollution and pulmonary embolism are also triggers. Typically, patients with AECOPD present to the ED up to several times a year2 and represent the third most common cause of 30-day readmissions to the hospital.8 Prior exacerbations, dyspnea, and other medical comorbidities are also risk factors for more frequent hospital visits.
Presenting Signs and Symptoms
Each occurrence of AECOPD represents a worsening of a patient’s respiratory symptoms beyond normal variations. This might include increases in cough, sputum production, and dyspnea. The goal in caring for a person with an AECOPD is to stabilize the acute event and provide a treatment plan. The range of acuity for moderate to severe disease makes devising an appropriate treatment plan challenging, and after implementing the best plans, the patient’s course may be characterized by a prolonged cycle of admissions and readmissions without substantial return to baseline.
Management
In practice, ED management of AECOPD in older adults typically differs significantly from published guideline recommendations,9 which may result in pooroutcomes related to shortcomings in quality of care. Better adherence to guideline recommendations when caring for elderly patients with COPD may lead to improved clinical outcomes and better resource usage.
Risk Stratification
Complicating ED management is the challenge of determining the severity of illness and degree of the exacerbation. Airflow obstruction alone is not sufficient to predict outcomes, as any particular measure of obstruction is associated with a spectrum of forced expiratory volume in the first second (FEV1) and varying performance. Moreover, peak-flow measurements are not useful in the setting of AECOPD, as opposed to their use in acute asthma exacerbations, and are not predictive of changes in clinical status.
GOLD and NICE Criteria
Guidelines have been developed and widely promoted to assist ED and hospital and community clinicians in providing evidence-based management for COPD patients. The GOLD Criteria and the National Institute for Clinical Excellence (NICE) are both clinical guidelines on management of COPD.10
Although well recognized and commonly used, the original GOLD criteria did not take into account the frequency and importance of the extrapulmonary manifestations of COPD in predicting outcome. Typically, those with severe or very severe COPD have an average of 12 co-occurring symptoms, an even greater number of signs and symptoms than those occurring in patients with cancer or heart or renal disease.11
BODE Criteria
The body mass index, airflow obstruction, dyspnea and exercise capacity (BODE) criteria assess and predict the health-related QOL and mortality risk for patients with COPD. Risk is adjusted based on four factors—weight, airway obstruction, dyspnea, and exercise capacity (ie, 6-minute walk distance).13
Initial Evaluation and Work-Up
As previously noted, when an AECOPD patient arrives to the ED, the first priority is to stabilize the patient and initiate treatment. In this respect, initial identification of the patient’s pulse oxygen saturation (SpO2) is important.
Laboratory Evaluation
In cases of respiratory failure, obtaining arterial blood gas (ABG) values are critical. The ABG test will assist in determining acute exacerbations of chronic hypercapnia and the need for ventilatory support. When considering CHF, a plasma B-type natriuretic peptide is useful to assess for CHF.
Imaging Studies
A chest radiograph may be useful in the initial evaluation to identify abnormalities, including barotrauma (ie, pneumothorax) and infiltrates. Additionally, in patients with comorbidities, it is important to assess cardiac status, and a chest X-ray may assist in identification of pulmonary edema, pleural effusions, and cardiomegaly. If the radiograph does show a pulmonary infiltrate (ie, pneumonia), it will help identify the probable triggers, but even in these instances, a sputum gram stain will not assist in the diagnosis.
Treatment
Relieving airflow obstruction is achieved with inhaled short-acting bronchodilators and systemic glucocorticoids, by treating infection, and by providing supplemental oxygen and ventilatory support.
Bronchodilators
The short-acting beta-adrenergic agonists (eg, albuterol) act rapidly and are effective in producing bronchodilation. Nebulized therapy may be most comfortable for the acutely ill patient. Typical dosing is 2.5 mg albuterol diluted to 3 cc by nebulizer every hour. Higher doses are not more effective, and there is no evidence of a higher response rate from constant nebulized therapy, which can cause anxiety and tachycardia in patients.14 Anticholinergic agents (eg, ipratropium) are often added despite unclear data regarding clinical advantage. In one study evaluating the effectiveness of adding ipratropium to albuterol, patients receiving a combination had the same improvement in FEV1 at 90 minutes.15 Patients receiving ipratropium alone had the lowest rate of reported side effects.15
Systemic Glucocorticoids
Short-course systemic glucocorticoids are an important addition to treatment and have been found to improve spirometry and decrease relapse rate. The oral and intravenous (IV) routes provide the same benefit. For the acutely ill patient with challenges swallowing, the IV route is preferred. The optimal dose is not clear, but hydrocortisone doses of 100 mg to 125 mg every 6 hours for 3 days are effective, as is oral prednisone 30 mg per day for 14 days, or 60 mg per day for 3 days with a taper.
Antibiotic Therapy
Antibiotics are indicated for patients with moderate to severe AECOPD who are ill enough to be admitted to the hospital. Empiric broad spectrum treatment is recommended. The initial antibiotic regimen should target likely bacterial pathogens (Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in most patients) and take into account local patterns of antibiotic resistance. Flouroquinolones or third-generation cephalosporins generally provide sufficient coverage. For patients experiencing only a mild exacerbation, antibiotics are not warranted.
Magnesium Sulfate
Other supplemental medications that have been evaluated include magnesium sulfate for bronchial smooth muscle relaxation. Studies have found that while magnesium is helpful in asthma, results are mixed with COPD.16
Supplemental Oxygen
Oxygen therapy is important during an AECOPD episode. Often, concerns arise about decreasing respiratory drive, which is typically driven by hypoxia in patients who have chronic hypercapnia. Arterial blood gas determinations are important in managing a patient’s respiratory status and will assist in determining actual oxygenation and any coexistent metabolic disturbances.
Noninvasive Ventilation. Oxygen can be administered efficiently by a venturi mask, which delivers precise fractions of oxygen, or by nasal cannula. A facemask is less comfortable, but is available for higher oxygen requirements, providing up to 55% oxygen, while a nonrebreather mask delivers up to 90% oxygen.
When necessary, noninvasive positive pressure ventilation (NPPV) improves outcomes for those with severe dyspnea and signs of respiratory fatigue manifested as increased work of breathing. Noninvasive positive pressure ventilation can improve clinical outcomes and is the ventilator mode of choice for those patients with COPD. Indications include severe dyspnea with signs of increased work of breathing and respiratory acidosis (arterial pH <7.35) and partial pressure of arterial carbon dioxide (PaCO2) >45 mm Hg.
Whenever possible, NPPV should be initiated with a triggered mode to allow spontaneous breaths. Inspiratory pressure of 8 cm to 12 cm H2O and expiratory pressure of 3 cm to 5 cm of H2 are recommended.
