User login
How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
Anxiety is a necessary and natural reaction to trauma, but, sometimes, anxiety symptoms become excessive and problematic, as experienced with posttraumatic stress disorder (PTSD). Some patients who struggle with PTSD endure a relentless apprehension so intense that it keeps them from participating in everyday activities, such as attending work and partaking in social activities. Associated anxiety symptoms severely impair everyday function and include increased heart rate, sweating, intrusive images, poor attention, fear, or insomnia. Posttraumatic stress disorder symptoms often lead to occupational dysfunction, relationship difficulty, and numerous other functional impairments.
Approximately 300,000 veterans meet the criteria for PTSD related to ongoing or recent wars.1 The veteran does not bear the personal and functional burden alone; however, the financial load is felt throughout society. One recent study suggests that for veterans diagnosed with PTSD, the first 2 years after deployment cost society an estimated $7,000 per individial.2 Current research suggests that this potentially debilitating disorder occurs in about 14% of Operation Iraqi Freedom/Operation Enduring Freedom combat troops, whereas the similar U.S. demographic population experiences PTSD at a rate of about 7%.1,3 The ongoing military trauma exposures are compelling the mental health community to establish efficient and effective treatment options.4,5
Several treatment strategies exist to reduce PTSD symptoms, but health care professionals must seek a balance between therapeutic benefit and cost. The treatment of PTSD is diverse and variable; however, in the most recent Clinical Practice Guideline (CPG) for PTSD, the VA and DoD specifically endorse some psychotherapeutic interventions while dissuading the use of others.6 Of note, the VA and DoD CPG strongly encourages Stress Inoculation Training (SIT) and similar cognitive therapies aimed at guiding patients through the process of consciously understanding the relationship between thoughts and feelings and then modifying thoughts to appropriately manage stressors.6 Meanwhile, group psychotherapy has been determined to be “somewhat helpful.”6 Even though cognitive- and group-based therapies have long been established as efficacious for numerous psychological disorders (depression, obsessive compulsive disorder, eating disorders, etc), neither the American Group Psychotherapy Association nor the VA and DoD CPG directly endorse the use of group cognitive behavioral therapy (GCBT) for the treatment of PTSD.6,7 However, both VA and DoD mental health providers commonly practice CBT and various group psychotherapies for the treatment of PTSD.
Despite the widespread use of CBT, there is a gap in the clinical understanding of the evidence supporting GCBT for PTSD. The goal of this synthesis was to understand the efficacy of treating PTSD symptoms with group psychotherapy. To begin this investigation, the following PICO (population, intervention, comparison, outcome) question was asked: In adults diagnosed with PTSD, how effective is group cognitive behavioral therapy in reducing PTSD-related symptoms?
Methods
Research articles addressing the use of GCBT in PTSD were obtained via database searches that took place during October 2012 (Table). Searched databases included the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews Randomized Controlled Trials, Psychological Information (PsycINFO), and Public Medicine (PubMed).
The PubMed database was searched using the following MeSH (medical subject heading) terms: “psychotherapy, group” and “stress disorders, post-traumatic” and “cognitive therapy.” Limitations were set to include only patients aged ≥ 18 years, results in English, those involving human subjects, and articles published within the past 5 years. A manual search of references was also conducted, and relevant articles were retained.
Articles that addressed primary substance abuse, other DSM Axis I disorders, intimate partner violence, or family issues were excluded from the evidence sample due to concerns of an alternate treatment focus. Articles with a focus on telehealth or alternative medicine were considered confounding to the scope of this review were also excluded. It was also noted that the term CBT is used collectively for an umbrella of treatments; however, treatments that focused on elements other than the components of CBT being delivered in a group were not included. To prevent duplication of the results, research from an inclusive review was not considered individually.
SUMMARY OF EVIDENCE
Six works fulfilled the PICO criteria and were of sufficient quality to be synthesized. Of the 6 articles retained for synthesis, 2 were high-level reviews. Both reviews supported the use of GCBT for PTSD treatment. Barrera and colleagues reported an overall large effect size regardless of the presence of exposure in-group among the 12 treatment conditions and 651 study participants.8 These researchers also reported that in-group exposure did not further traumatize other group members.
Similarly, although a notably older and smaller review, Bisson and Andrew reported a significant standard mean deviation between 4 GCBT treatment and wait list controls. These reviewers did not find a significant difference between trauma- and nontrauma-focused treatment groups. The reviews also noted that individual psychotherapy and/or pharmacotherapy was most often continued throughout the reviewed studies.8,9
The 4 other studies contribute substantively to this synthesis but arguably represent lower evidence quality. A large longitudinal study of 496 Australian veterans reported a large effect size that was sustained 9 months after treatment began.10 These researchers used an intensive outpatient program that included medication and other treatment modalities as the basis for GCBT delivery. They reported that the majority of the patients revealed improvement in PTSD symptoms.
Another study sampled a similar group of 10 combat veterans but focused particular attention on sleep-related PTSD symptoms of insomnia, nightmares, and sleep quality.11 Although these researchers were unable to report a significant difference in overall PTSD symptoms for the 8 subjects who completed the protocol, they did find a large effect size on insomnia severity and a medium effect size on sleep quality. Regular treatment, including medication, continued throughout this study.
Other researchers reported a medium effect size on PTSD symptoms while using GCBT in a heterogeneous group with various anxiety disorders, including obsessive compulsive disorder, generalized anxiety disorder, social phobia, panic disorders, and PTSD.12 Although reporting similar results as all other included studies, this study has some significant limitations, including a 26% dropout rate among the 152 participants. The final study included for synthesis reported a remarkable 67% elimination of the PTSD diagnosis among 6 motor vehicle accident survivors in the small, uncontrolled study.13 Concomitant treatments, including medications, were not reported in detail for these 4 studies except as mentioned.
As a whole, the 6 studies revealed some appreciable commonalities. Time since diagnosis did not seem to influence the results. Attrition was consistently found to be similar to other PTSD treatments. The reported session topics were loosely based on common CBT tenets (ie, education, challenging cognitions, and relaxation techniques) and were typically similar among treatment groups, including the use of homework.
DISCUSSION
As the diagnosis of PTSD increases to unfamiliar levels, GCBT has the potential to be helpful to clinicians and patients seeking alternatives to their current treatments.1,4,14 The reported results imply that GCBT can be useful in PTSD symptom reduction. This could be particularly useful to VA and military providers or rural providers operating with limited resources.
Treatment protocols are not well established and should be approached with care prior to the establishment of CBT treatment groups for those diagnosed with PTSD. Session overviews and descriptions, such as those mentioned in Thompson and colleagues, could provide a reference point for future use.13
Also worth considering, CBT can be an ambiguous term requiring deliberate definition within treatment protocols. As noted in the VA and DoD CPG, exposure- and trauma-focused treatment designs can be efficacious, but these elements do not seem to be required within the GCBT treatment setting.
The current research also suggests GCBT efficacy regardless of the index trauma. This does not suggest that heterogeneous groups were frequently studied nor can conclusions be drawn regarding heterogeneous treatment groups. Elements such as group size and session length are inconsistently reported and require specific consideration as well. There is a distinct lack of research directly comparing individual CBT with GCBT directly, which prohibits meaningful conclusions regarding PTSD symptom reduction. This research gap may well have influenced the recommendations within the VA and DoD CPG. Although some higher quality studies exist, many of the published reports on GCBT have noteworthy design flaw, such as inadequate controls and statistical analysis.
LIMITATIONS
There are some limitations to this literature synthesis. Although the search was limited to the past 5 years, the inclusion of reviews accounts for older evidence. As alluded to earlier, the lack of a standardized GCBT treatment protocol challenges results comparisons as well. The consequent treatment variations make direct interstudy comparison and synthesis difficult. Similarly, outcome measures varied between studies. Also, group psychotherapy is well established and accepted. Therefore, much of the supporting research was accomplished outside the parameters of this literature search. This empirical view of group psychotherapy among mental health providers may also contribute to the lack of available research.
It is also worth noting that studies finding neutral or negative results are often unpublished. This publication bias could account for the lack of available evidence. The research reports do not consistently report therapist qualifications; however, board certificates in group psychotherapy and CBT are undeniably variables available for debate. The inclusion of uncontrolled trials limits these findings as well. Although the above limitations are not exhaustive, they do provide necessary caveats to future generalizations.
FUTURE IMPLICATIONS
Perhaps the most important information to gain from future research is that of treatment outcomes. Studies that include a detailed outcome evaluation could reveal patient satisfaction, efficacy, and financial considerations. In the presence of adequate supportive data, GCBT could contribute outcome data regarding trauma survivor symptom normalization, peer support formation, access to care, treatment efficiency, and health care resources utilization. As noted in Barrera and colleagues, future analysis will require a greater volume of trials with an overall increase in methodological rigor.8
Current research has demonstrated a solid base from which to spawn specific treatment protocols. The available research is investigational in terms of treatment procedures. Replication of these studies could dictate treatment protocol and contribute substantively to future VA and DoD CPG updates. Future researchers should consider the use of a standard PTSD symptom assessment tool to make interstudy comparisons more meaningful. The length of treatment and exposure elements should be targeted specifically in future research as these components currently vary the most.
The military represents an obvious avenue for future research due to increased PTSD diagnosis in recent years. Although the etiology of the increase in PTSD is unclear and most likely multifactorial (decreased resilience, increased awareness, increased pursuit of secondary gains, etc), the need for treatment options is apparent.1 Group cohesion has been shown to be a core component of successful group psychotherapy, so military members who are accustomed to unit cohesion might represent a uniquely suitable population for this modality.15 Interestingly and for reasons not currently understood, veterans do not see effects of therapy as large as their civilian counterparts.8 This underscores the need for further evaluation of military-specific outcomes.
CONCLUSIONS
Although the available evidence is not robust, results do support the careful use of GCBT as an effective treatment for PTSD symptom reduction.8 Group psychotherapy has been generally regarded as an efficacious and cost-effective method to achieve similar outcomes to individual therapy. Increasing PTSD prevalence compels mental health care providers to explore all available treatment options. The potential for GCBT as an option is exciting, especially for mental health providers and those with limited resources. Rising health care standards and the current national fiscal situation is dictating a reevaluation of treatment options; so perhaps all health care providers will soon consider the use of GCBT.
As with any group assignment, the clinician should carefully consider the individual’s suitability and desire for group participation.16 With GCBT, providers could facilitate the relief of relentless apprehension and functional impairment for several patients simultaneously. Although there are many details left to explore regarding the use of GCBT for PTSD, the therapy’s foundation for use as a PTSD treatment is apparent.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Litz B, Schlenger W. Posttraumatic stress disorder in service members and new veterans of the Iraq and Afghanistan wars: A bibliography and critique. PTSD Res Q. 2009;20(1):1-3.
2. Tanielian T. Assessing combat exposure and post-traumatic stress disorder in troops and estimating the costs to society: Implications from the RAND Invisible Wounds of War Study. http://www.rand.org/pubs/testimonies/CT321.html. Published 2009. Accessed September 29, 2014.
3. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychol Rev. 2009;29(8):715-726.
5. Hoge CW. Interventions for war-related posttraumatic stress disorder: Meeting veterans where they are. JAMA. 2011;306(5):549-551.
6. Veterans Health Administration, Department of Defense. VA/DoD Clinical Practice Guideline: Management of Post-Traumatic Stress, Version 2.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010.
7. Burlingame GM, Fuhriman A, Mosier J. The differential effectiveness of group psychotherapy: A meta-analytic perspective. Group Dyn. 2003;7(1):3-12.
8. Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24-32.
9. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
10. Khoo A, Dent MT, Oei TS. (2011). Group cognitive behaviour therapy for military service-related post-traumatic stress disorder: Effectiveness, sustainability and repeatability. Aust N Z J Psychiatry. 2011;45(8):663-672.
11. Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. J Trauma Stress. 2009;22(6):639-642.
12. Erickson DH, Janeck A, Tallman K. A cognitive-behavioral group for patients with various anxiety disorders. Psychiatr Serv. 2007;58(9):1205-1211.
13. Thompson AR, Wilde E, Boon K. The development of group CBT for the treatment of road-traffic-accident-related post-traumatic stress disorder: A pilot study. Cognitive Behav Therapist. 2010;2(1):32-42.
14. Slade T, Johnston A, Oakley-Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594-605.
15. Crowe TP, Grenyer BF. Is therapist alliance or whole group cohesion more influential in group psychotherapy outcomes? Clin Psychol Psychother. 2008;15(4):239-246.
16. Leszcz M, Kobos JC. Evidence-based group psychotherapy: Using AGPA’s practice guidelines to enhance clinical effectiveness. J Clin Psychol. 2008;64(11):1238-1260.
Anxiety is a necessary and natural reaction to trauma, but, sometimes, anxiety symptoms become excessive and problematic, as experienced with posttraumatic stress disorder (PTSD). Some patients who struggle with PTSD endure a relentless apprehension so intense that it keeps them from participating in everyday activities, such as attending work and partaking in social activities. Associated anxiety symptoms severely impair everyday function and include increased heart rate, sweating, intrusive images, poor attention, fear, or insomnia. Posttraumatic stress disorder symptoms often lead to occupational dysfunction, relationship difficulty, and numerous other functional impairments.
Approximately 300,000 veterans meet the criteria for PTSD related to ongoing or recent wars.1 The veteran does not bear the personal and functional burden alone; however, the financial load is felt throughout society. One recent study suggests that for veterans diagnosed with PTSD, the first 2 years after deployment cost society an estimated $7,000 per individial.2 Current research suggests that this potentially debilitating disorder occurs in about 14% of Operation Iraqi Freedom/Operation Enduring Freedom combat troops, whereas the similar U.S. demographic population experiences PTSD at a rate of about 7%.1,3 The ongoing military trauma exposures are compelling the mental health community to establish efficient and effective treatment options.4,5
Several treatment strategies exist to reduce PTSD symptoms, but health care professionals must seek a balance between therapeutic benefit and cost. The treatment of PTSD is diverse and variable; however, in the most recent Clinical Practice Guideline (CPG) for PTSD, the VA and DoD specifically endorse some psychotherapeutic interventions while dissuading the use of others.6 Of note, the VA and DoD CPG strongly encourages Stress Inoculation Training (SIT) and similar cognitive therapies aimed at guiding patients through the process of consciously understanding the relationship between thoughts and feelings and then modifying thoughts to appropriately manage stressors.6 Meanwhile, group psychotherapy has been determined to be “somewhat helpful.”6 Even though cognitive- and group-based therapies have long been established as efficacious for numerous psychological disorders (depression, obsessive compulsive disorder, eating disorders, etc), neither the American Group Psychotherapy Association nor the VA and DoD CPG directly endorse the use of group cognitive behavioral therapy (GCBT) for the treatment of PTSD.6,7 However, both VA and DoD mental health providers commonly practice CBT and various group psychotherapies for the treatment of PTSD.
Despite the widespread use of CBT, there is a gap in the clinical understanding of the evidence supporting GCBT for PTSD. The goal of this synthesis was to understand the efficacy of treating PTSD symptoms with group psychotherapy. To begin this investigation, the following PICO (population, intervention, comparison, outcome) question was asked: In adults diagnosed with PTSD, how effective is group cognitive behavioral therapy in reducing PTSD-related symptoms?
Methods
Research articles addressing the use of GCBT in PTSD were obtained via database searches that took place during October 2012 (Table). Searched databases included the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews Randomized Controlled Trials, Psychological Information (PsycINFO), and Public Medicine (PubMed).
The PubMed database was searched using the following MeSH (medical subject heading) terms: “psychotherapy, group” and “stress disorders, post-traumatic” and “cognitive therapy.” Limitations were set to include only patients aged ≥ 18 years, results in English, those involving human subjects, and articles published within the past 5 years. A manual search of references was also conducted, and relevant articles were retained.
Articles that addressed primary substance abuse, other DSM Axis I disorders, intimate partner violence, or family issues were excluded from the evidence sample due to concerns of an alternate treatment focus. Articles with a focus on telehealth or alternative medicine were considered confounding to the scope of this review were also excluded. It was also noted that the term CBT is used collectively for an umbrella of treatments; however, treatments that focused on elements other than the components of CBT being delivered in a group were not included. To prevent duplication of the results, research from an inclusive review was not considered individually.
SUMMARY OF EVIDENCE
Six works fulfilled the PICO criteria and were of sufficient quality to be synthesized. Of the 6 articles retained for synthesis, 2 were high-level reviews. Both reviews supported the use of GCBT for PTSD treatment. Barrera and colleagues reported an overall large effect size regardless of the presence of exposure in-group among the 12 treatment conditions and 651 study participants.8 These researchers also reported that in-group exposure did not further traumatize other group members.
Similarly, although a notably older and smaller review, Bisson and Andrew reported a significant standard mean deviation between 4 GCBT treatment and wait list controls. These reviewers did not find a significant difference between trauma- and nontrauma-focused treatment groups. The reviews also noted that individual psychotherapy and/or pharmacotherapy was most often continued throughout the reviewed studies.8,9
The 4 other studies contribute substantively to this synthesis but arguably represent lower evidence quality. A large longitudinal study of 496 Australian veterans reported a large effect size that was sustained 9 months after treatment began.10 These researchers used an intensive outpatient program that included medication and other treatment modalities as the basis for GCBT delivery. They reported that the majority of the patients revealed improvement in PTSD symptoms.
Another study sampled a similar group of 10 combat veterans but focused particular attention on sleep-related PTSD symptoms of insomnia, nightmares, and sleep quality.11 Although these researchers were unable to report a significant difference in overall PTSD symptoms for the 8 subjects who completed the protocol, they did find a large effect size on insomnia severity and a medium effect size on sleep quality. Regular treatment, including medication, continued throughout this study.
Other researchers reported a medium effect size on PTSD symptoms while using GCBT in a heterogeneous group with various anxiety disorders, including obsessive compulsive disorder, generalized anxiety disorder, social phobia, panic disorders, and PTSD.12 Although reporting similar results as all other included studies, this study has some significant limitations, including a 26% dropout rate among the 152 participants. The final study included for synthesis reported a remarkable 67% elimination of the PTSD diagnosis among 6 motor vehicle accident survivors in the small, uncontrolled study.13 Concomitant treatments, including medications, were not reported in detail for these 4 studies except as mentioned.
As a whole, the 6 studies revealed some appreciable commonalities. Time since diagnosis did not seem to influence the results. Attrition was consistently found to be similar to other PTSD treatments. The reported session topics were loosely based on common CBT tenets (ie, education, challenging cognitions, and relaxation techniques) and were typically similar among treatment groups, including the use of homework.
DISCUSSION
As the diagnosis of PTSD increases to unfamiliar levels, GCBT has the potential to be helpful to clinicians and patients seeking alternatives to their current treatments.1,4,14 The reported results imply that GCBT can be useful in PTSD symptom reduction. This could be particularly useful to VA and military providers or rural providers operating with limited resources.
Treatment protocols are not well established and should be approached with care prior to the establishment of CBT treatment groups for those diagnosed with PTSD. Session overviews and descriptions, such as those mentioned in Thompson and colleagues, could provide a reference point for future use.13
Also worth considering, CBT can be an ambiguous term requiring deliberate definition within treatment protocols. As noted in the VA and DoD CPG, exposure- and trauma-focused treatment designs can be efficacious, but these elements do not seem to be required within the GCBT treatment setting.
The current research also suggests GCBT efficacy regardless of the index trauma. This does not suggest that heterogeneous groups were frequently studied nor can conclusions be drawn regarding heterogeneous treatment groups. Elements such as group size and session length are inconsistently reported and require specific consideration as well. There is a distinct lack of research directly comparing individual CBT with GCBT directly, which prohibits meaningful conclusions regarding PTSD symptom reduction. This research gap may well have influenced the recommendations within the VA and DoD CPG. Although some higher quality studies exist, many of the published reports on GCBT have noteworthy design flaw, such as inadequate controls and statistical analysis.
LIMITATIONS
There are some limitations to this literature synthesis. Although the search was limited to the past 5 years, the inclusion of reviews accounts for older evidence. As alluded to earlier, the lack of a standardized GCBT treatment protocol challenges results comparisons as well. The consequent treatment variations make direct interstudy comparison and synthesis difficult. Similarly, outcome measures varied between studies. Also, group psychotherapy is well established and accepted. Therefore, much of the supporting research was accomplished outside the parameters of this literature search. This empirical view of group psychotherapy among mental health providers may also contribute to the lack of available research.
It is also worth noting that studies finding neutral or negative results are often unpublished. This publication bias could account for the lack of available evidence. The research reports do not consistently report therapist qualifications; however, board certificates in group psychotherapy and CBT are undeniably variables available for debate. The inclusion of uncontrolled trials limits these findings as well. Although the above limitations are not exhaustive, they do provide necessary caveats to future generalizations.
FUTURE IMPLICATIONS
Perhaps the most important information to gain from future research is that of treatment outcomes. Studies that include a detailed outcome evaluation could reveal patient satisfaction, efficacy, and financial considerations. In the presence of adequate supportive data, GCBT could contribute outcome data regarding trauma survivor symptom normalization, peer support formation, access to care, treatment efficiency, and health care resources utilization. As noted in Barrera and colleagues, future analysis will require a greater volume of trials with an overall increase in methodological rigor.8
Current research has demonstrated a solid base from which to spawn specific treatment protocols. The available research is investigational in terms of treatment procedures. Replication of these studies could dictate treatment protocol and contribute substantively to future VA and DoD CPG updates. Future researchers should consider the use of a standard PTSD symptom assessment tool to make interstudy comparisons more meaningful. The length of treatment and exposure elements should be targeted specifically in future research as these components currently vary the most.
The military represents an obvious avenue for future research due to increased PTSD diagnosis in recent years. Although the etiology of the increase in PTSD is unclear and most likely multifactorial (decreased resilience, increased awareness, increased pursuit of secondary gains, etc), the need for treatment options is apparent.1 Group cohesion has been shown to be a core component of successful group psychotherapy, so military members who are accustomed to unit cohesion might represent a uniquely suitable population for this modality.15 Interestingly and for reasons not currently understood, veterans do not see effects of therapy as large as their civilian counterparts.8 This underscores the need for further evaluation of military-specific outcomes.
CONCLUSIONS
Although the available evidence is not robust, results do support the careful use of GCBT as an effective treatment for PTSD symptom reduction.8 Group psychotherapy has been generally regarded as an efficacious and cost-effective method to achieve similar outcomes to individual therapy. Increasing PTSD prevalence compels mental health care providers to explore all available treatment options. The potential for GCBT as an option is exciting, especially for mental health providers and those with limited resources. Rising health care standards and the current national fiscal situation is dictating a reevaluation of treatment options; so perhaps all health care providers will soon consider the use of GCBT.
As with any group assignment, the clinician should carefully consider the individual’s suitability and desire for group participation.16 With GCBT, providers could facilitate the relief of relentless apprehension and functional impairment for several patients simultaneously. Although there are many details left to explore regarding the use of GCBT for PTSD, the therapy’s foundation for use as a PTSD treatment is apparent.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Anxiety is a necessary and natural reaction to trauma, but, sometimes, anxiety symptoms become excessive and problematic, as experienced with posttraumatic stress disorder (PTSD). Some patients who struggle with PTSD endure a relentless apprehension so intense that it keeps them from participating in everyday activities, such as attending work and partaking in social activities. Associated anxiety symptoms severely impair everyday function and include increased heart rate, sweating, intrusive images, poor attention, fear, or insomnia. Posttraumatic stress disorder symptoms often lead to occupational dysfunction, relationship difficulty, and numerous other functional impairments.
