User login
Impact of Standardized Screening Protocols for Cystic Fibrosis–Related Diabetes in a Pediatric Population
From Children’s Hospitals and Clinics of Minnesota, and Children’s Respiratory and Critical Care Specialists, Minneapolis, MN.
Abstract
- Objective: In an effort to improve our pediatric center’s processes for screening, identifying, and treating cystic fibrosis–related diabetes (CFRD), we aimed to create outpatient and inpatient CFRD screening protocols.
- Methods: We identified barriers in our existing screening processes. The lab protocol for outpatients receiving oral glucose tolerance tests was streamlined. Inpatient screening order sets were developed. Interdisciplinary communication between pulmonary and endocrine care teams was improved. A protocol was developed for endocrinology consultation and follow-up for CFRD patients. Staff and families received additional education.
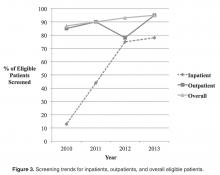
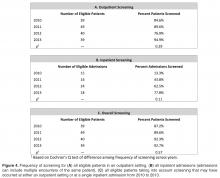
- Results: Outpatient screening was 85% in 2010, 90% in 2011, 77% in 2012, and 95% in 2013 (P = 0.29). Inpatient screening was 13% in 2010, 44% in 2011, 63% in 2012, and 78% 2013 (P = 0.11). Therefore, the combined screening protocols improved overall screening from 87% in 2010 to 90% in 2011, 92% in 2012, and 93% in 2013 (P = 0.57).
- Conclusion: Development of screening protocols improved identification of patients with CFRD.
The most prevalent comorbidity of cystic fibrosis (CF) is cystic fibrosis–related diabetes (CFRD) [1]. The incidence of CFRD increases with age and disease progression. In 2009, Moran et al noted a CFRD prevalence of approximately 20% in adolescents and 40% to 50% in adults [1]. An early diagnosis is especially important due to the correlation of an insulin-deficient state with pulmonary decline, increased pulmonary exacerbations, nutritional impairment, and increased mortality [2–6].
In 2009, the CF Foundation (CFF), the American Diabetes Association, and the Pediatric Endocrine Society updated the clinical care guidelines for the screening, diagnosis, and medical management of CFRD [7]. Soon after, the CFF, the Pediatric Endocrine Society, and the Dartmouth Institute Microsystem Academy sponsored a Learning and Leadership Collaborative (LLC) focusing on CFRD with the purpose of standardizing evidence-based clinical care processes to improve outcomes for patients with CF [8]. Our institution was selected to participate in this endeavor along with 6 other accredited CF centers in the United States.
We report how our pediatric institution established CFRD screening processes in the areas of outpatient care and inpatient care, thereby increasing screening rates.
Methods
Context
Our center cares for approximately 140 pediatric patients with CF. Our CF multidisciplinary program provides outpatient care within a private practice as well as inpatient hospital care within an independent, not-for-profit health care system. The clinic and hospital collaboratively provide services for our patients. Our center has a comprehensive annual clinic day for each child including annual laboratory tests, x-rays, pulmonary function tests, and interaction with the multidisciplinary CF team.
This year-long CFRD screening project was conducted from 2011 to 2012. We began this project with an outpatient screening rate of 85% in 2010. While this rate is high, we identified room for improvement in our screening processes for both outpatients and inpatients. The LLC provided tools and coaching during the project. Locally, we received support and leadership from our institution.
Reflecting on our initial high outpatient screening rate, we identified 2 pre-existing elements:
- As the affiliate center of the University of Minnesota, we have been influenced by their leadership in the field of CFRD. Accordingly, we have made screening for CFRD a priority, which included integrating an endocrinologist into our CF team.
- A review of our established annual patient standard-of-care laboratory results demonstrated 75% of patients in 2010 were completing laboratory testing. Therefore, the timing of the oral glucose tolerance test (OGTT) was changed from an unscheduled basis to becoming part of the outpatient annual lab protocol in an effort to screen the majority of patients.