Mechanical Ventilation. Mechanical ventilation is often undesirable because it may be extraordinarily difficult to wean a patient off the device and permit safe extubation. However, if a patient cannot be stabilized with NPPV, intubation and mechanical ventilation must be considered. Typically, this occurs when there is severe respiratory distress, tachypnea >30 breaths/min, accessory muscle use, and altered mentation.
Goals of intubation/mechanical respiration include correcting oxygenation and severe respiratory acidosis as well as reducing the work of breathing. Barotrauma is a significant risk when patients with COPD require mechanical ventilation. Volume-limited modes of ventilation are commonly used, while pressure support or pressure-limited modes are less suitable for patients with airflow limitation. Again, invasive ventilation should only be administered if a patient cannot tolerate NPPV.
Palliative Care in the ED
Palliative care is an approach that improves the QOL of patients and their families facing the issues associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and accurate assessment and treatment of pain and physical, psychosocial, and spiritual problems.3 This approach to care is warranted for COPD patients given the myriad of burdensome symptoms and functional decline that occurs.17
Palliative care expands traditional treatment goals to include enhancing QOL; helping with medical decision making; and identifying the goals of care. Palliative care is provided by board-certified physicians for the most complex of cases. However, the primary practice of palliative care must be delivered at the bedside by the treating provider. Managing pain, dyspnea, nausea, vomiting, and changes in bowel habits, as well as discussing goals of care, are among the basic palliative care skills all providers need to have and apply when indicated.
Palliative Care for Dyspnea
Opioids. Primary palliative care in the ED includes the appropriate use of low-dose oral and parenteral opioids to treat dyspnea in AECOPD. The use of a low-dose opioid, such as morphine 2 mg IV, titrated up to a desired response, is a safe and effective practice.18 Note the 2-mg starting dose is considered low-dose.19
With respect to managing dyspnea in AECOPD patients, nebulized opioids have not been found to be better than nebulized saline. More specific data regarding the use of oral opioids for managing refractory dyspnea in patients with predominantly COPD have been recently published: Long-acting morphine 20 mg once daily provides symptomatic relief in refractory dyspnea in the community setting. For the opioid-naïve patient, a lower dose is recommended.20
Oxygenation. There is no hard evidence of the effectiveness of oxygen in the palliation of breathlessness. Humidified air is effective initially, as is providing a fan at the bedside. Short-burst oxygen therapy should only be considered for episodes of severe breathlessness in patients whose COPD is not relieved by other treatments. Oxygen should continue to be prescribed only if an improvement in breathlessness following therapy has been documented. The American Thoracic Society recommends continuous oxygen therapy in patients with COPD who have severe resting hypoxemia (PaCO2 ≤55 mm Hg or SpO2 ≤88%).21
POLST Form
The Physicians Order for Life-Sustaining Treatment (POLST) form is a set of medical orders, similar to the “do not resuscitate” (allow natural death) order. A POLST form is not an advance directive and does not serve as a substitute for a patient’s assignation of a health care agent or durable power of attorney for health care.22
The POLST form enables physicians to order treatments patients would want, identify those treatments that patients would not want, and not provide those the patient considers “extraordinary” and excessively burdensome. A POLST form does not allow for active euthanasia or physician-assisted suicide.
Identifying treatment preferences is an important part of the initial evaluation of all patients. When dealing with an airway issue in a COPD patient, management can become complex. Ideally, the POLST form should arrive with the patient in the ED and list preferences regarding possible intensive interventions such as intubation and chest compressions. Discussing these issues with a patient in extreme distress is difficult or impossible, and in these cases, access to pertinent medical records, discussing preferences with family caregivers, and availability of a POLST form are much better ways to determine therapy.
Palliative Home Care
Patient Safety Considerations
Weight loss and associated muscle wasting are common features in patients with severe COPD, creating a high-risk situation for falls and a need for assistance with activities of daily living. The patient who is frail when discharged home from the ED requires a home-care plan before leaving the ED, and strict follow-up with the patient’s primary care provider will typically be needed within 2 to 4 weeks.
Psychological Considerations
Being mindful of the anxiety and depression that accompany the physical limitations of those with COPD is important. Mood disturbances serve as risk factors for re-hospitalization and mortality.13Multiple palliative care interventions provide patients assistance with these issues, including the use of antidepressants that may aid sleep, stabilize mood, and stimulate appetite.
Early referral to the palliative care team will provide improved care for the patient and family. Palliative care referral will provide continued management of the physical symptoms and evaluation and treatment of the psychosocial issues that accompany COPD. Additionally, the palliative care team can assist with safe discharge planning and follow-up, including the provision of the patient’s home needs as well as the family’s ability to cope with the home setting.
Prognosis
Predicting prognosis is difficult for the COPD patient due to the highly variable illness trajectory. Some patients have a low FEV1 and yet are very functional. However, assessment of severity of lung function impairment, frequency of exacerbations, and need for long-term oxygen therapy helps identify those patients who are entering the final 12 months of life. Evaluating symptom burden and impact on activities of daily living for patients with COPD is comparable to those of cancer patients, and in both cases, palliative care approaches are necessary.
Predicting Morbidity and Mortality
A profile developed from observational studies can help predict 6- to 12-month morbidity and mortality in patients with advanced COPD. This profile includes the following criteria:
- Significant dyspnea;
- FEV1 <30%;
- Number of exacerbations;
- Left heart failure or other comorbidities;
- Weight loss or cachexia;
- Decreasing performance status;
- Age older than 70 years; and
- Depression.
Although additional research is required to refine and verify this profile, reviewing these data points can prompt providers to initiate discussions with patients about treatment preferences and end-of-life care.23,24
Palliative Performance Scale
The Palliative Performance Scale (PPS) is another scale used to predict prognosis and eligibility for hospice care.25 This score provides a patient’s estimated survival.25 For a patient with a PPS score of 50%, hospice education may be appropriate.
Case Scenario Continued
Both the BODE and GOLD criteria scores assisted in determining prognosis and risk profiles of the patient in our case scenario. By applying the BODE criteria, our patient had a 4-year survival benefit of under 18%. The GOLD criteria results for this patient also were consistent with the BODE criteria and reflected end-stage COPD. Since this patient also had a PPS score of 50%, hospice education and care were discussed and initiated.
Conclusion
Patients with AECOPD commonly present to the ED. Such patients suffer with a high burden of illness and a need for immediate symptom management. However, after these measures have been instituted, strong evidence suggests that these patients typically do not receive palliative care with the same frequency compared to cancer or heart disease patients.
Management of AECOPD in the ED must include rapid treatment of dyspnea and pain, but also a determination of treatment preferences and an understanding of the prognosis. Several criteria are available to guide prognostic awareness and may help further the goals of care and disposition. Primary palliative care should be started by the ED provider for appropriate patients, with early referral to the palliative care team.