Approximately 300,000 veterans meet the criteria for PTSD related to ongoing or recent wars.1 The veteran does not bear the personal and functional burden alone; however, the financial load is felt throughout society. One recent study suggests that for veterans diagnosed with PTSD, the first 2 years after deployment cost society an estimated $7,000 per individial.2 Current research suggests that this potentially debilitating disorder occurs in about 14% of Operation Iraqi Freedom/Operation Enduring Freedom combat troops, whereas the similar U.S. demographic population experiences PTSD at a rate of about 7%.1,3 The ongoing military trauma exposures are compelling the mental health community to establish efficient and effective treatment options.4,5
Several treatment strategies exist to reduce PTSD symptoms, but health care professionals must seek a balance between therapeutic benefit and cost. The treatment of PTSD is diverse and variable; however, in the most recent Clinical Practice Guideline (CPG) for PTSD, the VA and DoD specifically endorse some psychotherapeutic interventions while dissuading the use of others.6 Of note, the VA and DoD CPG strongly encourages Stress Inoculation Training (SIT) and similar cognitive therapies aimed at guiding patients through the process of consciously understanding the relationship between thoughts and feelings and then modifying thoughts to appropriately manage stressors.6 Meanwhile, group psychotherapy has been determined to be “somewhat helpful.”6 Even though cognitive- and group-based therapies have long been established as efficacious for numerous psychological disorders (depression, obsessive compulsive disorder, eating disorders, etc), neither the American Group Psychotherapy Association nor the VA and DoD CPG directly endorse the use of group cognitive behavioral therapy (GCBT) for the treatment of PTSD.6,7 However, both VA and DoD mental health providers commonly practice CBT and various group psychotherapies for the treatment of PTSD.
Despite the widespread use of CBT, there is a gap in the clinical understanding of the evidence supporting GCBT for PTSD. The goal of this synthesis was to understand the efficacy of treating PTSD symptoms with group psychotherapy. To begin this investigation, the following PICO (population, intervention, comparison, outcome) question was asked: In adults diagnosed with PTSD, how effective is group cognitive behavioral therapy in reducing PTSD-related symptoms?
Methods
Research articles addressing the use of GCBT in PTSD were obtained via database searches that took place during October 2012 (Table). Searched databases included the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews Randomized Controlled Trials, Psychological Information (PsycINFO), and Public Medicine (PubMed).
The PubMed database was searched using the following MeSH (medical subject heading) terms: “psychotherapy, group” and “stress disorders, post-traumatic” and “cognitive therapy.” Limitations were set to include only patients aged ≥ 18 years, results in English, those involving human subjects, and articles published within the past 5 years. A manual search of references was also conducted, and relevant articles were retained.
Articles that addressed primary substance abuse, other DSM Axis I disorders, intimate partner violence, or family issues were excluded from the evidence sample due to concerns of an alternate treatment focus. Articles with a focus on telehealth or alternative medicine were considered confounding to the scope of this review were also excluded. It was also noted that the term CBT is used collectively for an umbrella of treatments; however, treatments that focused on elements other than the components of CBT being delivered in a group were not included. To prevent duplication of the results, research from an inclusive review was not considered individually.
SUMMARY OF EVIDENCE
Six works fulfilled the PICO criteria and were of sufficient quality to be synthesized. Of the 6 articles retained for synthesis, 2 were high-level reviews. Both reviews supported the use of GCBT for PTSD treatment. Barrera and colleagues reported an overall large effect size regardless of the presence of exposure in-group among the 12 treatment conditions and 651 study participants.8 These researchers also reported that in-group exposure did not further traumatize other group members.
Similarly, although a notably older and smaller review, Bisson and Andrew reported a significant standard mean deviation between 4 GCBT treatment and wait list controls. These reviewers did not find a significant difference between trauma- and nontrauma-focused treatment groups. The reviews also noted that individual psychotherapy and/or pharmacotherapy was most often continued throughout the reviewed studies.8,9
The 4 other studies contribute substantively to this synthesis but arguably represent lower evidence quality. A large longitudinal study of 496 Australian veterans reported a large effect size that was sustained 9 months after treatment began.10 These researchers used an intensive outpatient program that included medication and other treatment modalities as the basis for GCBT delivery. They reported that the majority of the patients revealed improvement in PTSD symptoms.
Another study sampled a similar group of 10 combat veterans but focused particular attention on sleep-related PTSD symptoms of insomnia, nightmares, and sleep quality.11 Although these researchers were unable to report a significant difference in overall PTSD symptoms for the 8 subjects who completed the protocol, they did find a large effect size on insomnia severity and a medium effect size on sleep quality. Regular treatment, including medication, continued throughout this study.
Other researchers reported a medium effect size on PTSD symptoms while using GCBT in a heterogeneous group with various anxiety disorders, including obsessive compulsive disorder, generalized anxiety disorder, social phobia, panic disorders, and PTSD.12 Although reporting similar results as all other included studies, this study has some significant limitations, including a 26% dropout rate among the 152 participants. The final study included for synthesis reported a remarkable 67% elimination of the PTSD diagnosis among 6 motor vehicle accident survivors in the small, uncontrolled study.13 Concomitant treatments, including medications, were not reported in detail for these 4 studies except as mentioned.
As a whole, the 6 studies revealed some appreciable commonalities. Time since diagnosis did not seem to influence the results. Attrition was consistently found to be similar to other PTSD treatments. The reported session topics were loosely based on common CBT tenets (ie, education, challenging cognitions, and relaxation techniques) and were typically similar among treatment groups, including the use of homework.
DISCUSSION
As the diagnosis of PTSD increases to unfamiliar levels, GCBT has the potential to be helpful to clinicians and patients seeking alternatives to their current treatments.1,4,14 The reported results imply that GCBT can be useful in PTSD symptom reduction. This could be particularly useful to VA and military providers or rural providers operating with limited resources.
Treatment protocols are not well established and should be approached with care prior to the establishment of CBT treatment groups for those diagnosed with PTSD. Session overviews and descriptions, such as those mentioned in Thompson and colleagues, could provide a reference point for future use.13
Also worth considering, CBT can be an ambiguous term requiring deliberate definition within treatment protocols. As noted in the VA and DoD CPG, exposure- and trauma-focused treatment designs can be efficacious, but these elements do not seem to be required within the GCBT treatment setting.
The current research also suggests GCBT efficacy regardless of the index trauma. This does not suggest that heterogeneous groups were frequently studied nor can conclusions be drawn regarding heterogeneous treatment groups. Elements such as group size and session length are inconsistently reported and require specific consideration as well. There is a distinct lack of research directly comparing individual CBT with GCBT directly, which prohibits meaningful conclusions regarding PTSD symptom reduction. This research gap may well have influenced the recommendations within the VA and DoD CPG. Although some higher quality studies exist, many of the published reports on GCBT have noteworthy design flaw, such as inadequate controls and statistical analysis.
LIMITATIONS
There are some limitations to this literature synthesis. Although the search was limited to the past 5 years, the inclusion of reviews accounts for older evidence. As alluded to earlier, the lack of a standardized GCBT treatment protocol challenges results comparisons as well. The consequent treatment variations make direct interstudy comparison and synthesis difficult. Similarly, outcome measures varied between studies. Also, group psychotherapy is well established and accepted. Therefore, much of the supporting research was accomplished outside the parameters of this literature search. This empirical view of group psychotherapy among mental health providers may also contribute to the lack of available research.
It is also worth noting that studies finding neutral or negative results are often unpublished. This publication bias could account for the lack of available evidence. The research reports do not consistently report therapist qualifications; however, board certificates in group psychotherapy and CBT are undeniably variables available for debate. The inclusion of uncontrolled trials limits these findings as well. Although the above limitations are not exhaustive, they do provide necessary caveats to future generalizations.
FUTURE IMPLICATIONS
Perhaps the most important information to gain from future research is that of treatment outcomes. Studies that include a detailed outcome evaluation could reveal patient satisfaction, efficacy, and financial considerations. In the presence of adequate supportive data, GCBT could contribute outcome data regarding trauma survivor symptom normalization, peer support formation, access to care, treatment efficiency, and health care resources utilization. As noted in Barrera and colleagues, future analysis will require a greater volume of trials with an overall increase in methodological rigor.8
Current research has demonstrated a solid base from which to spawn specific treatment protocols. The available research is investigational in terms of treatment procedures. Replication of these studies could dictate treatment protocol and contribute substantively to future VA and DoD CPG updates. Future researchers should consider the use of a standard PTSD symptom assessment tool to make interstudy comparisons more meaningful. The length of treatment and exposure elements should be targeted specifically in future research as these components currently vary the most.
The military represents an obvious avenue for future research due to increased PTSD diagnosis in recent years. Although the etiology of the increase in PTSD is unclear and most likely multifactorial (decreased resilience, increased awareness, increased pursuit of secondary gains, etc), the need for treatment options is apparent.1 Group cohesion has been shown to be a core component of successful group psychotherapy, so military members who are accustomed to unit cohesion might represent a uniquely suitable population for this modality.15 Interestingly and for reasons not currently understood, veterans do not see effects of therapy as large as their civilian counterparts.8 This underscores the need for further evaluation of military-specific outcomes.
CONCLUSIONS
Although the available evidence is not robust, results do support the careful use of GCBT as an effective treatment for PTSD symptom reduction.8 Group psychotherapy has been generally regarded as an efficacious and cost-effective method to achieve similar outcomes to individual therapy. Increasing PTSD prevalence compels mental health care providers to explore all available treatment options. The potential for GCBT as an option is exciting, especially for mental health providers and those with limited resources. Rising health care standards and the current national fiscal situation is dictating a reevaluation of treatment options; so perhaps all health care providers will soon consider the use of GCBT.
As with any group assignment, the clinician should carefully consider the individual’s suitability and desire for group participation.16 With GCBT, providers could facilitate the relief of relentless apprehension and functional impairment for several patients simultaneously. Although there are many details left to explore regarding the use of GCBT for PTSD, the therapy’s foundation for use as a PTSD treatment is apparent.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Litz B, Schlenger W. Posttraumatic stress disorder in service members and new veterans of the Iraq and Afghanistan wars: A bibliography and critique. PTSD Res Q. 2009;20(1):1-3.
2. Tanielian T. Assessing combat exposure and post-traumatic stress disorder in troops and estimating the costs to society: Implications from the RAND Invisible Wounds of War Study. http://www.rand.org/pubs/testimonies/CT321.html. Published 2009. Accessed September 29, 2014.
3. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychol Rev. 2009;29(8):715-726.
5. Hoge CW. Interventions for war-related posttraumatic stress disorder: Meeting veterans where they are. JAMA. 2011;306(5):549-551.
6. Veterans Health Administration, Department of Defense. VA/DoD Clinical Practice Guideline: Management of Post-Traumatic Stress, Version 2.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010.
7. Burlingame GM, Fuhriman A, Mosier J. The differential effectiveness of group psychotherapy: A meta-analytic perspective. Group Dyn. 2003;7(1):3-12.
8. Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24-32.
9. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
10. Khoo A, Dent MT, Oei TS. (2011). Group cognitive behaviour therapy for military service-related post-traumatic stress disorder: Effectiveness, sustainability and repeatability. Aust N Z J Psychiatry. 2011;45(8):663-672.
11. Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. J Trauma Stress. 2009;22(6):639-642.
12. Erickson DH, Janeck A, Tallman K. A cognitive-behavioral group for patients with various anxiety disorders. Psychiatr Serv. 2007;58(9):1205-1211.