Target Patient Population
The 2010 CFF guidelines recommend screening patients for CFRD beginning at age 10 years; however, it has been our practice to initiate screening beginning at age 8 years. For the purpose of this project and to increase generalizability for other centers, our results were adapted to include only patients 10 years of age and older.
Definitions
Successful outpatient screening was defined as completion of a 2-hour OGTT obtained by the following process: 1) The patient fasted for 8 hours, 2) a venous blood sample was drawn for a fasting serum glucose, 3) the patient consumed an oral glucose dose of 75 g within 5 minutes of the fasting blood draw, 4) a venous blood sample was drawn 2 hours after glucose administration for the postprandial serum glucose [9]. Patients weighing less than 43 kg received 1.75 g/kg of oral glucose. Outpatients eligible for screening were 10 years of age or older at their first quarterly visit of the year when annual laboratory tests including serum glucose are drawn.
Inpatient screening parameters were determined with guidance from the clinical care guidelines for cystic fibrosis-related diabetes [7], which recommends “monitoring fasting and 2-hour postprandial plasma glucose levels for the first 48 hours.” As this recommendation leaves room for interpretation as far as the quantity and interval of testing, we elected to define a successful screening as completion of one 2-hour postprandial plasma glucose level within 24 hours of admission plus one fasting glucose level within 48 hours of admission. If a patient was identified as having a fasting level ≥ 125 mg/dL or a 2-hour postprandial ≥ 200 mg/dL, additional glucose testing continued beyond 48 hours. However, for the purpose of identifying a successful screening, only the criteria of completing one 2-hour postprandial glucose plus one fasting glucose was considered. Successful screening for inpatients who received a course of steroids was defined as completion of three 2-hour postprandial plasma glucose levels within 24, 48, and 72 hours of admission or initiation of steroids. Inpatients eligible for screening were ten years of age or older at the time of admission.
Patients were not included if they were lost to follow-up for longer than 1 year or were seen for 2 or more quarterly visits at another CF center. Additionally, patients were not eligible if they had been previously diagnosed with CFRD.
Ethical Considerations
Ethical approval for this project was provided by Children’s Hospitals and Clinics of Minnesota Institutional Review Board.
Strategy for Change
We applied the principles of clinical microsystems and conducted numerous tests of change following the Plan-Do-Study-Act (PDSA) technique [10,11]. We organized a core team consisting of 6 individuals: our CF center director (pulmonologist); a hospital-employed pediatric endocrinologist; an outpatient CF clinic nurse coordinator; the hospital’s CF coordinator (pediatric nurse practitioner); a hospital-employed, certified diabetes educator (nurse); and the hospital’s CF dietitian. The core team met weekly throughout the year-long project and ensured other CF care providers were kept up-to-date on the changes implemented with the project.
Interventions
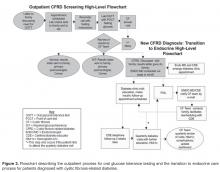
Outpatient Screening
The OGTT was added to a previously established protocol for all patients to complete their annual labs at their first visit of the year. We stressed the importance and rationale for the OGTT in an annual clinic letter sent to families. The family was also sent a reminder letter with fasting instructions, a copy of the annual lab orders, and a reminder telephone call before the appointment. Based on family input regarding delays in the turn-around time for venous blood samples, a point of care (POC) glucose protocol was implemented. Laboratory personnel drew annual blood work, completed a POC blood glucose (in addition to the serum glucose), and administered the oral glucose load if the POC glucose was < 200 mg/dL.
Subsequent to completion of our project, we learned that our lab had inadvertently administered a 37.5-g dose for the OGTT instead of the recommended 75-g dose. To identify patients who may have had CFRD but were not diagnosed due to the low oral glucose dose, we have screened all patients with the corrected dose since January 2013.