1. National Center for Health Statistics. Health, United States 2015 With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: US Dept. Health and Human Services; 2016. http://www.cdc.gov/nchs/hus/. Accessed October 17, 2016.
2. Khialani B, Sivakumaran P, Keijzers G, Sriram KB. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci . 2014;19(4):297-303.
3. World Health Organization Web site. Chronic respiratory diseases. COPD: Definition. http://www.who.int/respiratory/copd/definition/en/. Accessed October 17, 2016.
4. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med . 2007;176(6):532-555.
5. Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD . 2007;4(1):29-39.
6. Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr; MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc . 2003;51(7):908-916.
7. Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest . 2001;119:3.
8. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med . 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
9. Rowe BH, Bhutani M, Stickland MK, Cydulka R. Assessment and management of chronic obstructive pulmonary disease in the emergency department and beyond. Expert Rev Respir Med . 2011;5(4):549-559. doi:10.1586/ers.11.43.
10. National Institute for Clinical Excellence Web site. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline CG101. https://www.nice.org.uk/Guidance/cg101. Published June 2010. Accessed October 17, 2016.
11. Christensen VL, Holm AM, Cooper B, Paul SM, Miaskowski C, Rustøen T. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage . 2016;51(5):849-859. doi:10.1016/j.jpainsymman.2015.12.324.
12. GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease Web site. http://goldcopd.org/gold-reports/. Accessed October 17, 2016.
13. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res . 2009;10:1. doi:10.1186/1465-9921-10-1.
14. Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med . 2001;134(7):600-620.
15. McCrory DC, Brown CD. Inhaled short-acting beta 2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2001;(2):CD002984.
16. Shivanthan MC, Rajapakse S. Magnesium for acute exacerbation of chronic obstructive pulmonary disease: A systematic review of randomised trials. Ann Thorac Med . 2014;9(2):77-80. doi:10.4103/1817-1737.128844.
17. Curtis JR. Palliative and end of life care for patients with severe COPD. Eur Respir J . 2008;32(3):796-803.
18. Rocker GM, Simpson AC, Young J, et al. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open . 2013;1(1):E27-E36.
19. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax . 2002;57(11):939-944.
20. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ . 2003;327(7414):523-528.
21. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008.
22. National POLST Paradigm. http://polst.org/professionals-page/?pro=1. Accessed October 17, 2016.
23. Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49(1):90-97; discussion 97-98.
24. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis . 2008;3(1):11-29.
25. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care . 1996;12(1):5-11.
Case Scenario
A 62-year-old man who regularly presented to the ED for exacerbations of chronic obstructive pulmonary disease (COPD) after running out of his medications presented again for evaluation and treatment. His outpatient care had been poorly coordinated, and he relied on the ED to provide him with the support he needed. This presentation represented his fifth visit to the ED over the past 3 months.
The patient’s medical history was positive for asthma since childhood, tobacco use, hypertension, and a recent diagnosis of congestive heart failure (CHF). Over the past year, he had four hospital admissions, and was currently unable to walk from his bedroom to another room without becoming short of breath. He also had recently experienced a 20-lb weight loss.
At this visit, the patient complained of chest pain and lightheadedness, which he described as suffocating. Prior to these recent symptoms, he enjoyed walking in his neighborhood and talking with friends. He was an avid reader and sports fan, but admitted that he now had trouble focusing on reading and following games on television. He lived alone, and his family lived across the country. The patient further admitted that although he had attempted to quit cigarette smoking, he was unable to give up his 50-pack per year habit. He had no completed advance health care directive and had significant challenges tending to his basic needs.
The Trajectory of COPD
Chronic obstructive pulmonary disease is a common chronic illness that causes significant morbidity and mortality. A 2016 National Health Services report cited respiratory illness, primarily from COPD, as the third leading cause of death in the United States in 2014.1The trajectory of this disease is marked by frequent exacerbations with partial recovery to baseline function. The burden of those living with COPD is significant and marked by a poor overall health-related quality of life (QOL). The ED has become a staging area for patients seeking care for exacerbations of COPD.2
The World Health Organization (WHO) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have defined COPD as a spectrum of diseases including emphysema, chronic bronchitis, and chronic obstructive asthma characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs.3 Exacerbations and comorbidities contribute to the overall severity of COPD in individual patients.4
The case presented in this article illustrates the common scenario of a patient whose COPD has become severe and highly symptomatic with declining function to the point where he requires home support. His physical decline had been rapid and resulted in many unmet needs. When a patient such as this presents for emergent care, he must first be stabilized; then a care plan will need to be developed prior to discharge.
Management Goals
The overall goals of treating COPD are based on preserving function and are not curative in nature. Chronic obstructive pulmonary disease is a progressive illness that will intensify over time.5 As such, palliative care services are warranted. However, many patients with COPD do not receive palliative care services compared to patients with such other serious and life-limiting disease as cancer and heart disease.
Acute Exacerbations of COPD
Incidence
The frequency of acute exacerbations of COPD (AECOPD) increases with age, productive cough, long-standing COPD, previous hospitalizations related to COPD, eosinophilia, and comorbidities (eg, CHF). Patients with moderate to severe COPD and a history of prior exacerbations were found to have a higher likelihood of future exacerbations. From a quality and cost perspective, it may be useful to identify high-risk patients and strengthen their outpatient program to lessen the need for ED care and more intensive support.6,7
In our case scenario, the patient could have been stabilized at home with a well-controlled plan and home support, which would have resulted in an improved QOL and more time free from his high symptom burden.
Causes
Bacterial and viral respiratory infections are the most likely cause of AECOPD. Environmental pollution and pulmonary embolism are also triggers. Typically, patients with AECOPD present to the ED up to several times a year2 and represent the third most common cause of 30-day readmissions to the hospital.8 Prior exacerbations, dyspnea, and other medical comorbidities are also risk factors for more frequent hospital visits.
Presenting Signs and Symptoms
Each occurrence of AECOPD represents a worsening of a patient’s respiratory symptoms beyond normal variations. This might include increases in cough, sputum production, and dyspnea. The goal in caring for a person with an AECOPD is to stabilize the acute event and provide a treatment plan. The range of acuity for moderate to severe disease makes devising an appropriate treatment plan challenging, and after implementing the best plans, the patient’s course may be characterized by a prolonged cycle of admissions and readmissions without substantial return to baseline.
Management
In practice, ED management of AECOPD in older adults typically differs significantly from published guideline recommendations,9 which may result in pooroutcomes related to shortcomings in quality of care. Better adherence to guideline recommendations when caring for elderly patients with COPD may lead to improved clinical outcomes and better resource usage.