13. Thompson AR, Wilde E, Boon K. The development of group CBT for the treatment of road-traffic-accident-related post-traumatic stress disorder: A pilot study. Cognitive Behav Therapist. 2010;2(1):32-42.
14. Slade T, Johnston A, Oakley-Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594-605.
15. Crowe TP, Grenyer BF. Is therapist alliance or whole group cohesion more influential in group psychotherapy outcomes? Clin Psychol Psychother. 2008;15(4):239-246.
16. Leszcz M, Kobos JC. Evidence-based group psychotherapy: Using AGPA’s practice guidelines to enhance clinical effectiveness. J Clin Psychol. 2008;64(11):1238-1260.
1. Litz B, Schlenger W. Posttraumatic stress disorder in service members and new veterans of the Iraq and Afghanistan wars: A bibliography and critique. PTSD Res Q. 2009;20(1):1-3.
2. Tanielian T. Assessing combat exposure and post-traumatic stress disorder in troops and estimating the costs to society: Implications from the RAND Invisible Wounds of War Study. http://www.rand.org/pubs/testimonies/CT321.html. Published 2009. Accessed September 29, 2014.
3. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychol Rev. 2009;29(8):715-726.
5. Hoge CW. Interventions for war-related posttraumatic stress disorder: Meeting veterans where they are. JAMA. 2011;306(5):549-551.
6. Veterans Health Administration, Department of Defense. VA/DoD Clinical Practice Guideline: Management of Post-Traumatic Stress, Version 2.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010.
7. Burlingame GM, Fuhriman A, Mosier J. The differential effectiveness of group psychotherapy: A meta-analytic perspective. Group Dyn. 2003;7(1):3-12.
8. Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24-32.
9. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
10. Khoo A, Dent MT, Oei TS. (2011). Group cognitive behaviour therapy for military service-related post-traumatic stress disorder: Effectiveness, sustainability and repeatability. Aust N Z J Psychiatry. 2011;45(8):663-672.
11. Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. J Trauma Stress. 2009;22(6):639-642.
12. Erickson DH, Janeck A, Tallman K. A cognitive-behavioral group for patients with various anxiety disorders. Psychiatr Serv. 2007;58(9):1205-1211.
13. Thompson AR, Wilde E, Boon K. The development of group CBT for the treatment of road-traffic-accident-related post-traumatic stress disorder: A pilot study. Cognitive Behav Therapist. 2010;2(1):32-42.
14. Slade T, Johnston A, Oakley-Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594-605.
15. Crowe TP, Grenyer BF. Is therapist alliance or whole group cohesion more influential in group psychotherapy outcomes? Clin Psychol Psychother. 2008;15(4):239-246.
16. Leszcz M, Kobos JC. Evidence-based group psychotherapy: Using AGPA’s practice guidelines to enhance clinical effectiveness. J Clin Psychol. 2008;64(11):1238-1260.
Infectious Arthritis of Native and Prosthetic Joints
Introduction
Acute bacterial arthritis is a potentially serious and rapidly progressive infection that may involve native or prosthetic joints. The epidemiology, pathophysiology, repertoire of potential infecting pathogens, clinical presentation and treatment differ for these two forms of infectious arthritis, but both are associated with significant morbidity and mortality. Infectious arthritis of native and prosthetic joints may be caused by viruses, or fungi, but the most common cause is bacteria.
Acute Bacterial Arthritis
Epidemiology
The burden of septic arthritis in the general population is considerable. The incidence of native joint septic arthritis is approximately 5 cases per 100,000 persons per year and is much higher in patients with rheumatoid arthritis (1,2). Between 1% and 5% of joints with indwelling prostheses become infected and the total number of infections per year is increasing due to a rise in the number of patients who have had prosthetic replacement surgery (3). The mortality from joint infection is difficult to estimate due to differing comorbidity in afflicted patients, but is likely between 15% and 30% (4-6). There is substantial morbidity from these infections because of decreased joint function and mobility, and in cases involving joint prostheses from the excisional or exchange arthroplastic surgery that is often required for treatment.
The most common route of infection for native joint infection is hematogenous (1), but may also be a result of direct inoculation of bacteria through trauma or joint surgery (including arthrocentesis, corticosteroid injection, or arthroscopy) (7), or via contiguous spread from adjacent infected soft tissue or bone (1,8). While hematogenous infection of prosthetic joints occurs, the majority of these infections are the result of joint contamination in the course of implantation surgery or post-surgical wound infection (3). Host factors that increase the risk of septic arthritis include pre-existing joint disease (especially rheumatoid arthritis), immunosuppression, diabetes mellitus, malignancy, chronic renal failure, intravenous drug use, severe skin diseases, and advanced age (1,2,4,6). The extent of joint injury resulting from infection depends on the virulence of the infecting pathogen and degree of host immune response (9).
Microbiology
Native Joint
The most common causes of bacterial septic arthritis are outlined in Table 1. In adults, the most frequent etiology is S. aureus (37–65% of cases) (1,4,6,8,12,15,16) followed by Streptococcus sp. (12,15). An increasing percentage of S. aureus isolated from septic joints are resistant to antistaphylococcal penicillins and cephalosporins (methicillin-resistant S. aureus, MRSA). In adults with diabetes, malignancy, and genitourinary structural abnormalities, group B Streptococcus is a frequently isolated pathogen (5,6,17). Gram-negative bacilli are commonly found in neonates, intravenous drug users, and immunocompromised hosts (18). N. gonorrhoeae is a significant cause of bacterial arthritis in sexually active adults and adolescents (19) and Kingella kingae and Haemophilus influenzae are likely pediatric isolates (20,21). Joint infections that follow bite trauma usually are seen in the small joints of the hand and involve Pasteurella multocida in the case of animal bites, and Eikenella corrodens in the case of humans bites (22-24). Polymicrobial floras are found in up to 8% of cases of septic arthritis.
Prosthetic Joint
The bacteria that cause prosthetic joint arthritis vary depending on the stage of infection as defined by the elapsed time after implantation surgery (Table 1 on page 31). The coagulase negative staphylococci are the most common (30–43% of cases) (3,10), followed by S. aureus (12–23%) (25).
Nonbacterial Pathogens
Nonbacterial pathogens that may cause septic arthritis include viruses, fungi, and mycobacteria. Viral arthritis is often associated with a systemic febrile illness and other manifestations of infection such as rash. Parvovirus B19 is the most common viral arthritide, presenting as a symmetric polyarticular arthritis involving the joints of the hand as well as larger joints (26). The classic red “slapped cheeks” associated with this viral infection in children is usually not present in adults, although a faint lacy reticular rash may be seen.
Fungi and mycobacteria usually cause a subacute or chronic mono- or oligoarticular arthritis (27). Candida species are an increasing cause of both native and prosthetic joint septic arthritis. Risk factors for this infection include loss of skin integrity, diabetes, malignancy, intravenous drug use, and immunosuppressive therapy including glucocorticoids (28). Patients are often chronically ill and have exposure to broad-spectrum antimicrobials, hyperalimentation fluid, and/or indwelling central intravenous catheters. Other fungi, including Cryptococcus, Blastomyces, Histoplasma, Coccidioides, and Sporothrix are rare causes of septic arthritis (29,30). Mycobacterium tuberculosis is the most common cause of mycobacterial arthritis worldwide and should be considered in a patient presenting with chronic arthritis with risk factors for tuberculosis, including being foreign-born (31).
Clinical Features
The clinical manifestations, severity, treatment, and prognosis of septic arthritis are dependent on the identity and virulence of the bacterium, source of joint infection, and underlying host factors. Nongonococcal septic arthritis is monoarticular in 80% to 90% of cases. The knee is usually affected (50% of cases) (27) followed by the hip, wrists, and ankles (2). In adults, the majority of hip infections involve prosthetic or osteosynthetic material (1). Arthritis of the small joints of the foot is most often seen in diabetic patients and is usually secondary to contiguous skin and soft tissue ulcerations or adjacent osteomyelitis.
Gonococcal arthritis may present as febrile monoarticular arthritis, usually of the knees, wrists, and ankles (27), or as one of the manifestations of disseminated gonococcal infection. The latter is characterized by fever, dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis (19). Skin lesions are often pustular and occur simultaneously with tenosynovitis, predominately affecting the fingers, hands, wrists, or feet. Concomitant mucosal infection of the urethra or cervix is often present but usually asymptomatic. Urethral and cervical cultures or a nucleic amplification test will frequently yield N. gonorrhoeae (19,32).
Symptoms of acute septic arthritis include pain and loss of joint function. Fever and chills are often present. The acutely infected native joint is usually red, warm, and swollen with an obvious effusion. Range of motion is limited and extremely painful. For deep and axial joint, pain is often the only focal symptom. More subtle symptoms and signs may result in a delay of diagnosis and are particularly seen in patients receiving systemic or intra-articular steroids, and in those with immunocompromised status, advanced comorbidities (including rheumatoid arthritis), and extreme age (33). A thorough physical examination may reveal a distant source of joint infection in up to 50% of patients (27).
Prosthetic joint infection may present acutely as above, particularly in early stage infection, or more indolently with progressive joint pain, minimal swelling or effusion, and absence of fever (34). In late infection a cutaneous draining sinus tract may be present. Rarely, the involved prosthesis may be visible beneath an ulceration or focus of soft-tissue breakdown.
Diseases that can mimic septic arthritis are crystalinduced arthritis, rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, Still’s disease, rheumatic fever, and Kawasaki syndrome.
Diagnostic Approach
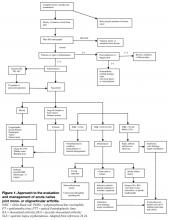
A diagnostic approach to acute native joint arthritis is outlined in Figure 1 on page 32 (35,36). Important aspects include exclusion of other causes of arthritis including trauma, rheumatic diseases, and crystalline arthritis. The most important diagnostic test upon which management hinges is diagnostic arthrocentesis. Fluoroscopic or CT-guided arthrocentesis is indicated for axial and deep joints (e.g., sacroiliac or pubic symphysis) or in the event of a “dry tap” of a peripheral joint. Synovial fluid analysis will often suggest whether an acutely painful joint is due to noninflammatory, sterile inflammatory, or septic causes (Table 2 on page 33). In addition, it will provide fluid for culture and gram stain, a rapid test that can guide early empiric antibiotic therapy. Bacterial, fungal, and mycobacterial cultures should always be performed in order to direct pathogen-specific antimicrobial therapy, which is often given as a prolonged course. Antimicrobial therapy should be delayed until arthrocentesis and other appropriate diagnostic cultures are obtained unless the patient shows signs of sepsis.
For prosthetic joint infections the diagnostic approach is essentially the same although early radiographic imaging is more important than in native joint infection as it may show signs of prosthesis failure or loosening (seen in many late prosthesis infections). Additionally, the synovial fluid white blood cell (WBC) is often lower than in nativejoint infection, with a diagnostic cutoff suggested as greater than 1,700 cells/mm3 or >65% neutrophils (37).
Nonspecific blood tests such as a white blood count, erythrocyte sedimentation rate, or C-reactive protein argue against joint infection if they are normal, but do not specifically suggest septic arthritis if elevated. Other important diagnostic tests include blood cultures (positive in 50–70% of acute bacterial arthritides) (27), but in only 30% or less of gonococcal arthritis cases) (38), wound cultures (although these often correlate poorly with synovial fluid culture results, except when the pathogen is S. aureus), and serologic testing for B. burgdorferi in selected cases with clinical features of Lyme arthritis in endemic areas. If gonococcal arthritis is suspected urethral and cervical specimens should be sent for N. gonorrhoeae culture and nucleic acid amplification tests. Radiographic and scintillographic imaging may yield additional information that will assist in identifying preexisting joint disease or for confirming a diagnosis of native or prosthetic joint infection or its complications (Table 3 on page 33).