To improve communication between the pulmonary and endocrine teams, weekly meetings were scheduled. The teams reviewed patients who had recently completed their annual laboratory tests or recently saw an endocrinology provider. After review of lab values, the patient families were mailed letters informing them of their child’s glucose test results which were categorized as normal, impaired, or abnormal suggesting CFRD. If the patient did not complete the OGTT, the family was mailed a letter reiterating the importance of screening. In the event of an abnormal OGTT suggesting CFRD, the results were discussed with the family during a clinic appointment. The endocrinologist and diabetes educator were subsequently notified, and an endocrine clinic appointment was arranged with the family to discuss the results and care plan. An electronic dashboard (spreadsheet) was created to track lab values and clinic visits for patients with impaired blood glucose tolerance as well as CFRD. The dashboard was reviewed and updated on a quarterly basis.
Inpatient Screening
To improve screening of hospitalized patients with CF not known to have CFRD, 4 standardized order sets detailing blood glucose testing schedules were developed in our electronic medical record as listed below. Our team educated physicians and nurses on the standardized order sets as well as patient families about the additional testing needed during hospitalization. An endocrine nurse practitioner conducted daily rounds on weekdays. All glucose results were verified by the laboratory. If a patient was identified to have a fasting blood glucose ≥ 125 mg/dL or 2-hour postprandial ≥ 200 mg/dL, the endocrine service was notified, and additional glucose testing was ordered. If the patient’s glucose levels persisted at high levels, meeting the criteria for a diagnosis of CFRD, further education was provided for the family and follow-up care was arranged.
Order Sets
1) Not receiving G-tube feedings
Day 1: 2-hour postprandial
Day 2: Fasting
2) Receiving G-tube feedings
Day 1: 2-hour postprandial, 2-hours after start of
G-tube feeds and at end of G-tube feeds
3) Receiving low-dose steroid therapy (IV methylprednisolone up to 4 mg/kg per day, administered every 6 hours and oral prednisolone 1 mg/kg twice per day for 5 days) [12]
Day 1: 2-hour postprandial
Day 2: Fasting and 2-hour postprandial
Day 3: 2-hour postprandial
4) Receiving high-dose steroid therapy per the allergic bronchopulmonary aspergillosis protocol (IV methylprednisolone 10–15 mg/kg once per day for 3 days) [12]
Days 1-4: Point of care prior to every meal, at bedtime, and at 0200 hours
Analysis
All patients with CF who are seen at our clinic were included in this project. Approximately 96% of our patients have consented to be part of the CFF supported patient registry, PortCF [13]. Data are entered by the CF clinic nurse coordinator for these patients to document clinic visits, hospital admissions, medications, lab values, and other parameters. We retrieved data from PortCF documenting the number of patients eligible for the OGTT and the number of patients diagnosed with CFRD. Individual medical records were cross-referenced with these results and also reviewed for patients who do not participate in PortCF. We retrieved data reports from our hospital informatics department to identify the number of patients with CF who were hospitalized each year and to obtain all glucose levels obtained during hospitalization. From these data, we calculated screening rates and the annual number of patients diagnosed with CFRD. We used the Cochran’s Q test to compare the differences in frequency of screening across years.
Results
The newly implemented inpatient protocol, formally instituted in the third quarter of 2011, yielded a 43.8% screening rate in 2011 compared to a rate of 13.3% in 2010. Inpatient screening rates improved to 62.5% in 2012, and 77.8% in 2013 (Figures 3 and 4).
In 2011, 10 patients with CFRD were identified: 6 patients were diagnosed retrospectively based on a review of glucose levels collected in 2010 prior to the release of the new guidelines; 1 was diagnosed via inpatient screening; 3 were diagnosed via outpatient screening. In 2012, 5 patients with CFRD were identified: 4 were diagnosed via inpatient screening and one was diagnosed via outpatient screening. In 2013, 5 patients were identified: 1 diagnosed via inpatient screening and 4 diagnosed via outpatient screening. All diagnosed patients met the criteria of having a fasting blood sugar ≥ 126 mg/dL or a 2-hour postprandial ≥ 200 mg/dL that persisted for more than 48 hours.