Risk Stratification
Complicating ED management is the challenge of determining the severity of illness and degree of the exacerbation. Airflow obstruction alone is not sufficient to predict outcomes, as any particular measure of obstruction is associated with a spectrum of forced expiratory volume in the first second (FEV1) and varying performance. Moreover, peak-flow measurements are not useful in the setting of AECOPD, as opposed to their use in acute asthma exacerbations, and are not predictive of changes in clinical status.
GOLD and NICE Criteria
Guidelines have been developed and widely promoted to assist ED and hospital and community clinicians in providing evidence-based management for COPD patients. The GOLD Criteria and the National Institute for Clinical Excellence (NICE) are both clinical guidelines on management of COPD.10
Although well recognized and commonly used, the original GOLD criteria did not take into account the frequency and importance of the extrapulmonary manifestations of COPD in predicting outcome. Typically, those with severe or very severe COPD have an average of 12 co-occurring symptoms, an even greater number of signs and symptoms than those occurring in patients with cancer or heart or renal disease.11
BODE Criteria
The body mass index, airflow obstruction, dyspnea and exercise capacity (BODE) criteria assess and predict the health-related QOL and mortality risk for patients with COPD. Risk is adjusted based on four factors—weight, airway obstruction, dyspnea, and exercise capacity (ie, 6-minute walk distance).13
Initial Evaluation and Work-Up
As previously noted, when an AECOPD patient arrives to the ED, the first priority is to stabilize the patient and initiate treatment. In this respect, initial identification of the patient’s pulse oxygen saturation (SpO2) is important.
Laboratory Evaluation
In cases of respiratory failure, obtaining arterial blood gas (ABG) values are critical. The ABG test will assist in determining acute exacerbations of chronic hypercapnia and the need for ventilatory support. When considering CHF, a plasma B-type natriuretic peptide is useful to assess for CHF.
Imaging Studies
A chest radiograph may be useful in the initial evaluation to identify abnormalities, including barotrauma (ie, pneumothorax) and infiltrates. Additionally, in patients with comorbidities, it is important to assess cardiac status, and a chest X-ray may assist in identification of pulmonary edema, pleural effusions, and cardiomegaly. If the radiograph does show a pulmonary infiltrate (ie, pneumonia), it will help identify the probable triggers, but even in these instances, a sputum gram stain will not assist in the diagnosis.
Treatment
Relieving airflow obstruction is achieved with inhaled short-acting bronchodilators and systemic glucocorticoids, by treating infection, and by providing supplemental oxygen and ventilatory support.
Bronchodilators
The short-acting beta-adrenergic agonists (eg, albuterol) act rapidly and are effective in producing bronchodilation. Nebulized therapy may be most comfortable for the acutely ill patient. Typical dosing is 2.5 mg albuterol diluted to 3 cc by nebulizer every hour. Higher doses are not more effective, and there is no evidence of a higher response rate from constant nebulized therapy, which can cause anxiety and tachycardia in patients.14 Anticholinergic agents (eg, ipratropium) are often added despite unclear data regarding clinical advantage. In one study evaluating the effectiveness of adding ipratropium to albuterol, patients receiving a combination had the same improvement in FEV1 at 90 minutes.15 Patients receiving ipratropium alone had the lowest rate of reported side effects.15
Systemic Glucocorticoids
Short-course systemic glucocorticoids are an important addition to treatment and have been found to improve spirometry and decrease relapse rate. The oral and intravenous (IV) routes provide the same benefit. For the acutely ill patient with challenges swallowing, the IV route is preferred. The optimal dose is not clear, but hydrocortisone doses of 100 mg to 125 mg every 6 hours for 3 days are effective, as is oral prednisone 30 mg per day for 14 days, or 60 mg per day for 3 days with a taper.
Antibiotic Therapy
Antibiotics are indicated for patients with moderate to severe AECOPD who are ill enough to be admitted to the hospital. Empiric broad spectrum treatment is recommended. The initial antibiotic regimen should target likely bacterial pathogens (Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in most patients) and take into account local patterns of antibiotic resistance. Flouroquinolones or third-generation cephalosporins generally provide sufficient coverage. For patients experiencing only a mild exacerbation, antibiotics are not warranted.
Magnesium Sulfate
Other supplemental medications that have been evaluated include magnesium sulfate for bronchial smooth muscle relaxation. Studies have found that while magnesium is helpful in asthma, results are mixed with COPD.16
Supplemental Oxygen
Oxygen therapy is important during an AECOPD episode. Often, concerns arise about decreasing respiratory drive, which is typically driven by hypoxia in patients who have chronic hypercapnia. Arterial blood gas determinations are important in managing a patient’s respiratory status and will assist in determining actual oxygenation and any coexistent metabolic disturbances.
Noninvasive Ventilation. Oxygen can be administered efficiently by a venturi mask, which delivers precise fractions of oxygen, or by nasal cannula. A facemask is less comfortable, but is available for higher oxygen requirements, providing up to 55% oxygen, while a nonrebreather mask delivers up to 90% oxygen.
When necessary, noninvasive positive pressure ventilation (NPPV) improves outcomes for those with severe dyspnea and signs of respiratory fatigue manifested as increased work of breathing. Noninvasive positive pressure ventilation can improve clinical outcomes and is the ventilator mode of choice for those patients with COPD. Indications include severe dyspnea with signs of increased work of breathing and respiratory acidosis (arterial pH <7.35) and partial pressure of arterial carbon dioxide (PaCO2) >45 mm Hg.
Whenever possible, NPPV should be initiated with a triggered mode to allow spontaneous breaths. Inspiratory pressure of 8 cm to 12 cm H2O and expiratory pressure of 3 cm to 5 cm of H2 are recommended.
Mechanical Ventilation. Mechanical ventilation is often undesirable because it may be extraordinarily difficult to wean a patient off the device and permit safe extubation. However, if a patient cannot be stabilized with NPPV, intubation and mechanical ventilation must be considered. Typically, this occurs when there is severe respiratory distress, tachypnea >30 breaths/min, accessory muscle use, and altered mentation.
Goals of intubation/mechanical respiration include correcting oxygenation and severe respiratory acidosis as well as reducing the work of breathing. Barotrauma is a significant risk when patients with COPD require mechanical ventilation. Volume-limited modes of ventilation are commonly used, while pressure support or pressure-limited modes are less suitable for patients with airflow limitation. Again, invasive ventilation should only be administered if a patient cannot tolerate NPPV.
Palliative Care in the ED
Palliative care is an approach that improves the QOL of patients and their families facing the issues associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and accurate assessment and treatment of pain and physical, psychosocial, and spiritual problems.3 This approach to care is warranted for COPD patients given the myriad of burdensome symptoms and functional decline that occurs.17
Palliative care expands traditional treatment goals to include enhancing QOL; helping with medical decision making; and identifying the goals of care. Palliative care is provided by board-certified physicians for the most complex of cases. However, the primary practice of palliative care must be delivered at the bedside by the treating provider. Managing pain, dyspnea, nausea, vomiting, and changes in bowel habits, as well as discussing goals of care, are among the basic palliative care skills all providers need to have and apply when indicated.
