User login
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
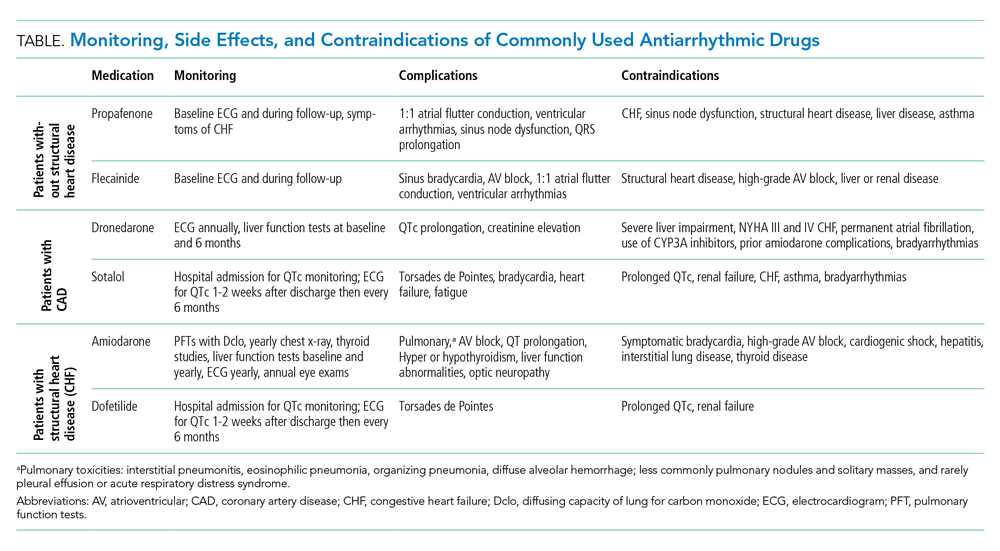
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3
Catheter Ablation
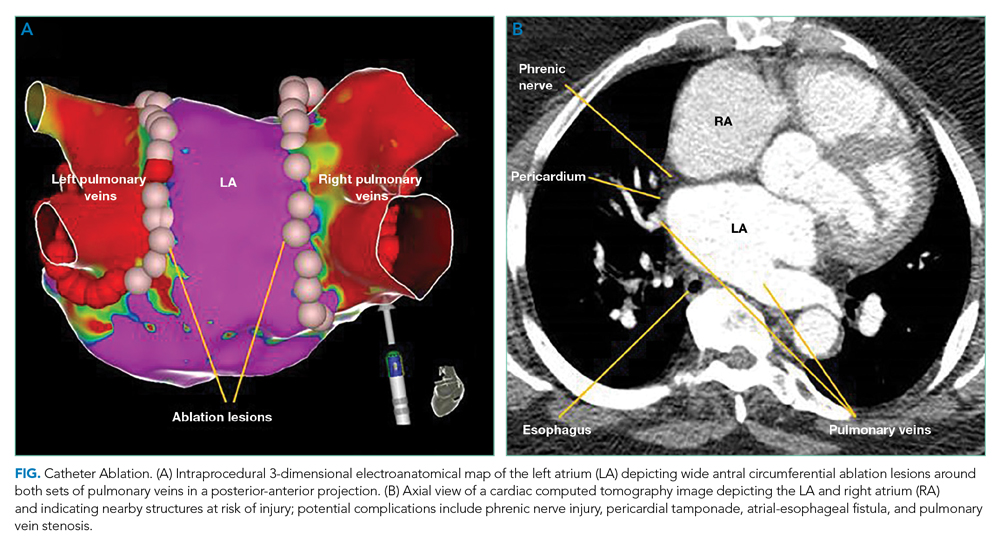
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7
Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
© 2021 Society of Hospital Medicine