Discussion
This collaborative initiative resulted in the development of structured screening protocols leading to overall screening improvement. A standardized screening protocol is fundamental to the identification and subsequent treatment of a patient with CFRD. Patients with CFRD may have decreased mortality when diagnosed early and treated aggressively, underscoring the necessity for CF centers to improve screening protocols [1,6].
Determining the best methods for implementation may vary from center to center, but the key factors we have identified from this project include strong leadership and team commitment—elements that have previously been identified as being predictive of positive quality improvement outcomes [14].
Education of staff, families, and patients is also an important factor. Education played a significant role during the inpatient screening PDSA cycles. For example, a time intensive but worthwhile training was conducted for the physicians and nurses on the order sets. Education for the patients and families during inpatient stays led to a better understanding of the rationale for, and support of, the additional blood draws.
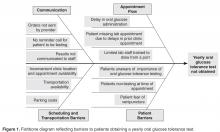
The importance of family involvement was evident as we refined our outpatient protocol. Input from the phone surveys allowed us to identify barriers in our process that led us to establish more efficient clinic visits with reduced time in the lab, improved clinic flow and increased patient satisfaction. Other important factors were co-location of the endocrine and pulmonary clinics and flexibility of scheduling to facilitate seeing patients in both clinics on the same day.
Although we uncovered an error in oral glucose dosing, we were able to appropriately screen the majority of these patients within the next year. At the conclusion of 2013, our outpatient protocol had facilitated a screening rate of 95%, surpassing the 2012 rate of 77%. Interestingly, 2 of the patients diagnosed with CFRD in 2011 were diagnosed by HbA1c criteria, with normal OGTT results, perhaps due to the substandard glucose dose. Only 5 additional patients screened positive for CFRD at the end of 2013, which is the same number identified in 2012. It is unclear whether they would have been diagnosed earlier with the recommended dose of oral glucose or if they developed CFRD due to the progression of their disease.
A limitation of this project may be its reproducibility at other institutions. It is likely that the success of this project was due to the strong relationship between the endocrinology and pulmonary teams, committed leadership, institutional support for co-location of the clinics, and the team’s competency in quality improvement techniques with guidance from the LLC. The process of developing, implementing, and sustaining screening protocols was time-intensive and may be difficult to replicate in another center without similar resources available.
A strong motivation behind this project was the LLC, and while other centers may not have the same opportunity, the CFRD Evidence-Based Practice and Smart Change Idea Compendium was published as result of the LLC to serve as a guide for other centers [8]. Kern et al successfully demonstrated that a process based on the ideas from this compendium can help achieve higher OGTT outpatient screening rates in a CF center [15]. Kern et al’s project was similar to ours although the duration was shorter (< 1 year) and it began with a 47% screening rate prior to implementation. Our center extended the efforts of Kern et al by implementing our initiative over the course of 3 years, but unlike Kern et al, we included patients with failed or rescheduled appointments. Kern et al defined, identified, and excluded patients with moderate or severe pulmonary exacerbation from their eligible screening pool. We included all patients over the course of our year-long screening periods, as we have created an opportunity to screen ill patients at subsequent visits or as inpatients.
As with any quality improvement project, there is the risk of not sustaining improvements. In 2012 our outpatient screening rates were lower than the previous year. However, the coordination of the outpatient and inpatient protocols helped us achieve an improved overall screening rate of 92% in 2012. One possible explanation for the decrease in 2012 outpatient screening is that several patients eligible for screening in 2012 were less adherent with attending clinic visits. Five of these patients transitioned to an adult center or were diagnosed with CFRD in 2013. With this shift in the eligibility pool, our outpatient screening rate for 2013 surpassed our rate from 2012. In an attempt to sustain our gains, members of our team continue to review patient screening and endocrine referrals at monthly meetings. The decrease in outpatient screening demonstrates the importance of ongoing evaluation and monitoring of quality improvement projects after the initial objectives have been achieved.