Palliative Care for Dyspnea
Opioids. Primary palliative care in the ED includes the appropriate use of low-dose oral and parenteral opioids to treat dyspnea in AECOPD. The use of a low-dose opioid, such as morphine 2 mg IV, titrated up to a desired response, is a safe and effective practice.18 Note the 2-mg starting dose is considered low-dose.19
With respect to managing dyspnea in AECOPD patients, nebulized opioids have not been found to be better than nebulized saline. More specific data regarding the use of oral opioids for managing refractory dyspnea in patients with predominantly COPD have been recently published: Long-acting morphine 20 mg once daily provides symptomatic relief in refractory dyspnea in the community setting. For the opioid-naïve patient, a lower dose is recommended.20
Oxygenation. There is no hard evidence of the effectiveness of oxygen in the palliation of breathlessness. Humidified air is effective initially, as is providing a fan at the bedside. Short-burst oxygen therapy should only be considered for episodes of severe breathlessness in patients whose COPD is not relieved by other treatments. Oxygen should continue to be prescribed only if an improvement in breathlessness following therapy has been documented. The American Thoracic Society recommends continuous oxygen therapy in patients with COPD who have severe resting hypoxemia (PaCO2 ≤55 mm Hg or SpO2 ≤88%).21
POLST Form
The Physicians Order for Life-Sustaining Treatment (POLST) form is a set of medical orders, similar to the “do not resuscitate” (allow natural death) order. A POLST form is not an advance directive and does not serve as a substitute for a patient’s assignation of a health care agent or durable power of attorney for health care.22
The POLST form enables physicians to order treatments patients would want, identify those treatments that patients would not want, and not provide those the patient considers “extraordinary” and excessively burdensome. A POLST form does not allow for active euthanasia or physician-assisted suicide.
Identifying treatment preferences is an important part of the initial evaluation of all patients. When dealing with an airway issue in a COPD patient, management can become complex. Ideally, the POLST form should arrive with the patient in the ED and list preferences regarding possible intensive interventions such as intubation and chest compressions. Discussing these issues with a patient in extreme distress is difficult or impossible, and in these cases, access to pertinent medical records, discussing preferences with family caregivers, and availability of a POLST form are much better ways to determine therapy.
Palliative Home Care
Patient Safety Considerations
Weight loss and associated muscle wasting are common features in patients with severe COPD, creating a high-risk situation for falls and a need for assistance with activities of daily living. The patient who is frail when discharged home from the ED requires a home-care plan before leaving the ED, and strict follow-up with the patient’s primary care provider will typically be needed within 2 to 4 weeks.
Psychological Considerations
Being mindful of the anxiety and depression that accompany the physical limitations of those with COPD is important. Mood disturbances serve as risk factors for re-hospitalization and mortality.13Multiple palliative care interventions provide patients assistance with these issues, including the use of antidepressants that may aid sleep, stabilize mood, and stimulate appetite.
Early referral to the palliative care team will provide improved care for the patient and family. Palliative care referral will provide continued management of the physical symptoms and evaluation and treatment of the psychosocial issues that accompany COPD. Additionally, the palliative care team can assist with safe discharge planning and follow-up, including the provision of the patient’s home needs as well as the family’s ability to cope with the home setting.
Prognosis
Predicting prognosis is difficult for the COPD patient due to the highly variable illness trajectory. Some patients have a low FEV1 and yet are very functional. However, assessment of severity of lung function impairment, frequency of exacerbations, and need for long-term oxygen therapy helps identify those patients who are entering the final 12 months of life. Evaluating symptom burden and impact on activities of daily living for patients with COPD is comparable to those of cancer patients, and in both cases, palliative care approaches are necessary.
Predicting Morbidity and Mortality
A profile developed from observational studies can help predict 6- to 12-month morbidity and mortality in patients with advanced COPD. This profile includes the following criteria:
- Significant dyspnea;
- FEV1 <30%;
- Number of exacerbations;
- Left heart failure or other comorbidities;
- Weight loss or cachexia;
- Decreasing performance status;
- Age older than 70 years; and
- Depression.
Although additional research is required to refine and verify this profile, reviewing these data points can prompt providers to initiate discussions with patients about treatment preferences and end-of-life care.23,24
Palliative Performance Scale
The Palliative Performance Scale (PPS) is another scale used to predict prognosis and eligibility for hospice care.25 This score provides a patient’s estimated survival.25 For a patient with a PPS score of 50%, hospice education may be appropriate.
Case Scenario Continued
Both the BODE and GOLD criteria scores assisted in determining prognosis and risk profiles of the patient in our case scenario. By applying the BODE criteria, our patient had a 4-year survival benefit of under 18%. The GOLD criteria results for this patient also were consistent with the BODE criteria and reflected end-stage COPD. Since this patient also had a PPS score of 50%, hospice education and care were discussed and initiated.
Conclusion
Patients with AECOPD commonly present to the ED. Such patients suffer with a high burden of illness and a need for immediate symptom management. However, after these measures have been instituted, strong evidence suggests that these patients typically do not receive palliative care with the same frequency compared to cancer or heart disease patients.
Management of AECOPD in the ED must include rapid treatment of dyspnea and pain, but also a determination of treatment preferences and an understanding of the prognosis. Several criteria are available to guide prognostic awareness and may help further the goals of care and disposition. Primary palliative care should be started by the ED provider for appropriate patients, with early referral to the palliative care team.
Case Scenario
A 62-year-old man who regularly presented to the ED for exacerbations of chronic obstructive pulmonary disease (COPD) after running out of his medications presented again for evaluation and treatment. His outpatient care had been poorly coordinated, and he relied on the ED to provide him with the support he needed. This presentation represented his fifth visit to the ED over the past 3 months.
The patient’s medical history was positive for asthma since childhood, tobacco use, hypertension, and a recent diagnosis of congestive heart failure (CHF). Over the past year, he had four hospital admissions, and was currently unable to walk from his bedroom to another room without becoming short of breath. He also had recently experienced a 20-lb weight loss.
At this visit, the patient complained of chest pain and lightheadedness, which he described as suffocating. Prior to these recent symptoms, he enjoyed walking in his neighborhood and talking with friends. He was an avid reader and sports fan, but admitted that he now had trouble focusing on reading and following games on television. He lived alone, and his family lived across the country. The patient further admitted that although he had attempted to quit cigarette smoking, he was unable to give up his 50-pack per year habit. He had no completed advance health care directive and had significant challenges tending to his basic needs.