Treatment
Native joint
Prompt joint drainage and antimicrobial therapy are the mainstays of treatment in bacterial, fungal, or mycobacterial joint infection. Drainage can be through closed needle aspiration performed daily, or arthroscopy. The former modality allows direct visual inspection of the joint with concomitant irrigation, lysis of adhesions, and removal of necrotic tissue and purulent material (42). Open surgical drainage is recommended for septic arthritis of the hip and when less invasive methods fail to control infection.
Initial antimicrobial therapy should be withheld until synovial fluid has been obtained and should be based on synovial fluid gram staining (Table 4). In the case of a nondiagnostic gram stain, empiric antimicrobial coverage of likely infecting pathogens is indicated. Therapy should be narrowed based on identification and antimicrobial susceptibility testing of bacteria cultured from synovial fluid, blood, or in some cases from ancillary cultures. For patients with MRSA-related infection who are allergic to or intolerant of vancomycin, linezolid or daptomycin are potential alternatives, although not approved by the U.S. Food and Drug Administration for this indication. Linezolid is a potentially attractive option for treatment as it is available as an oral tablet, but for bone and joint infection treatment experience is limited. For septic arthritis related to animal or human bites ampicillin-sulbactam or amoxicillin-clavulanate (clindamycin plus ciprofloxacin in penicillin-allergic patients) provides activity against Pasteurella multocida and other oral bacteria. Gonococcal arthritis is best treated initially with ceftriaxone or cefotaxime; oral ciprofloxacin or levofloxacin may be substituted in regions without fluoroquinolone resistance as the patient improves (Table 4). Septic arthritis due to Candida sp. should be treated initially with an amphotericin B preparation followed by a prolonged course of fluconazole if susceptibility testing confirms activity against the cultured yeast isolate (43).
Duration of intravenous antimicrobials for bacterial joint infections is usually 2 to 4 weeks, while for gonococcal arthritis 2 weeks is sufficient. Antimicrobial therapy that continues for 2 weeks or longer should have weekly followup and laboratory monitoring for hematologic, renal, and liver toxicity.
Prosthetic Joint
Treatment of prosthetic joint septic arthritis is complex, and early consultation with an orthopedic surgeon and infectious diseases physician is recommended. Extensive surgical debridement of the afflicted joint and effective, prolonged antimicrobial therapy is necessary in almost all cases. In order to achieve an optimal synovial fluid and tissue culture yield, antimicrobial therapy should be delayed until the time of debridement surgery unless the patient is septic or exhibiting serious systemic complications of infection. Suggestions for early empiric therapy while awaiting culture results are given in Table 4. Final antimicrobial choices should be based on culture results with assistance from an infectious diseases consultant.
Carefully selected cases of prosthetic joint infection may be treated with simple surgical debridement of the joint with prosthesis retention and at least 3 months of antimicrobial therapy that includes rifampin if the organism is gram positive (44). Patients who present with a short duration of symptoms within 1 month of joint implantation, or those with acute hematogenous infection, are the best candidates for such a treatment strategy. Unfortunately, relapse is common in these cases, particularly if the infection is due to S. aureus, gram-negative bacilli, or drug-resistant pathogens. Thus, the optimal treatment protocols involve surgical excision of the infected prosthesis and prolonged antimicrobial therapy.
Surgical prosthesis extraction and reimplantation can be performed in either a one- or two-stage approach. The two-stage procedure is the more successful strategy and involves removal of the prosthesis and cement followed by a 6-week course of bactericidal antimicrobial therapy. Subsequently a new prosthesis is reimplanted. Using this approach, a 90% to 96% success rate in total hip replacement infections and a 97% success rate in total knee infections has been realized (45-47). An alternative tactic is a one-stage surgical procedure that excises the infected prosthesis with immediate reimplantation of a new joint using antibiotic-impregnated methacrylate cement. This method is effective in 77% to 83% of cases (48-50). Higher failure rates are observed for S. aureus and gram-negative bacillary infections (51). One-stage procedures are often used for elderly or infirm patients who might not tolerate protracted bed rest and a second major operation (52). A recent review article by Zimmerli et al. provides an excellent overview of antimicrobial and surgical treatment options for prosthetic joint infections (34).
Suppressive Antibiotic Therapy
Lifelong oral antimicrobial therapy plays a limited role for definitive therapy but is useful when a surgical approach is not possible because of medical or surgical contraindications. The goal of suppressive therapy is to control the infection and retain prosthesis function. It is important that patients and their families understand that the intention of such treatment is not to cure but to suppress the infection. Generally, oral suppressive therapy is initiated after a course of intravenous therapy. Goulet et al. (53) demonstrated a 63% success rate in maintaining function of hip arthroplasty in patients who met 5 criteria: 1) prostheses removal is not possible, 2) the pathogen is avirulent, 3) the pathogen is sensitive to oral antibiotics, 4) the patient is adherent to and tolerates antibiotics, and 5) the prosthesis is not loose. Patients being treated with lifelong suppressive therapy are at risk for the development of antibiotic resistance (in either the joint infecting pathogen or other commensal organism), local or systemic progression of infection, and adverse effects from chronic antibiotic usage.
Antimicrobial Prophylaxis to Prevent Joint Prosthesis Infection
Patients undergoing elective total joint replacement surgery should be evaluated for symptoms or signs of local infection that predispose to occult or overt bacteremia (particularly odontogenic, urologic, and dermatologic). Surgery should be delayed until such infections and coexisting medical conditions have been treated. Perioperative antibiotic prophylaxis has been shown to reduce deep wound infection and prosthetic joint infection in joint reimplant surgery but should not be continued for more than 24 hours after the preoperative dose (54,55). In order to decrease the risk of hematogenous seeding of established implants, early recognition and treatment of overt infection is crucial. The use of prophylactic antibiotics for patients with joint implants prior to or after dental or other procedures such as colonoscopy or cystoscopy is controversial. The American Academy of Orthopedic Surgeons recommends that a single dose of prophylactic antibiotic be given to certain patients undergoing urologic instrumentation or dental procedures that are accompanied by significant bleeding (56,57). Patients who are candidates for such prophylaxis include those with rheumatoid arthritis or other inflammatory arthropathy, immunosuppression, diabetes, malnutrition, hemophilia, or who have had a previous joint infection.
Dr. Ohl can be contacted at cohl@wfubmc.edu.
References
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-5.
- Ohl C. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1311-1322.
- Brause B. Infections with prostheses in bones and joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1332-7.
- Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30.
- Nolla JM, Gomez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: a detailed analysis of 10 cases and literature review. Semin Arthritis Rheum 2000;30: 121-6.
- Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis 1999;58:214-219.
- Kuzmanova SI, Atanassov AN, Andreev SA, Solakov PT. Minor and major complications of arthroscopic synovectomy of the knee joint performed by rheumatologist. Folia Med (Plovdiv). 2003;45:55-9.
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996;117:423-8.
- Mader JT, Shirtliff M, Calhoun JH. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol 1999;13:1-20.
- Berendt A. Infections of prosthetic joints and related problems. In: Cohen J, Powderly W, eds. Infectious Diseases. Edinburgh: Mosby, 2005: 583-589.
- Raymond NJ, Henry J, Workowski KA. Enterococcal arthritis: case report and review. Clin Infect Dis. 1995;21: 516-522.
- Ross JJ, Saltzman CL, Carling P, Shapiro DS. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:319-27.
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21:283-8.
- Sack K. Monarthritis: differential diagnosis. Am J Med. 1997; 102(1A):30S-34S.
- Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267-9.
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-3.
- Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore). 2003;82: 119-28.
- Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-44.
- Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201-8.
- Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860-66.
- Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to haemophilus influenzae type b. Clin Orthop. 1997;(341):128-33.
- Ewing R, Fainstein V, Musher DM, Lidsky M, Clarridge J. Articular and skeletal infections caused by Pasteurella multocida. South Med J. 1980;73:1349-52.
- Murray PM. Septic arthritis of the hand and wrist. Hand Clin. 1998;14:579-87, viii.
- Resnick D, Pineda CJ, Weisman MH, Kerr R. Osteomyelitis and septic arthritis of the hand following human bites. Skeletal Radiol. 1985;14:263-6.
- Murdoch DR, Roberts SA, Fowler JV Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647-9.
- Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-6.
- Goldenberg DL. Septic arthritis. Lancet 1998; 351:197-202.
- Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. Candida arthritis. Rheum Dis Clin North Am. 1993;19:427-37.
- Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-7.
- Cuellar ML, Silveira LH, Citera G, Cabrera GE, Valle R. Other fungal arthritides. Rheum Dis Clin North Am. 1993;19:439-55.
- Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-43.
- Van Der PB, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008-16.
- Kaandorp CJ, van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38:1819-25.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54.
- Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8.
- Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68:83-90.
- Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556-62.
- Cucurull E, Espinoza LR. Gonococcal arthritis. Rheum Dis Clin North Am. 1998; 24:305-22.
- Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275-289.
- Learch TJ, Farooki S. Magnetic resonance imaging of septic arthritis. Clin Imaging. 2000;24:236-42.
- Mohana-Borges AV, Chung CB, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42:135-49.
- Donatto KC. Orthopedic management of septic arthritis. Rheum Dis Clin North Am. 1998;24:275-86.
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-89.
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537-41.
- Garvin KL, Salvati EA, Brause BD. Role of gentamicin-impregnated cement in total joint arthroplasty. Orthop Clin North Am. 1988;19:605-10.
- Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;205-12.
- Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Twostage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272-8.
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981; 63-B(3):342-53.
- Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-64.
- Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;(381):101-5.
- Fitzgerald RH Jr, Jones DR. Hip implant infection. Treatment with resection arthroplasty and late total hip arthroplasty. Am J Med. 1985; 78(6B):225-8.
- Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-88.
- Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty. 1988; 3:109-16.
- Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003; 74:644-51.
- Norden CW. A critical review of antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis 1983;5:928-32.
- Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003; 169:1796-7.
- Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003; 134:895-9.
Introduction
Acute bacterial arthritis is a potentially serious and rapidly progressive infection that may involve native or prosthetic joints. The epidemiology, pathophysiology, repertoire of potential infecting pathogens, clinical presentation and treatment differ for these two forms of infectious arthritis, but both are associated with significant morbidity and mortality. Infectious arthritis of native and prosthetic joints may be caused by viruses, or fungi, but the most common cause is bacteria.
Acute Bacterial Arthritis
Epidemiology
The burden of septic arthritis in the general population is considerable. The incidence of native joint septic arthritis is approximately 5 cases per 100,000 persons per year and is much higher in patients with rheumatoid arthritis (1,2). Between 1% and 5% of joints with indwelling prostheses become infected and the total number of infections per year is increasing due to a rise in the number of patients who have had prosthetic replacement surgery (3). The mortality from joint infection is difficult to estimate due to differing comorbidity in afflicted patients, but is likely between 15% and 30% (4-6). There is substantial morbidity from these infections because of decreased joint function and mobility, and in cases involving joint prostheses from the excisional or exchange arthroplastic surgery that is often required for treatment.
The most common route of infection for native joint infection is hematogenous (1), but may also be a result of direct inoculation of bacteria through trauma or joint surgery (including arthrocentesis, corticosteroid injection, or arthroscopy) (7), or via contiguous spread from adjacent infected soft tissue or bone (1,8). While hematogenous infection of prosthetic joints occurs, the majority of these infections are the result of joint contamination in the course of implantation surgery or post-surgical wound infection (3). Host factors that increase the risk of septic arthritis include pre-existing joint disease (especially rheumatoid arthritis), immunosuppression, diabetes mellitus, malignancy, chronic renal failure, intravenous drug use, severe skin diseases, and advanced age (1,2,4,6). The extent of joint injury resulting from infection depends on the virulence of the infecting pathogen and degree of host immune response (9).