Typically, our less adherent patients are only seen in clinic when experiencing an exacerbation when we cannot administer the OGTT. As a result, these patients are generally admitted for treatment of their exacerbation, and we are able to screen them during their hospitalization. Of the 9 patients not screened as outpatients in 2012, 7 were screened as inpatients. Three patients were unscreened that year: 1 patient failed to fast, another refused the test, and the last patient was inadvertently missed. While our intention is to screen all patients as an outpatient with OGTT, we have found that the only opportunity we have for screening less adherent patients is often while hospitalized, emphasizing the importance of a dual screening approach. This approach has allowed us to screen the majority of our patients as reflected in our overall screening rate.
One of our major challenges moving forward is to help the patients diagnosed with CFRD and their families accept yet another diagnosis and the burden of care associated with it. Our focus has shifted now to determine the best methods to motivate patients with CFRD to regularly attend endocrine clinic appointments, recognizing the challenges of additional clinic visits, monitoring, and medications. It is interesting to speculate whether improved CFRD clinical outcomes may correlate with improved screening rates. In 2012 our patients had a median HbA1c of 5.7 when the national average is 6.6 [16]. Further research is needed to delineate a possible relationship between an effective screening protocol and favorable clinical outcome measures.
Conclusion
The use of a structured process developed by a multidisciplinary team resulted in improved CFRD screening rates. In addition to outpatient protocols, it is critical to develop inpatient glucose testing protocols in order to capture patients who are only seen at times of exacerbation. Even in this era of treatment at a cellular level with correctors and potentiators, early detection and treatment of CFRD is essential for optimal clinical outcomes [1,5,17]. The next step for us is to sustain our gains and to improve endocrine care facilitation. We hope this report may guide other teams and institutional leadership in their efforts to improve identification of individuals with CFRD.
Acknowledgments: We would like to acknowledge Gautham Suresh, MD, Jennifer Abuzzahab, MD, Robert Payne, MD, and Andrew Flood, PhD, for their assistance in the preparation of our manuscript. We also acknowledge John Nash, MSW, LMSW, who provided us tools and coaching throughout the Learning and Leadership Collaborative.
Corresponding author: Lisa Read, MPH, 2525 Chicago Ave. South, MS 17-750, Minneapolis, MN 55404, lisa.read@childrensmn.org.
Funding/support. This work was supported by a grant from the Cystic Fibrosis Foundation for the Learning and Leadership Collaborative: Cystic Fibrosis-Related Diabetes Care (MCNAMA11Q10, to Dr. McNamara).
1. Moran A, Dunitz J, Nathan B, et al. Cystic fibrosis–related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–31.
2. Cawood TJ, McKenna MJ, Gallagher CG, et al. Cystic fibrosis-related diabetes in adults. Ir Med J 2006;99:83–6.
3. Koch C, Rainisio M, Madessani U, et al. Investigators of the European Epidemiologic Registry of Cystic Fibrosis. Presence of cystic fibrosis-related diabetes mellitus tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001;32:343–50.
4. Marshall BC, Butler SM, Stoddard M, et al. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–7.
5. Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141–4.
6. Lewis C, Blackman SM, Nelson A, et al. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med 2015;191:194–200.
7. Moran A, Brunzell C, Cohen, RC, et al. Clinical Care guidelines for cystic fibrosis–related diabetes. A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–708.
8. Nash J, Messier R, Casella SJ, et al. Learning and Leadership Collaborative: CFRD. Cystic fibrosis related diabetes (CFRD) evidence-based practice and smart change idea compendium 2012. Bethesda, MD: Cystic Fibrosis Foundation; McLean, VA: Pediatric Endocrine Society; Lebanon, NH: Dartmouth Institute Microsystem Academy; May 2012.