The Trajectory of COPD
Chronic obstructive pulmonary disease is a common chronic illness that causes significant morbidity and mortality. A 2016 National Health Services report cited respiratory illness, primarily from COPD, as the third leading cause of death in the United States in 2014.1The trajectory of this disease is marked by frequent exacerbations with partial recovery to baseline function. The burden of those living with COPD is significant and marked by a poor overall health-related quality of life (QOL). The ED has become a staging area for patients seeking care for exacerbations of COPD.2
The World Health Organization (WHO) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have defined COPD as a spectrum of diseases including emphysema, chronic bronchitis, and chronic obstructive asthma characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs.3 Exacerbations and comorbidities contribute to the overall severity of COPD in individual patients.4
The case presented in this article illustrates the common scenario of a patient whose COPD has become severe and highly symptomatic with declining function to the point where he requires home support. His physical decline had been rapid and resulted in many unmet needs. When a patient such as this presents for emergent care, he must first be stabilized; then a care plan will need to be developed prior to discharge.
Management Goals
The overall goals of treating COPD are based on preserving function and are not curative in nature. Chronic obstructive pulmonary disease is a progressive illness that will intensify over time.5 As such, palliative care services are warranted. However, many patients with COPD do not receive palliative care services compared to patients with such other serious and life-limiting disease as cancer and heart disease.
Acute Exacerbations of COPD
Incidence
The frequency of acute exacerbations of COPD (AECOPD) increases with age, productive cough, long-standing COPD, previous hospitalizations related to COPD, eosinophilia, and comorbidities (eg, CHF). Patients with moderate to severe COPD and a history of prior exacerbations were found to have a higher likelihood of future exacerbations. From a quality and cost perspective, it may be useful to identify high-risk patients and strengthen their outpatient program to lessen the need for ED care and more intensive support.6,7
In our case scenario, the patient could have been stabilized at home with a well-controlled plan and home support, which would have resulted in an improved QOL and more time free from his high symptom burden.
Causes
Bacterial and viral respiratory infections are the most likely cause of AECOPD. Environmental pollution and pulmonary embolism are also triggers. Typically, patients with AECOPD present to the ED up to several times a year2 and represent the third most common cause of 30-day readmissions to the hospital.8 Prior exacerbations, dyspnea, and other medical comorbidities are also risk factors for more frequent hospital visits.
Presenting Signs and Symptoms
Each occurrence of AECOPD represents a worsening of a patient’s respiratory symptoms beyond normal variations. This might include increases in cough, sputum production, and dyspnea. The goal in caring for a person with an AECOPD is to stabilize the acute event and provide a treatment plan. The range of acuity for moderate to severe disease makes devising an appropriate treatment plan challenging, and after implementing the best plans, the patient’s course may be characterized by a prolonged cycle of admissions and readmissions without substantial return to baseline.
Management
In practice, ED management of AECOPD in older adults typically differs significantly from published guideline recommendations,9 which may result in pooroutcomes related to shortcomings in quality of care. Better adherence to guideline recommendations when caring for elderly patients with COPD may lead to improved clinical outcomes and better resource usage.
Risk Stratification
Complicating ED management is the challenge of determining the severity of illness and degree of the exacerbation. Airflow obstruction alone is not sufficient to predict outcomes, as any particular measure of obstruction is associated with a spectrum of forced expiratory volume in the first second (FEV1) and varying performance. Moreover, peak-flow measurements are not useful in the setting of AECOPD, as opposed to their use in acute asthma exacerbations, and are not predictive of changes in clinical status.
GOLD and NICE Criteria
Guidelines have been developed and widely promoted to assist ED and hospital and community clinicians in providing evidence-based management for COPD patients. The GOLD Criteria and the National Institute for Clinical Excellence (NICE) are both clinical guidelines on management of COPD.10
Although well recognized and commonly used, the original GOLD criteria did not take into account the frequency and importance of the extrapulmonary manifestations of COPD in predicting outcome. Typically, those with severe or very severe COPD have an average of 12 co-occurring symptoms, an even greater number of signs and symptoms than those occurring in patients with cancer or heart or renal disease.11
BODE Criteria
The body mass index, airflow obstruction, dyspnea and exercise capacity (BODE) criteria assess and predict the health-related QOL and mortality risk for patients with COPD. Risk is adjusted based on four factors—weight, airway obstruction, dyspnea, and exercise capacity (ie, 6-minute walk distance).13
Initial Evaluation and Work-Up
As previously noted, when an AECOPD patient arrives to the ED, the first priority is to stabilize the patient and initiate treatment. In this respect, initial identification of the patient’s pulse oxygen saturation (SpO2) is important.
Laboratory Evaluation
In cases of respiratory failure, obtaining arterial blood gas (ABG) values are critical. The ABG test will assist in determining acute exacerbations of chronic hypercapnia and the need for ventilatory support. When considering CHF, a plasma B-type natriuretic peptide is useful to assess for CHF.
Imaging Studies
A chest radiograph may be useful in the initial evaluation to identify abnormalities, including barotrauma (ie, pneumothorax) and infiltrates. Additionally, in patients with comorbidities, it is important to assess cardiac status, and a chest X-ray may assist in identification of pulmonary edema, pleural effusions, and cardiomegaly. If the radiograph does show a pulmonary infiltrate (ie, pneumonia), it will help identify the probable triggers, but even in these instances, a sputum gram stain will not assist in the diagnosis.
Treatment
Relieving airflow obstruction is achieved with inhaled short-acting bronchodilators and systemic glucocorticoids, by treating infection, and by providing supplemental oxygen and ventilatory support.
Bronchodilators
The short-acting beta-adrenergic agonists (eg, albuterol) act rapidly and are effective in producing bronchodilation. Nebulized therapy may be most comfortable for the acutely ill patient. Typical dosing is 2.5 mg albuterol diluted to 3 cc by nebulizer every hour. Higher doses are not more effective, and there is no evidence of a higher response rate from constant nebulized therapy, which can cause anxiety and tachycardia in patients.14 Anticholinergic agents (eg, ipratropium) are often added despite unclear data regarding clinical advantage. In one study evaluating the effectiveness of adding ipratropium to albuterol, patients receiving a combination had the same improvement in FEV1 at 90 minutes.15 Patients receiving ipratropium alone had the lowest rate of reported side effects.15
Systemic Glucocorticoids
Short-course systemic glucocorticoids are an important addition to treatment and have been found to improve spirometry and decrease relapse rate. The oral and intravenous (IV) routes provide the same benefit. For the acutely ill patient with challenges swallowing, the IV route is preferred. The optimal dose is not clear, but hydrocortisone doses of 100 mg to 125 mg every 6 hours for 3 days are effective, as is oral prednisone 30 mg per day for 14 days, or 60 mg per day for 3 days with a taper.