Microbiology
Native Joint
The most common causes of bacterial septic arthritis are outlined in Table 1. In adults, the most frequent etiology is S. aureus (37–65% of cases) (1,4,6,8,12,15,16) followed by Streptococcus sp. (12,15). An increasing percentage of S. aureus isolated from septic joints are resistant to antistaphylococcal penicillins and cephalosporins (methicillin-resistant S. aureus, MRSA). In adults with diabetes, malignancy, and genitourinary structural abnormalities, group B Streptococcus is a frequently isolated pathogen (5,6,17). Gram-negative bacilli are commonly found in neonates, intravenous drug users, and immunocompromised hosts (18). N. gonorrhoeae is a significant cause of bacterial arthritis in sexually active adults and adolescents (19) and Kingella kingae and Haemophilus influenzae are likely pediatric isolates (20,21). Joint infections that follow bite trauma usually are seen in the small joints of the hand and involve Pasteurella multocida in the case of animal bites, and Eikenella corrodens in the case of humans bites (22-24). Polymicrobial floras are found in up to 8% of cases of septic arthritis.
Prosthetic Joint
The bacteria that cause prosthetic joint arthritis vary depending on the stage of infection as defined by the elapsed time after implantation surgery (Table 1 on page 31). The coagulase negative staphylococci are the most common (30–43% of cases) (3,10), followed by S. aureus (12–23%) (25).
Nonbacterial Pathogens
Nonbacterial pathogens that may cause septic arthritis include viruses, fungi, and mycobacteria. Viral arthritis is often associated with a systemic febrile illness and other manifestations of infection such as rash. Parvovirus B19 is the most common viral arthritide, presenting as a symmetric polyarticular arthritis involving the joints of the hand as well as larger joints (26). The classic red “slapped cheeks” associated with this viral infection in children is usually not present in adults, although a faint lacy reticular rash may be seen.
Fungi and mycobacteria usually cause a subacute or chronic mono- or oligoarticular arthritis (27). Candida species are an increasing cause of both native and prosthetic joint septic arthritis. Risk factors for this infection include loss of skin integrity, diabetes, malignancy, intravenous drug use, and immunosuppressive therapy including glucocorticoids (28). Patients are often chronically ill and have exposure to broad-spectrum antimicrobials, hyperalimentation fluid, and/or indwelling central intravenous catheters. Other fungi, including Cryptococcus, Blastomyces, Histoplasma, Coccidioides, and Sporothrix are rare causes of septic arthritis (29,30). Mycobacterium tuberculosis is the most common cause of mycobacterial arthritis worldwide and should be considered in a patient presenting with chronic arthritis with risk factors for tuberculosis, including being foreign-born (31).
Clinical Features
The clinical manifestations, severity, treatment, and prognosis of septic arthritis are dependent on the identity and virulence of the bacterium, source of joint infection, and underlying host factors. Nongonococcal septic arthritis is monoarticular in 80% to 90% of cases. The knee is usually affected (50% of cases) (27) followed by the hip, wrists, and ankles (2). In adults, the majority of hip infections involve prosthetic or osteosynthetic material (1). Arthritis of the small joints of the foot is most often seen in diabetic patients and is usually secondary to contiguous skin and soft tissue ulcerations or adjacent osteomyelitis.
Gonococcal arthritis may present as febrile monoarticular arthritis, usually of the knees, wrists, and ankles (27), or as one of the manifestations of disseminated gonococcal infection. The latter is characterized by fever, dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis (19). Skin lesions are often pustular and occur simultaneously with tenosynovitis, predominately affecting the fingers, hands, wrists, or feet. Concomitant mucosal infection of the urethra or cervix is often present but usually asymptomatic. Urethral and cervical cultures or a nucleic amplification test will frequently yield N. gonorrhoeae (19,32).
Symptoms of acute septic arthritis include pain and loss of joint function. Fever and chills are often present. The acutely infected native joint is usually red, warm, and swollen with an obvious effusion. Range of motion is limited and extremely painful. For deep and axial joint, pain is often the only focal symptom. More subtle symptoms and signs may result in a delay of diagnosis and are particularly seen in patients receiving systemic or intra-articular steroids, and in those with immunocompromised status, advanced comorbidities (including rheumatoid arthritis), and extreme age (33). A thorough physical examination may reveal a distant source of joint infection in up to 50% of patients (27).
Prosthetic joint infection may present acutely as above, particularly in early stage infection, or more indolently with progressive joint pain, minimal swelling or effusion, and absence of fever (34). In late infection a cutaneous draining sinus tract may be present. Rarely, the involved prosthesis may be visible beneath an ulceration or focus of soft-tissue breakdown.
Diseases that can mimic septic arthritis are crystalinduced arthritis, rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, Still’s disease, rheumatic fever, and Kawasaki syndrome.
Diagnostic Approach
A diagnostic approach to acute native joint arthritis is outlined in Figure 1 on page 32 (35,36). Important aspects include exclusion of other causes of arthritis including trauma, rheumatic diseases, and crystalline arthritis. The most important diagnostic test upon which management hinges is diagnostic arthrocentesis. Fluoroscopic or CT-guided arthrocentesis is indicated for axial and deep joints (e.g., sacroiliac or pubic symphysis) or in the event of a “dry tap” of a peripheral joint. Synovial fluid analysis will often suggest whether an acutely painful joint is due to noninflammatory, sterile inflammatory, or septic causes (Table 2 on page 33). In addition, it will provide fluid for culture and gram stain, a rapid test that can guide early empiric antibiotic therapy. Bacterial, fungal, and mycobacterial cultures should always be performed in order to direct pathogen-specific antimicrobial therapy, which is often given as a prolonged course. Antimicrobial therapy should be delayed until arthrocentesis and other appropriate diagnostic cultures are obtained unless the patient shows signs of sepsis.
For prosthetic joint infections the diagnostic approach is essentially the same although early radiographic imaging is more important than in native joint infection as it may show signs of prosthesis failure or loosening (seen in many late prosthesis infections). Additionally, the synovial fluid white blood cell (WBC) is often lower than in nativejoint infection, with a diagnostic cutoff suggested as greater than 1,700 cells/mm3 or >65% neutrophils (37).
Nonspecific blood tests such as a white blood count, erythrocyte sedimentation rate, or C-reactive protein argue against joint infection if they are normal, but do not specifically suggest septic arthritis if elevated. Other important diagnostic tests include blood cultures (positive in 50–70% of acute bacterial arthritides) (27), but in only 30% or less of gonococcal arthritis cases) (38), wound cultures (although these often correlate poorly with synovial fluid culture results, except when the pathogen is S. aureus), and serologic testing for B. burgdorferi in selected cases with clinical features of Lyme arthritis in endemic areas. If gonococcal arthritis is suspected urethral and cervical specimens should be sent for N. gonorrhoeae culture and nucleic acid amplification tests. Radiographic and scintillographic imaging may yield additional information that will assist in identifying preexisting joint disease or for confirming a diagnosis of native or prosthetic joint infection or its complications (Table 3 on page 33).
Treatment
Native joint
Prompt joint drainage and antimicrobial therapy are the mainstays of treatment in bacterial, fungal, or mycobacterial joint infection. Drainage can be through closed needle aspiration performed daily, or arthroscopy. The former modality allows direct visual inspection of the joint with concomitant irrigation, lysis of adhesions, and removal of necrotic tissue and purulent material (42). Open surgical drainage is recommended for septic arthritis of the hip and when less invasive methods fail to control infection.
Initial antimicrobial therapy should be withheld until synovial fluid has been obtained and should be based on synovial fluid gram staining (Table 4). In the case of a nondiagnostic gram stain, empiric antimicrobial coverage of likely infecting pathogens is indicated. Therapy should be narrowed based on identification and antimicrobial susceptibility testing of bacteria cultured from synovial fluid, blood, or in some cases from ancillary cultures. For patients with MRSA-related infection who are allergic to or intolerant of vancomycin, linezolid or daptomycin are potential alternatives, although not approved by the U.S. Food and Drug Administration for this indication. Linezolid is a potentially attractive option for treatment as it is available as an oral tablet, but for bone and joint infection treatment experience is limited. For septic arthritis related to animal or human bites ampicillin-sulbactam or amoxicillin-clavulanate (clindamycin plus ciprofloxacin in penicillin-allergic patients) provides activity against Pasteurella multocida and other oral bacteria. Gonococcal arthritis is best treated initially with ceftriaxone or cefotaxime; oral ciprofloxacin or levofloxacin may be substituted in regions without fluoroquinolone resistance as the patient improves (Table 4). Septic arthritis due to Candida sp. should be treated initially with an amphotericin B preparation followed by a prolonged course of fluconazole if susceptibility testing confirms activity against the cultured yeast isolate (43).
Duration of intravenous antimicrobials for bacterial joint infections is usually 2 to 4 weeks, while for gonococcal arthritis 2 weeks is sufficient. Antimicrobial therapy that continues for 2 weeks or longer should have weekly followup and laboratory monitoring for hematologic, renal, and liver toxicity.
Prosthetic Joint
Treatment of prosthetic joint septic arthritis is complex, and early consultation with an orthopedic surgeon and infectious diseases physician is recommended. Extensive surgical debridement of the afflicted joint and effective, prolonged antimicrobial therapy is necessary in almost all cases. In order to achieve an optimal synovial fluid and tissue culture yield, antimicrobial therapy should be delayed until the time of debridement surgery unless the patient is septic or exhibiting serious systemic complications of infection. Suggestions for early empiric therapy while awaiting culture results are given in Table 4. Final antimicrobial choices should be based on culture results with assistance from an infectious diseases consultant.
Carefully selected cases of prosthetic joint infection may be treated with simple surgical debridement of the joint with prosthesis retention and at least 3 months of antimicrobial therapy that includes rifampin if the organism is gram positive (44). Patients who present with a short duration of symptoms within 1 month of joint implantation, or those with acute hematogenous infection, are the best candidates for such a treatment strategy. Unfortunately, relapse is common in these cases, particularly if the infection is due to S. aureus, gram-negative bacilli, or drug-resistant pathogens. Thus, the optimal treatment protocols involve surgical excision of the infected prosthesis and prolonged antimicrobial therapy.
Surgical prosthesis extraction and reimplantation can be performed in either a one- or two-stage approach. The two-stage procedure is the more successful strategy and involves removal of the prosthesis and cement followed by a 6-week course of bactericidal antimicrobial therapy. Subsequently a new prosthesis is reimplanted. Using this approach, a 90% to 96% success rate in total hip replacement infections and a 97% success rate in total knee infections has been realized (45-47). An alternative tactic is a one-stage surgical procedure that excises the infected prosthesis with immediate reimplantation of a new joint using antibiotic-impregnated methacrylate cement. This method is effective in 77% to 83% of cases (48-50). Higher failure rates are observed for S. aureus and gram-negative bacillary infections (51). One-stage procedures are often used for elderly or infirm patients who might not tolerate protracted bed rest and a second major operation (52). A recent review article by Zimmerli et al. provides an excellent overview of antimicrobial and surgical treatment options for prosthetic joint infections (34).
Suppressive Antibiotic Therapy
Lifelong oral antimicrobial therapy plays a limited role for definitive therapy but is useful when a surgical approach is not possible because of medical or surgical contraindications. The goal of suppressive therapy is to control the infection and retain prosthesis function. It is important that patients and their families understand that the intention of such treatment is not to cure but to suppress the infection. Generally, oral suppressive therapy is initiated after a course of intravenous therapy. Goulet et al. (53) demonstrated a 63% success rate in maintaining function of hip arthroplasty in patients who met 5 criteria: 1) prostheses removal is not possible, 2) the pathogen is avirulent, 3) the pathogen is sensitive to oral antibiotics, 4) the patient is adherent to and tolerates antibiotics, and 5) the prosthesis is not loose. Patients being treated with lifelong suppressive therapy are at risk for the development of antibiotic resistance (in either the joint infecting pathogen or other commensal organism), local or systemic progression of infection, and adverse effects from chronic antibiotic usage.
