User login
Female Runner, 47, with Inguinal Lump
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
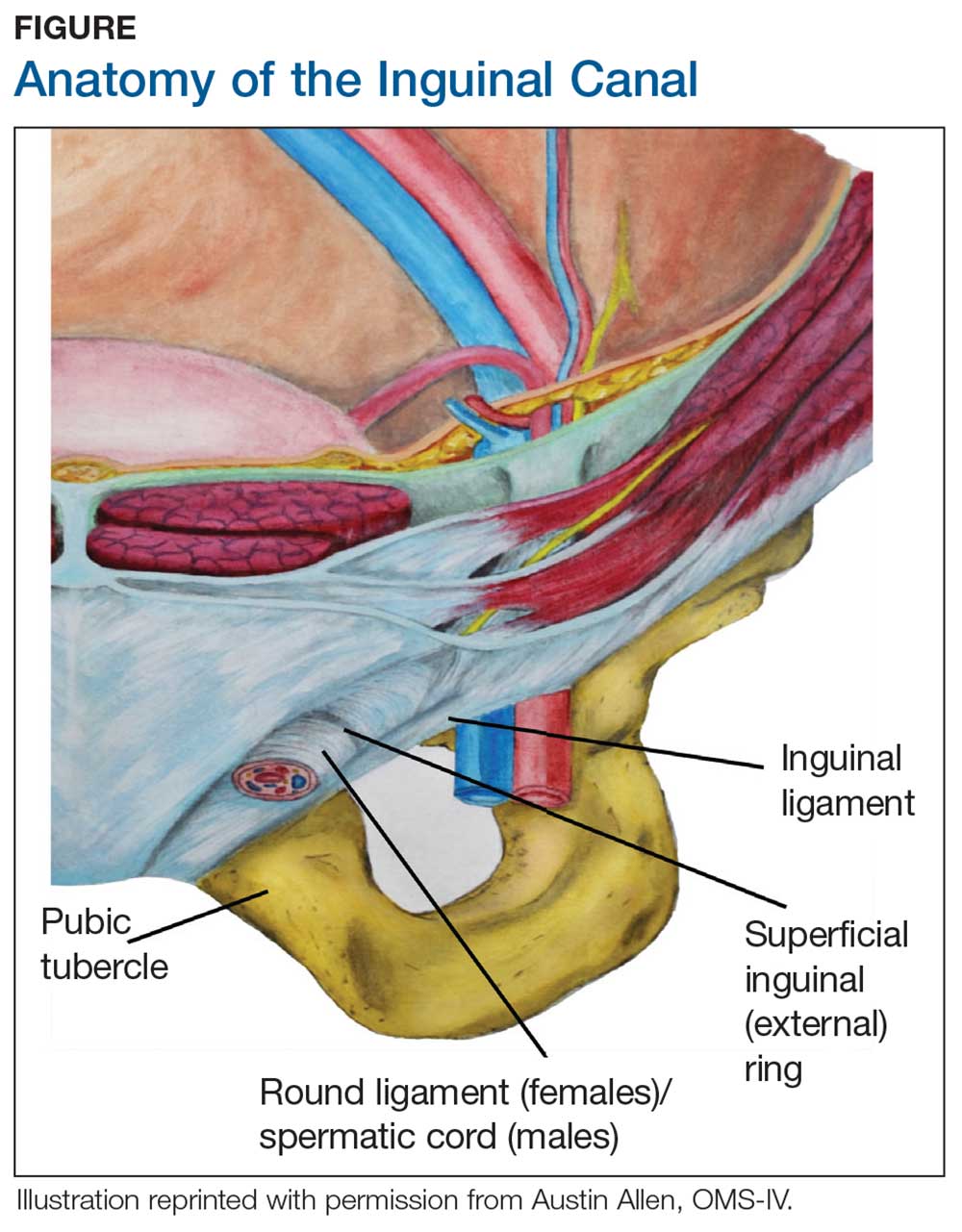
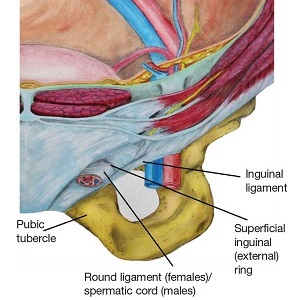
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.