9. WHO Expert Committee on Diabetes Mellitus: Second Report of the WHO Expert Committee on Diabetes Mellitus. Geneva: World Health Organization; 1980 (Tech. Rep. Ser no. 646).
10. Cystic Fibrosis Foundation, Dartmouth Medical School: Center for the Evaluative Clinical Sciences, Dartmouth/Hitchcock Medical Center. Action guide for accelerating improvement in cystic fibrosis care: clinical microsystems. 2006.
11. Langley GJ, Nolan KM, Nolan TW, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco, CA: Jossey-Bass; 1996.
12. Cohen-Cymberknoh M, Blau H, Shoseyov D, et al. Intravenous monthly pulse methylprednisolone treatment for ABPA in patients with cystic fibrosis. J Cyst Fibros 2009;8:253–7.
13. Center Specific Patient Registry Report for 2011. Bethesda, MD: Cystic Fibrosis Foundation; 2012.
14. Parker VA, Wubbenhorst WH, Young GJ, et al. Implementing quality improvement in hospitals: The role of leadership and culture. Am J Med Qual 1999; 14:64–9.
15. Kern AS, Prestridge AL. Improving screening for cystic fibrosis-related diabetes at a pediatric cystic fibrosis program. Pediatrics 2013;132:e512–8.
16. Center Specific Patient Registry Report for 2012. Bethesda, MD: Cystic Fibrosis Foundation; 2013.
17. Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–61.
From Children’s Hospitals and Clinics of Minnesota, and Children’s Respiratory and Critical Care Specialists, Minneapolis, MN.
Abstract
- Objective: In an effort to improve our pediatric center’s processes for screening, identifying, and treating cystic fibrosis–related diabetes (CFRD), we aimed to create outpatient and inpatient CFRD screening protocols.
- Methods: We identified barriers in our existing screening processes. The lab protocol for outpatients receiving oral glucose tolerance tests was streamlined. Inpatient screening order sets were developed. Interdisciplinary communication between pulmonary and endocrine care teams was improved. A protocol was developed for endocrinology consultation and follow-up for CFRD patients. Staff and families received additional education.
- Results: Outpatient screening was 85% in 2010, 90% in 2011, 77% in 2012, and 95% in 2013 (P = 0.29). Inpatient screening was 13% in 2010, 44% in 2011, 63% in 2012, and 78% 2013 (P = 0.11). Therefore, the combined screening protocols improved overall screening from 87% in 2010 to 90% in 2011, 92% in 2012, and 93% in 2013 (P = 0.57).
- Conclusion: Development of screening protocols improved identification of patients with CFRD.
The most prevalent comorbidity of cystic fibrosis (CF) is cystic fibrosis–related diabetes (CFRD) [1]. The incidence of CFRD increases with age and disease progression. In 2009, Moran et al noted a CFRD prevalence of approximately 20% in adolescents and 40% to 50% in adults [1]. An early diagnosis is especially important due to the correlation of an insulin-deficient state with pulmonary decline, increased pulmonary exacerbations, nutritional impairment, and increased mortality [2–6].
In 2009, the CF Foundation (CFF), the American Diabetes Association, and the Pediatric Endocrine Society updated the clinical care guidelines for the screening, diagnosis, and medical management of CFRD [7]. Soon after, the CFF, the Pediatric Endocrine Society, and the Dartmouth Institute Microsystem Academy sponsored a Learning and Leadership Collaborative (LLC) focusing on CFRD with the purpose of standardizing evidence-based clinical care processes to improve outcomes for patients with CF [8]. Our institution was selected to participate in this endeavor along with 6 other accredited CF centers in the United States.
We report how our pediatric institution established CFRD screening processes in the areas of outpatient care and inpatient care, thereby increasing screening rates.
Methods
Context
Our center cares for approximately 140 pediatric patients with CF. Our CF multidisciplinary program provides outpatient care within a private practice as well as inpatient hospital care within an independent, not-for-profit health care system. The clinic and hospital collaboratively provide services for our patients. Our center has a comprehensive annual clinic day for each child including annual laboratory tests, x-rays, pulmonary function tests, and interaction with the multidisciplinary CF team.