Antimicrobial Prophylaxis to Prevent Joint Prosthesis Infection
Patients undergoing elective total joint replacement surgery should be evaluated for symptoms or signs of local infection that predispose to occult or overt bacteremia (particularly odontogenic, urologic, and dermatologic). Surgery should be delayed until such infections and coexisting medical conditions have been treated. Perioperative antibiotic prophylaxis has been shown to reduce deep wound infection and prosthetic joint infection in joint reimplant surgery but should not be continued for more than 24 hours after the preoperative dose (54,55). In order to decrease the risk of hematogenous seeding of established implants, early recognition and treatment of overt infection is crucial. The use of prophylactic antibiotics for patients with joint implants prior to or after dental or other procedures such as colonoscopy or cystoscopy is controversial. The American Academy of Orthopedic Surgeons recommends that a single dose of prophylactic antibiotic be given to certain patients undergoing urologic instrumentation or dental procedures that are accompanied by significant bleeding (56,57). Patients who are candidates for such prophylaxis include those with rheumatoid arthritis or other inflammatory arthropathy, immunosuppression, diabetes, malnutrition, hemophilia, or who have had a previous joint infection.
Dr. Ohl can be contacted at cohl@wfubmc.edu.
References
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-5.
- Ohl C. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1311-1322.
- Brause B. Infections with prostheses in bones and joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1332-7.
- Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30.
- Nolla JM, Gomez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: a detailed analysis of 10 cases and literature review. Semin Arthritis Rheum 2000;30: 121-6.
- Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis 1999;58:214-219.
- Kuzmanova SI, Atanassov AN, Andreev SA, Solakov PT. Minor and major complications of arthroscopic synovectomy of the knee joint performed by rheumatologist. Folia Med (Plovdiv). 2003;45:55-9.
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996;117:423-8.
- Mader JT, Shirtliff M, Calhoun JH. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol 1999;13:1-20.
- Berendt A. Infections of prosthetic joints and related problems. In: Cohen J, Powderly W, eds. Infectious Diseases. Edinburgh: Mosby, 2005: 583-589.
- Raymond NJ, Henry J, Workowski KA. Enterococcal arthritis: case report and review. Clin Infect Dis. 1995;21: 516-522.
- Ross JJ, Saltzman CL, Carling P, Shapiro DS. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:319-27.
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21:283-8.
- Sack K. Monarthritis: differential diagnosis. Am J Med. 1997; 102(1A):30S-34S.
- Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267-9.
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-3.
- Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore). 2003;82: 119-28.
- Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-44.
- Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201-8.
- Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860-66.
- Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to haemophilus influenzae type b. Clin Orthop. 1997;(341):128-33.
- Ewing R, Fainstein V, Musher DM, Lidsky M, Clarridge J. Articular and skeletal infections caused by Pasteurella multocida. South Med J. 1980;73:1349-52.
- Murray PM. Septic arthritis of the hand and wrist. Hand Clin. 1998;14:579-87, viii.
- Resnick D, Pineda CJ, Weisman MH, Kerr R. Osteomyelitis and septic arthritis of the hand following human bites. Skeletal Radiol. 1985;14:263-6.
- Murdoch DR, Roberts SA, Fowler JV Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647-9.
- Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-6.
- Goldenberg DL. Septic arthritis. Lancet 1998; 351:197-202.
- Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. Candida arthritis. Rheum Dis Clin North Am. 1993;19:427-37.
- Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-7.
- Cuellar ML, Silveira LH, Citera G, Cabrera GE, Valle R. Other fungal arthritides. Rheum Dis Clin North Am. 1993;19:439-55.
- Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-43.
- Van Der PB, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008-16.
- Kaandorp CJ, van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38:1819-25.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54.
- Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8.
- Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68:83-90.
- Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556-62.
- Cucurull E, Espinoza LR. Gonococcal arthritis. Rheum Dis Clin North Am. 1998; 24:305-22.
- Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275-289.
- Learch TJ, Farooki S. Magnetic resonance imaging of septic arthritis. Clin Imaging. 2000;24:236-42.
- Mohana-Borges AV, Chung CB, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42:135-49.
- Donatto KC. Orthopedic management of septic arthritis. Rheum Dis Clin North Am. 1998;24:275-86.
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-89.
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537-41.
- Garvin KL, Salvati EA, Brause BD. Role of gentamicin-impregnated cement in total joint arthroplasty. Orthop Clin North Am. 1988;19:605-10.
- Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;205-12.
- Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Twostage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272-8.
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981; 63-B(3):342-53.
- Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-64.
- Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;(381):101-5.
- Fitzgerald RH Jr, Jones DR. Hip implant infection. Treatment with resection arthroplasty and late total hip arthroplasty. Am J Med. 1985; 78(6B):225-8.
- Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-88.
- Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty. 1988; 3:109-16.
- Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003; 74:644-51.
- Norden CW. A critical review of antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis 1983;5:928-32.
- Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003; 169:1796-7.
- Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003; 134:895-9.
Introduction
Acute bacterial arthritis is a potentially serious and rapidly progressive infection that may involve native or prosthetic joints. The epidemiology, pathophysiology, repertoire of potential infecting pathogens, clinical presentation and treatment differ for these two forms of infectious arthritis, but both are associated with significant morbidity and mortality. Infectious arthritis of native and prosthetic joints may be caused by viruses, or fungi, but the most common cause is bacteria.
Acute Bacterial Arthritis
Epidemiology
The burden of septic arthritis in the general population is considerable. The incidence of native joint septic arthritis is approximately 5 cases per 100,000 persons per year and is much higher in patients with rheumatoid arthritis (1,2). Between 1% and 5% of joints with indwelling prostheses become infected and the total number of infections per year is increasing due to a rise in the number of patients who have had prosthetic replacement surgery (3). The mortality from joint infection is difficult to estimate due to differing comorbidity in afflicted patients, but is likely between 15% and 30% (4-6). There is substantial morbidity from these infections because of decreased joint function and mobility, and in cases involving joint prostheses from the excisional or exchange arthroplastic surgery that is often required for treatment.
The most common route of infection for native joint infection is hematogenous (1), but may also be a result of direct inoculation of bacteria through trauma or joint surgery (including arthrocentesis, corticosteroid injection, or arthroscopy) (7), or via contiguous spread from adjacent infected soft tissue or bone (1,8). While hematogenous infection of prosthetic joints occurs, the majority of these infections are the result of joint contamination in the course of implantation surgery or post-surgical wound infection (3). Host factors that increase the risk of septic arthritis include pre-existing joint disease (especially rheumatoid arthritis), immunosuppression, diabetes mellitus, malignancy, chronic renal failure, intravenous drug use, severe skin diseases, and advanced age (1,2,4,6). The extent of joint injury resulting from infection depends on the virulence of the infecting pathogen and degree of host immune response (9).
Microbiology
Native Joint
The most common causes of bacterial septic arthritis are outlined in Table 1. In adults, the most frequent etiology is S. aureus (37–65% of cases) (1,4,6,8,12,15,16) followed by Streptococcus sp. (12,15). An increasing percentage of S. aureus isolated from septic joints are resistant to antistaphylococcal penicillins and cephalosporins (methicillin-resistant S. aureus, MRSA). In adults with diabetes, malignancy, and genitourinary structural abnormalities, group B Streptococcus is a frequently isolated pathogen (5,6,17). Gram-negative bacilli are commonly found in neonates, intravenous drug users, and immunocompromised hosts (18). N. gonorrhoeae is a significant cause of bacterial arthritis in sexually active adults and adolescents (19) and Kingella kingae and Haemophilus influenzae are likely pediatric isolates (20,21). Joint infections that follow bite trauma usually are seen in the small joints of the hand and involve Pasteurella multocida in the case of animal bites, and Eikenella corrodens in the case of humans bites (22-24). Polymicrobial floras are found in up to 8% of cases of septic arthritis.
Prosthetic Joint
The bacteria that cause prosthetic joint arthritis vary depending on the stage of infection as defined by the elapsed time after implantation surgery (Table 1 on page 31). The coagulase negative staphylococci are the most common (30–43% of cases) (3,10), followed by S. aureus (12–23%) (25).
Nonbacterial Pathogens
Nonbacterial pathogens that may cause septic arthritis include viruses, fungi, and mycobacteria. Viral arthritis is often associated with a systemic febrile illness and other manifestations of infection such as rash. Parvovirus B19 is the most common viral arthritide, presenting as a symmetric polyarticular arthritis involving the joints of the hand as well as larger joints (26). The classic red “slapped cheeks” associated with this viral infection in children is usually not present in adults, although a faint lacy reticular rash may be seen.
Fungi and mycobacteria usually cause a subacute or chronic mono- or oligoarticular arthritis (27). Candida species are an increasing cause of both native and prosthetic joint septic arthritis. Risk factors for this infection include loss of skin integrity, diabetes, malignancy, intravenous drug use, and immunosuppressive therapy including glucocorticoids (28). Patients are often chronically ill and have exposure to broad-spectrum antimicrobials, hyperalimentation fluid, and/or indwelling central intravenous catheters. Other fungi, including Cryptococcus, Blastomyces, Histoplasma, Coccidioides, and Sporothrix are rare causes of septic arthritis (29,30). Mycobacterium tuberculosis is the most common cause of mycobacterial arthritis worldwide and should be considered in a patient presenting with chronic arthritis with risk factors for tuberculosis, including being foreign-born (31).
Clinical Features
The clinical manifestations, severity, treatment, and prognosis of septic arthritis are dependent on the identity and virulence of the bacterium, source of joint infection, and underlying host factors. Nongonococcal septic arthritis is monoarticular in 80% to 90% of cases. The knee is usually affected (50% of cases) (27) followed by the hip, wrists, and ankles (2). In adults, the majority of hip infections involve prosthetic or osteosynthetic material (1). Arthritis of the small joints of the foot is most often seen in diabetic patients and is usually secondary to contiguous skin and soft tissue ulcerations or adjacent osteomyelitis.
Gonococcal arthritis may present as febrile monoarticular arthritis, usually of the knees, wrists, and ankles (27), or as one of the manifestations of disseminated gonococcal infection. The latter is characterized by fever, dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis (19). Skin lesions are often pustular and occur simultaneously with tenosynovitis, predominately affecting the fingers, hands, wrists, or feet. Concomitant mucosal infection of the urethra or cervix is often present but usually asymptomatic. Urethral and cervical cultures or a nucleic amplification test will frequently yield N. gonorrhoeae (19,32).
Symptoms of acute septic arthritis include pain and loss of joint function. Fever and chills are often present. The acutely infected native joint is usually red, warm, and swollen with an obvious effusion. Range of motion is limited and extremely painful. For deep and axial joint, pain is often the only focal symptom. More subtle symptoms and signs may result in a delay of diagnosis and are particularly seen in patients receiving systemic or intra-articular steroids, and in those with immunocompromised status, advanced comorbidities (including rheumatoid arthritis), and extreme age (33). A thorough physical examination may reveal a distant source of joint infection in up to 50% of patients (27).
Prosthetic joint infection may present acutely as above, particularly in early stage infection, or more indolently with progressive joint pain, minimal swelling or effusion, and absence of fever (34). In late infection a cutaneous draining sinus tract may be present. Rarely, the involved prosthesis may be visible beneath an ulceration or focus of soft-tissue breakdown.
Diseases that can mimic septic arthritis are crystalinduced arthritis, rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, Still’s disease, rheumatic fever, and Kawasaki syndrome.