Antibiotic Therapy
Antibiotics are indicated for patients with moderate to severe AECOPD who are ill enough to be admitted to the hospital. Empiric broad spectrum treatment is recommended. The initial antibiotic regimen should target likely bacterial pathogens (Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in most patients) and take into account local patterns of antibiotic resistance. Flouroquinolones or third-generation cephalosporins generally provide sufficient coverage. For patients experiencing only a mild exacerbation, antibiotics are not warranted.
Magnesium Sulfate
Other supplemental medications that have been evaluated include magnesium sulfate for bronchial smooth muscle relaxation. Studies have found that while magnesium is helpful in asthma, results are mixed with COPD.16
Supplemental Oxygen
Oxygen therapy is important during an AECOPD episode. Often, concerns arise about decreasing respiratory drive, which is typically driven by hypoxia in patients who have chronic hypercapnia. Arterial blood gas determinations are important in managing a patient’s respiratory status and will assist in determining actual oxygenation and any coexistent metabolic disturbances.
Noninvasive Ventilation. Oxygen can be administered efficiently by a venturi mask, which delivers precise fractions of oxygen, or by nasal cannula. A facemask is less comfortable, but is available for higher oxygen requirements, providing up to 55% oxygen, while a nonrebreather mask delivers up to 90% oxygen.
When necessary, noninvasive positive pressure ventilation (NPPV) improves outcomes for those with severe dyspnea and signs of respiratory fatigue manifested as increased work of breathing. Noninvasive positive pressure ventilation can improve clinical outcomes and is the ventilator mode of choice for those patients with COPD. Indications include severe dyspnea with signs of increased work of breathing and respiratory acidosis (arterial pH <7.35) and partial pressure of arterial carbon dioxide (PaCO2) >45 mm Hg.
Whenever possible, NPPV should be initiated with a triggered mode to allow spontaneous breaths. Inspiratory pressure of 8 cm to 12 cm H2O and expiratory pressure of 3 cm to 5 cm of H2 are recommended.
Mechanical Ventilation. Mechanical ventilation is often undesirable because it may be extraordinarily difficult to wean a patient off the device and permit safe extubation. However, if a patient cannot be stabilized with NPPV, intubation and mechanical ventilation must be considered. Typically, this occurs when there is severe respiratory distress, tachypnea >30 breaths/min, accessory muscle use, and altered mentation.
Goals of intubation/mechanical respiration include correcting oxygenation and severe respiratory acidosis as well as reducing the work of breathing. Barotrauma is a significant risk when patients with COPD require mechanical ventilation. Volume-limited modes of ventilation are commonly used, while pressure support or pressure-limited modes are less suitable for patients with airflow limitation. Again, invasive ventilation should only be administered if a patient cannot tolerate NPPV.
Palliative Care in the ED
Palliative care is an approach that improves the QOL of patients and their families facing the issues associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and accurate assessment and treatment of pain and physical, psychosocial, and spiritual problems.3 This approach to care is warranted for COPD patients given the myriad of burdensome symptoms and functional decline that occurs.17
Palliative care expands traditional treatment goals to include enhancing QOL; helping with medical decision making; and identifying the goals of care. Palliative care is provided by board-certified physicians for the most complex of cases. However, the primary practice of palliative care must be delivered at the bedside by the treating provider. Managing pain, dyspnea, nausea, vomiting, and changes in bowel habits, as well as discussing goals of care, are among the basic palliative care skills all providers need to have and apply when indicated.
Palliative Care for Dyspnea
Opioids. Primary palliative care in the ED includes the appropriate use of low-dose oral and parenteral opioids to treat dyspnea in AECOPD. The use of a low-dose opioid, such as morphine 2 mg IV, titrated up to a desired response, is a safe and effective practice.18 Note the 2-mg starting dose is considered low-dose.19
With respect to managing dyspnea in AECOPD patients, nebulized opioids have not been found to be better than nebulized saline. More specific data regarding the use of oral opioids for managing refractory dyspnea in patients with predominantly COPD have been recently published: Long-acting morphine 20 mg once daily provides symptomatic relief in refractory dyspnea in the community setting. For the opioid-naïve patient, a lower dose is recommended.20
Oxygenation. There is no hard evidence of the effectiveness of oxygen in the palliation of breathlessness. Humidified air is effective initially, as is providing a fan at the bedside. Short-burst oxygen therapy should only be considered for episodes of severe breathlessness in patients whose COPD is not relieved by other treatments. Oxygen should continue to be prescribed only if an improvement in breathlessness following therapy has been documented. The American Thoracic Society recommends continuous oxygen therapy in patients with COPD who have severe resting hypoxemia (PaCO2 ≤55 mm Hg or SpO2 ≤88%).21
POLST Form
The Physicians Order for Life-Sustaining Treatment (POLST) form is a set of medical orders, similar to the “do not resuscitate” (allow natural death) order. A POLST form is not an advance directive and does not serve as a substitute for a patient’s assignation of a health care agent or durable power of attorney for health care.22
The POLST form enables physicians to order treatments patients would want, identify those treatments that patients would not want, and not provide those the patient considers “extraordinary” and excessively burdensome. A POLST form does not allow for active euthanasia or physician-assisted suicide.
Identifying treatment preferences is an important part of the initial evaluation of all patients. When dealing with an airway issue in a COPD patient, management can become complex. Ideally, the POLST form should arrive with the patient in the ED and list preferences regarding possible intensive interventions such as intubation and chest compressions. Discussing these issues with a patient in extreme distress is difficult or impossible, and in these cases, access to pertinent medical records, discussing preferences with family caregivers, and availability of a POLST form are much better ways to determine therapy.
Palliative Home Care
Patient Safety Considerations
Weight loss and associated muscle wasting are common features in patients with severe COPD, creating a high-risk situation for falls and a need for assistance with activities of daily living. The patient who is frail when discharged home from the ED requires a home-care plan before leaving the ED, and strict follow-up with the patient’s primary care provider will typically be needed within 2 to 4 weeks.
Psychological Considerations
Being mindful of the anxiety and depression that accompany the physical limitations of those with COPD is important. Mood disturbances serve as risk factors for re-hospitalization and mortality.13Multiple palliative care interventions provide patients assistance with these issues, including the use of antidepressants that may aid sleep, stabilize mood, and stimulate appetite.
Early referral to the palliative care team will provide improved care for the patient and family. Palliative care referral will provide continued management of the physical symptoms and evaluation and treatment of the psychosocial issues that accompany COPD. Additionally, the palliative care team can assist with safe discharge planning and follow-up, including the provision of the patient’s home needs as well as the family’s ability to cope with the home setting.
Prognosis
Predicting prognosis is difficult for the COPD patient due to the highly variable illness trajectory. Some patients have a low FEV1 and yet are very functional. However, assessment of severity of lung function impairment, frequency of exacerbations, and need for long-term oxygen therapy helps identify those patients who are entering the final 12 months of life. Evaluating symptom burden and impact on activities of daily living for patients with COPD is comparable to those of cancer patients, and in both cases, palliative care approaches are necessary.