This year-long CFRD screening project was conducted from 2011 to 2012. We began this project with an outpatient screening rate of 85% in 2010. While this rate is high, we identified room for improvement in our screening processes for both outpatients and inpatients. The LLC provided tools and coaching during the project. Locally, we received support and leadership from our institution.
Reflecting on our initial high outpatient screening rate, we identified 2 pre-existing elements:
- As the affiliate center of the University of Minnesota, we have been influenced by their leadership in the field of CFRD. Accordingly, we have made screening for CFRD a priority, which included integrating an endocrinologist into our CF team.
- A review of our established annual patient standard-of-care laboratory results demonstrated 75% of patients in 2010 were completing laboratory testing. Therefore, the timing of the oral glucose tolerance test (OGTT) was changed from an unscheduled basis to becoming part of the outpatient annual lab protocol in an effort to screen the majority of patients.
Target Patient Population
The 2010 CFF guidelines recommend screening patients for CFRD beginning at age 10 years; however, it has been our practice to initiate screening beginning at age 8 years. For the purpose of this project and to increase generalizability for other centers, our results were adapted to include only patients 10 years of age and older.
Definitions
Successful outpatient screening was defined as completion of a 2-hour OGTT obtained by the following process: 1) The patient fasted for 8 hours, 2) a venous blood sample was drawn for a fasting serum glucose, 3) the patient consumed an oral glucose dose of 75 g within 5 minutes of the fasting blood draw, 4) a venous blood sample was drawn 2 hours after glucose administration for the postprandial serum glucose [9]. Patients weighing less than 43 kg received 1.75 g/kg of oral glucose. Outpatients eligible for screening were 10 years of age or older at their first quarterly visit of the year when annual laboratory tests including serum glucose are drawn.
Inpatient screening parameters were determined with guidance from the clinical care guidelines for cystic fibrosis-related diabetes [7], which recommends “monitoring fasting and 2-hour postprandial plasma glucose levels for the first 48 hours.” As this recommendation leaves room for interpretation as far as the quantity and interval of testing, we elected to define a successful screening as completion of one 2-hour postprandial plasma glucose level within 24 hours of admission plus one fasting glucose level within 48 hours of admission. If a patient was identified as having a fasting level ≥ 125 mg/dL or a 2-hour postprandial ≥ 200 mg/dL, additional glucose testing continued beyond 48 hours. However, for the purpose of identifying a successful screening, only the criteria of completing one 2-hour postprandial glucose plus one fasting glucose was considered. Successful screening for inpatients who received a course of steroids was defined as completion of three 2-hour postprandial plasma glucose levels within 24, 48, and 72 hours of admission or initiation of steroids. Inpatients eligible for screening were ten years of age or older at the time of admission.
Patients were not included if they were lost to follow-up for longer than 1 year or were seen for 2 or more quarterly visits at another CF center. Additionally, patients were not eligible if they had been previously diagnosed with CFRD.
Ethical Considerations
Ethical approval for this project was provided by Children’s Hospitals and Clinics of Minnesota Institutional Review Board.
Strategy for Change
We applied the principles of clinical microsystems and conducted numerous tests of change following the Plan-Do-Study-Act (PDSA) technique [10,11]. We organized a core team consisting of 6 individuals: our CF center director (pulmonologist); a hospital-employed pediatric endocrinologist; an outpatient CF clinic nurse coordinator; the hospital’s CF coordinator (pediatric nurse practitioner); a hospital-employed, certified diabetes educator (nurse); and the hospital’s CF dietitian. The core team met weekly throughout the year-long project and ensured other CF care providers were kept up-to-date on the changes implemented with the project.