Diagnostic Approach
A diagnostic approach to acute native joint arthritis is outlined in Figure 1 on page 32 (35,36). Important aspects include exclusion of other causes of arthritis including trauma, rheumatic diseases, and crystalline arthritis. The most important diagnostic test upon which management hinges is diagnostic arthrocentesis. Fluoroscopic or CT-guided arthrocentesis is indicated for axial and deep joints (e.g., sacroiliac or pubic symphysis) or in the event of a “dry tap” of a peripheral joint. Synovial fluid analysis will often suggest whether an acutely painful joint is due to noninflammatory, sterile inflammatory, or septic causes (Table 2 on page 33). In addition, it will provide fluid for culture and gram stain, a rapid test that can guide early empiric antibiotic therapy. Bacterial, fungal, and mycobacterial cultures should always be performed in order to direct pathogen-specific antimicrobial therapy, which is often given as a prolonged course. Antimicrobial therapy should be delayed until arthrocentesis and other appropriate diagnostic cultures are obtained unless the patient shows signs of sepsis.
For prosthetic joint infections the diagnostic approach is essentially the same although early radiographic imaging is more important than in native joint infection as it may show signs of prosthesis failure or loosening (seen in many late prosthesis infections). Additionally, the synovial fluid white blood cell (WBC) is often lower than in nativejoint infection, with a diagnostic cutoff suggested as greater than 1,700 cells/mm3 or >65% neutrophils (37).
Nonspecific blood tests such as a white blood count, erythrocyte sedimentation rate, or C-reactive protein argue against joint infection if they are normal, but do not specifically suggest septic arthritis if elevated. Other important diagnostic tests include blood cultures (positive in 50–70% of acute bacterial arthritides) (27), but in only 30% or less of gonococcal arthritis cases) (38), wound cultures (although these often correlate poorly with synovial fluid culture results, except when the pathogen is S. aureus), and serologic testing for B. burgdorferi in selected cases with clinical features of Lyme arthritis in endemic areas. If gonococcal arthritis is suspected urethral and cervical specimens should be sent for N. gonorrhoeae culture and nucleic acid amplification tests. Radiographic and scintillographic imaging may yield additional information that will assist in identifying preexisting joint disease or for confirming a diagnosis of native or prosthetic joint infection or its complications (Table 3 on page 33).
Treatment
Native joint
Prompt joint drainage and antimicrobial therapy are the mainstays of treatment in bacterial, fungal, or mycobacterial joint infection. Drainage can be through closed needle aspiration performed daily, or arthroscopy. The former modality allows direct visual inspection of the joint with concomitant irrigation, lysis of adhesions, and removal of necrotic tissue and purulent material (42). Open surgical drainage is recommended for septic arthritis of the hip and when less invasive methods fail to control infection.
Initial antimicrobial therapy should be withheld until synovial fluid has been obtained and should be based on synovial fluid gram staining (Table 4). In the case of a nondiagnostic gram stain, empiric antimicrobial coverage of likely infecting pathogens is indicated. Therapy should be narrowed based on identification and antimicrobial susceptibility testing of bacteria cultured from synovial fluid, blood, or in some cases from ancillary cultures. For patients with MRSA-related infection who are allergic to or intolerant of vancomycin, linezolid or daptomycin are potential alternatives, although not approved by the U.S. Food and Drug Administration for this indication. Linezolid is a potentially attractive option for treatment as it is available as an oral tablet, but for bone and joint infection treatment experience is limited. For septic arthritis related to animal or human bites ampicillin-sulbactam or amoxicillin-clavulanate (clindamycin plus ciprofloxacin in penicillin-allergic patients) provides activity against Pasteurella multocida and other oral bacteria. Gonococcal arthritis is best treated initially with ceftriaxone or cefotaxime; oral ciprofloxacin or levofloxacin may be substituted in regions without fluoroquinolone resistance as the patient improves (Table 4). Septic arthritis due to Candida sp. should be treated initially with an amphotericin B preparation followed by a prolonged course of fluconazole if susceptibility testing confirms activity against the cultured yeast isolate (43).
Duration of intravenous antimicrobials for bacterial joint infections is usually 2 to 4 weeks, while for gonococcal arthritis 2 weeks is sufficient. Antimicrobial therapy that continues for 2 weeks or longer should have weekly followup and laboratory monitoring for hematologic, renal, and liver toxicity.
Prosthetic Joint
Treatment of prosthetic joint septic arthritis is complex, and early consultation with an orthopedic surgeon and infectious diseases physician is recommended. Extensive surgical debridement of the afflicted joint and effective, prolonged antimicrobial therapy is necessary in almost all cases. In order to achieve an optimal synovial fluid and tissue culture yield, antimicrobial therapy should be delayed until the time of debridement surgery unless the patient is septic or exhibiting serious systemic complications of infection. Suggestions for early empiric therapy while awaiting culture results are given in Table 4. Final antimicrobial choices should be based on culture results with assistance from an infectious diseases consultant.
Carefully selected cases of prosthetic joint infection may be treated with simple surgical debridement of the joint with prosthesis retention and at least 3 months of antimicrobial therapy that includes rifampin if the organism is gram positive (44). Patients who present with a short duration of symptoms within 1 month of joint implantation, or those with acute hematogenous infection, are the best candidates for such a treatment strategy. Unfortunately, relapse is common in these cases, particularly if the infection is due to S. aureus, gram-negative bacilli, or drug-resistant pathogens. Thus, the optimal treatment protocols involve surgical excision of the infected prosthesis and prolonged antimicrobial therapy.
Surgical prosthesis extraction and reimplantation can be performed in either a one- or two-stage approach. The two-stage procedure is the more successful strategy and involves removal of the prosthesis and cement followed by a 6-week course of bactericidal antimicrobial therapy. Subsequently a new prosthesis is reimplanted. Using this approach, a 90% to 96% success rate in total hip replacement infections and a 97% success rate in total knee infections has been realized (45-47). An alternative tactic is a one-stage surgical procedure that excises the infected prosthesis with immediate reimplantation of a new joint using antibiotic-impregnated methacrylate cement. This method is effective in 77% to 83% of cases (48-50). Higher failure rates are observed for S. aureus and gram-negative bacillary infections (51). One-stage procedures are often used for elderly or infirm patients who might not tolerate protracted bed rest and a second major operation (52). A recent review article by Zimmerli et al. provides an excellent overview of antimicrobial and surgical treatment options for prosthetic joint infections (34).
Suppressive Antibiotic Therapy
Lifelong oral antimicrobial therapy plays a limited role for definitive therapy but is useful when a surgical approach is not possible because of medical or surgical contraindications. The goal of suppressive therapy is to control the infection and retain prosthesis function. It is important that patients and their families understand that the intention of such treatment is not to cure but to suppress the infection. Generally, oral suppressive therapy is initiated after a course of intravenous therapy. Goulet et al. (53) demonstrated a 63% success rate in maintaining function of hip arthroplasty in patients who met 5 criteria: 1) prostheses removal is not possible, 2) the pathogen is avirulent, 3) the pathogen is sensitive to oral antibiotics, 4) the patient is adherent to and tolerates antibiotics, and 5) the prosthesis is not loose. Patients being treated with lifelong suppressive therapy are at risk for the development of antibiotic resistance (in either the joint infecting pathogen or other commensal organism), local or systemic progression of infection, and adverse effects from chronic antibiotic usage.
Antimicrobial Prophylaxis to Prevent Joint Prosthesis Infection
Patients undergoing elective total joint replacement surgery should be evaluated for symptoms or signs of local infection that predispose to occult or overt bacteremia (particularly odontogenic, urologic, and dermatologic). Surgery should be delayed until such infections and coexisting medical conditions have been treated. Perioperative antibiotic prophylaxis has been shown to reduce deep wound infection and prosthetic joint infection in joint reimplant surgery but should not be continued for more than 24 hours after the preoperative dose (54,55). In order to decrease the risk of hematogenous seeding of established implants, early recognition and treatment of overt infection is crucial. The use of prophylactic antibiotics for patients with joint implants prior to or after dental or other procedures such as colonoscopy or cystoscopy is controversial. The American Academy of Orthopedic Surgeons recommends that a single dose of prophylactic antibiotic be given to certain patients undergoing urologic instrumentation or dental procedures that are accompanied by significant bleeding (56,57). Patients who are candidates for such prophylaxis include those with rheumatoid arthritis or other inflammatory arthropathy, immunosuppression, diabetes, malnutrition, hemophilia, or who have had a previous joint infection.
Dr. Ohl can be contacted at cohl@wfubmc.edu.
References
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-5.
- Ohl C. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1311-1322.
- Brause B. Infections with prostheses in bones and joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1332-7.
- Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30.
- Nolla JM, Gomez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: a detailed analysis of 10 cases and literature review. Semin Arthritis Rheum 2000;30: 121-6.
- Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis 1999;58:214-219.
- Kuzmanova SI, Atanassov AN, Andreev SA, Solakov PT. Minor and major complications of arthroscopic synovectomy of the knee joint performed by rheumatologist. Folia Med (Plovdiv). 2003;45:55-9.
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996;117:423-8.
- Mader JT, Shirtliff M, Calhoun JH. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol 1999;13:1-20.
- Berendt A. Infections of prosthetic joints and related problems. In: Cohen J, Powderly W, eds. Infectious Diseases. Edinburgh: Mosby, 2005: 583-589.
- Raymond NJ, Henry J, Workowski KA. Enterococcal arthritis: case report and review. Clin Infect Dis. 1995;21: 516-522.
- Ross JJ, Saltzman CL, Carling P, Shapiro DS. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:319-27.
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21:283-8.
- Sack K. Monarthritis: differential diagnosis. Am J Med. 1997; 102(1A):30S-34S.
- Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267-9.
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-3.
- Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore). 2003;82: 119-28.
- Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-44.
- Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201-8.
- Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860-66.
- Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to haemophilus influenzae type b. Clin Orthop. 1997;(341):128-33.
- Ewing R, Fainstein V, Musher DM, Lidsky M, Clarridge J. Articular and skeletal infections caused by Pasteurella multocida. South Med J. 1980;73:1349-52.
- Murray PM. Septic arthritis of the hand and wrist. Hand Clin. 1998;14:579-87, viii.
- Resnick D, Pineda CJ, Weisman MH, Kerr R. Osteomyelitis and septic arthritis of the hand following human bites. Skeletal Radiol. 1985;14:263-6.
- Murdoch DR, Roberts SA, Fowler JV Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647-9.
- Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-6.
- Goldenberg DL. Septic arthritis. Lancet 1998; 351:197-202.
- Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. Candida arthritis. Rheum Dis Clin North Am. 1993;19:427-37.
- Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-7.
- Cuellar ML, Silveira LH, Citera G, Cabrera GE, Valle R. Other fungal arthritides. Rheum Dis Clin North Am. 1993;19:439-55.
- Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-43.
- Van Der PB, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008-16.
- Kaandorp CJ, van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38:1819-25.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54.
- Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8.
- Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68:83-90.
- Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556-62.
- Cucurull E, Espinoza LR. Gonococcal arthritis. Rheum Dis Clin North Am. 1998; 24:305-22.
- Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275-289.
- Learch TJ, Farooki S. Magnetic resonance imaging of septic arthritis. Clin Imaging. 2000;24:236-42.
- Mohana-Borges AV, Chung CB, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42:135-49.
- Donatto KC. Orthopedic management of septic arthritis. Rheum Dis Clin North Am. 1998;24:275-86.
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-89.
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537-41.
- Garvin KL, Salvati EA, Brause BD. Role of gentamicin-impregnated cement in total joint arthroplasty. Orthop Clin North Am. 1988;19:605-10.
- Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;205-12.
- Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Twostage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272-8.
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981; 63-B(3):342-53.
- Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-64.
- Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;(381):101-5.
- Fitzgerald RH Jr, Jones DR. Hip implant infection. Treatment with resection arthroplasty and late total hip arthroplasty. Am J Med. 1985; 78(6B):225-8.
- Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-88.
- Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty. 1988; 3:109-16.
- Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003; 74:644-51.
- Norden CW. A critical review of antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis 1983;5:928-32.
- Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003; 169:1796-7.
- Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003; 134:895-9.