Predicting Morbidity and Mortality
A profile developed from observational studies can help predict 6- to 12-month morbidity and mortality in patients with advanced COPD. This profile includes the following criteria:
- Significant dyspnea;
- FEV1 <30%;
- Number of exacerbations;
- Left heart failure or other comorbidities;
- Weight loss or cachexia;
- Decreasing performance status;
- Age older than 70 years; and
- Depression.
Although additional research is required to refine and verify this profile, reviewing these data points can prompt providers to initiate discussions with patients about treatment preferences and end-of-life care.23,24
Palliative Performance Scale
The Palliative Performance Scale (PPS) is another scale used to predict prognosis and eligibility for hospice care.25 This score provides a patient’s estimated survival.25 For a patient with a PPS score of 50%, hospice education may be appropriate.
Case Scenario Continued
Both the BODE and GOLD criteria scores assisted in determining prognosis and risk profiles of the patient in our case scenario. By applying the BODE criteria, our patient had a 4-year survival benefit of under 18%. The GOLD criteria results for this patient also were consistent with the BODE criteria and reflected end-stage COPD. Since this patient also had a PPS score of 50%, hospice education and care were discussed and initiated.
Conclusion
Patients with AECOPD commonly present to the ED. Such patients suffer with a high burden of illness and a need for immediate symptom management. However, after these measures have been instituted, strong evidence suggests that these patients typically do not receive palliative care with the same frequency compared to cancer or heart disease patients.
Management of AECOPD in the ED must include rapid treatment of dyspnea and pain, but also a determination of treatment preferences and an understanding of the prognosis. Several criteria are available to guide prognostic awareness and may help further the goals of care and disposition. Primary palliative care should be started by the ED provider for appropriate patients, with early referral to the palliative care team.
1. National Center for Health Statistics. Health, United States 2015 With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: US Dept. Health and Human Services; 2016. http://www.cdc.gov/nchs/hus/. Accessed October 17, 2016.
2. Khialani B, Sivakumaran P, Keijzers G, Sriram KB. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci . 2014;19(4):297-303.
3. World Health Organization Web site. Chronic respiratory diseases. COPD: Definition. http://www.who.int/respiratory/copd/definition/en/. Accessed October 17, 2016.
4. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med . 2007;176(6):532-555.
5. Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD . 2007;4(1):29-39.
6. Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr; MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc . 2003;51(7):908-916.
7. Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest . 2001;119:3.
8. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med . 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
9. Rowe BH, Bhutani M, Stickland MK, Cydulka R. Assessment and management of chronic obstructive pulmonary disease in the emergency department and beyond. Expert Rev Respir Med . 2011;5(4):549-559. doi:10.1586/ers.11.43.
10. National Institute for Clinical Excellence Web site. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline CG101. https://www.nice.org.uk/Guidance/cg101. Published June 2010. Accessed October 17, 2016.
11. Christensen VL, Holm AM, Cooper B, Paul SM, Miaskowski C, Rustøen T. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage . 2016;51(5):849-859. doi:10.1016/j.jpainsymman.2015.12.324.
12. GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease Web site. http://goldcopd.org/gold-reports/. Accessed October 17, 2016.
13. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res . 2009;10:1. doi:10.1186/1465-9921-10-1.
14. Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med . 2001;134(7):600-620.
15. McCrory DC, Brown CD. Inhaled short-acting beta 2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2001;(2):CD002984.
16. Shivanthan MC, Rajapakse S. Magnesium for acute exacerbation of chronic obstructive pulmonary disease: A systematic review of randomised trials. Ann Thorac Med . 2014;9(2):77-80. doi:10.4103/1817-1737.128844.
17. Curtis JR. Palliative and end of life care for patients with severe COPD. Eur Respir J . 2008;32(3):796-803.
18. Rocker GM, Simpson AC, Young J, et al. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open . 2013;1(1):E27-E36.
19. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax . 2002;57(11):939-944.
20. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ . 2003;327(7414):523-528.
21. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008.
22. National POLST Paradigm. http://polst.org/professionals-page/?pro=1. Accessed October 17, 2016.
23. Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49(1):90-97; discussion 97-98.
24. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis . 2008;3(1):11-29.
25. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care . 1996;12(1):5-11.
1. National Center for Health Statistics. Health, United States 2015 With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: US Dept. Health and Human Services; 2016. http://www.cdc.gov/nchs/hus/. Accessed October 17, 2016.
2. Khialani B, Sivakumaran P, Keijzers G, Sriram KB. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci . 2014;19(4):297-303.
3. World Health Organization Web site. Chronic respiratory diseases. COPD: Definition. http://www.who.int/respiratory/copd/definition/en/. Accessed October 17, 2016.
4. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med . 2007;176(6):532-555.
5. Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD . 2007;4(1):29-39.
6. Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr; MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc . 2003;51(7):908-916.
7. Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest . 2001;119:3.
8. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med . 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
9. Rowe BH, Bhutani M, Stickland MK, Cydulka R. Assessment and management of chronic obstructive pulmonary disease in the emergency department and beyond. Expert Rev Respir Med . 2011;5(4):549-559. doi:10.1586/ers.11.43.
10. National Institute for Clinical Excellence Web site. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline CG101. https://www.nice.org.uk/Guidance/cg101. Published June 2010. Accessed October 17, 2016.
11. Christensen VL, Holm AM, Cooper B, Paul SM, Miaskowski C, Rustøen T. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage . 2016;51(5):849-859. doi:10.1016/j.jpainsymman.2015.12.324.
12. GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease Web site. http://goldcopd.org/gold-reports/. Accessed October 17, 2016.
13. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res . 2009;10:1. doi:10.1186/1465-9921-10-1.
14. Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med . 2001;134(7):600-620.
15. McCrory DC, Brown CD. Inhaled short-acting beta 2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2001;(2):CD002984.
16. Shivanthan MC, Rajapakse S. Magnesium for acute exacerbation of chronic obstructive pulmonary disease: A systematic review of randomised trials. Ann Thorac Med . 2014;9(2):77-80. doi:10.4103/1817-1737.128844.
17. Curtis JR. Palliative and end of life care for patients with severe COPD. Eur Respir J . 2008;32(3):796-803.
18. Rocker GM, Simpson AC, Young J, et al. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open . 2013;1(1):E27-E36.
19. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax . 2002;57(11):939-944.
20. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ . 2003;327(7414):523-528.
21. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008.
22. National POLST Paradigm. http://polst.org/professionals-page/?pro=1. Accessed October 17, 2016.
23. Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49(1):90-97; discussion 97-98.
24. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis . 2008;3(1):11-29.
25. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care . 1996;12(1):5-11.
Treating agitation in schizophrenia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel