User login
Opioid use disorder in pregnancy: A strategy for using methadone
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
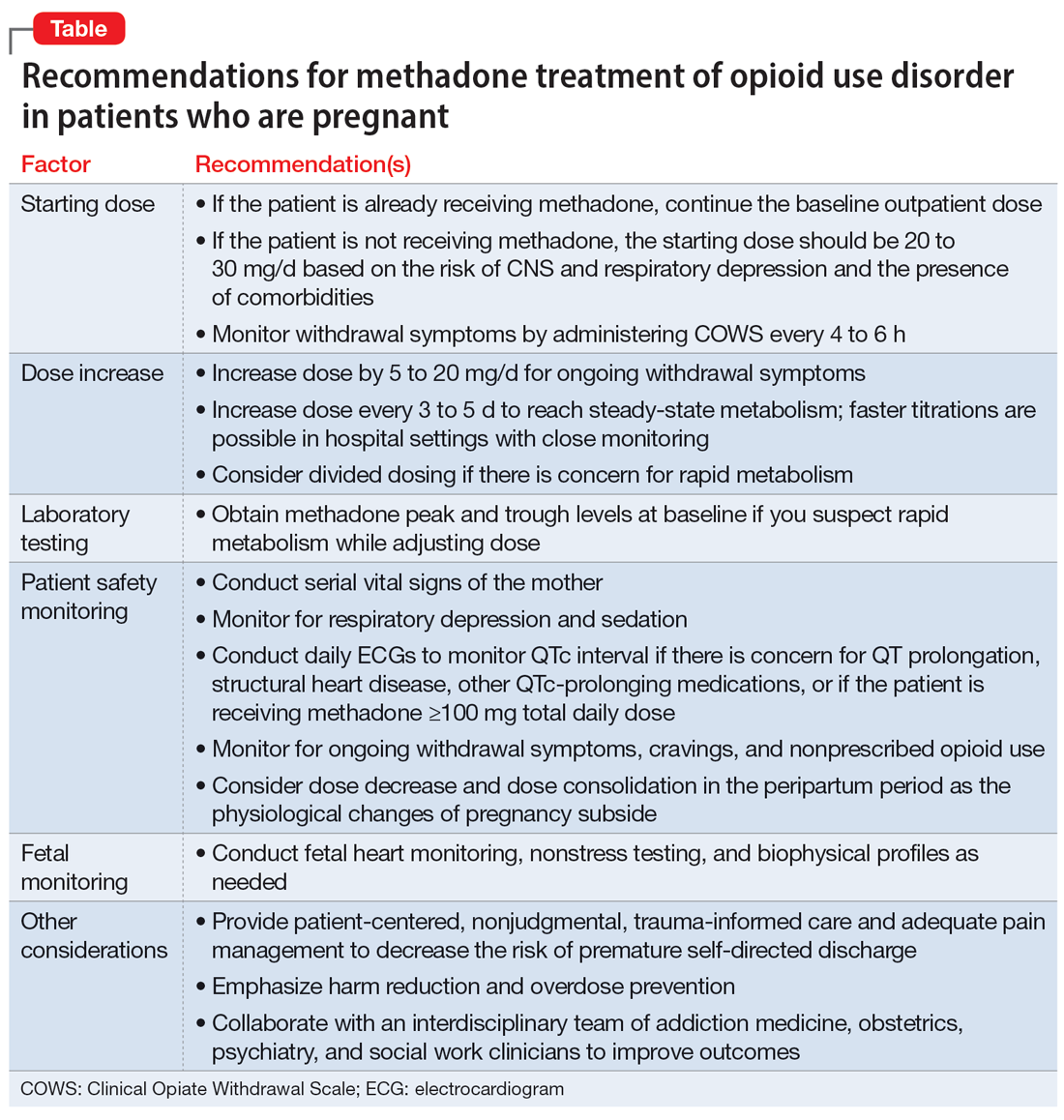
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.
Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