Interventions
Outpatient Screening
The OGTT was added to a previously established protocol for all patients to complete their annual labs at their first visit of the year. We stressed the importance and rationale for the OGTT in an annual clinic letter sent to families. The family was also sent a reminder letter with fasting instructions, a copy of the annual lab orders, and a reminder telephone call before the appointment. Based on family input regarding delays in the turn-around time for venous blood samples, a point of care (POC) glucose protocol was implemented. Laboratory personnel drew annual blood work, completed a POC blood glucose (in addition to the serum glucose), and administered the oral glucose load if the POC glucose was < 200 mg/dL.
Subsequent to completion of our project, we learned that our lab had inadvertently administered a 37.5-g dose for the OGTT instead of the recommended 75-g dose. To identify patients who may have had CFRD but were not diagnosed due to the low oral glucose dose, we have screened all patients with the corrected dose since January 2013.
To improve communication between the pulmonary and endocrine teams, weekly meetings were scheduled. The teams reviewed patients who had recently completed their annual laboratory tests or recently saw an endocrinology provider. After review of lab values, the patient families were mailed letters informing them of their child’s glucose test results which were categorized as normal, impaired, or abnormal suggesting CFRD. If the patient did not complete the OGTT, the family was mailed a letter reiterating the importance of screening. In the event of an abnormal OGTT suggesting CFRD, the results were discussed with the family during a clinic appointment. The endocrinologist and diabetes educator were subsequently notified, and an endocrine clinic appointment was arranged with the family to discuss the results and care plan. An electronic dashboard (spreadsheet) was created to track lab values and clinic visits for patients with impaired blood glucose tolerance as well as CFRD. The dashboard was reviewed and updated on a quarterly basis.
Inpatient Screening
To improve screening of hospitalized patients with CF not known to have CFRD, 4 standardized order sets detailing blood glucose testing schedules were developed in our electronic medical record as listed below. Our team educated physicians and nurses on the standardized order sets as well as patient families about the additional testing needed during hospitalization. An endocrine nurse practitioner conducted daily rounds on weekdays. All glucose results were verified by the laboratory. If a patient was identified to have a fasting blood glucose ≥ 125 mg/dL or 2-hour postprandial ≥ 200 mg/dL, the endocrine service was notified, and additional glucose testing was ordered. If the patient’s glucose levels persisted at high levels, meeting the criteria for a diagnosis of CFRD, further education was provided for the family and follow-up care was arranged.
Order Sets
1) Not receiving G-tube feedings
Day 1: 2-hour postprandial
Day 2: Fasting
2) Receiving G-tube feedings
Day 1: 2-hour postprandial, 2-hours after start of
G-tube feeds and at end of G-tube feeds
3) Receiving low-dose steroid therapy (IV methylprednisolone up to 4 mg/kg per day, administered every 6 hours and oral prednisolone 1 mg/kg twice per day for 5 days) [12]
Day 1: 2-hour postprandial
Day 2: Fasting and 2-hour postprandial
Day 3: 2-hour postprandial
4) Receiving high-dose steroid therapy per the allergic bronchopulmonary aspergillosis protocol (IV methylprednisolone 10–15 mg/kg once per day for 3 days) [12]
Days 1-4: Point of care prior to every meal, at bedtime, and at 0200 hours
Analysis
All patients with CF who are seen at our clinic were included in this project. Approximately 96% of our patients have consented to be part of the CFF supported patient registry, PortCF [13]. Data are entered by the CF clinic nurse coordinator for these patients to document clinic visits, hospital admissions, medications, lab values, and other parameters. We retrieved data from PortCF documenting the number of patients eligible for the OGTT and the number of patients diagnosed with CFRD. Individual medical records were cross-referenced with these results and also reviewed for patients who do not participate in PortCF. We retrieved data reports from our hospital informatics department to identify the number of patients with CF who were hospitalized each year and to obtain all glucose levels obtained during hospitalization. From these data, we calculated screening rates and the annual number of patients diagnosed with CFRD. We used the Cochran’s Q test to compare the differences in frequency of screening across years.
Results
The newly implemented inpatient protocol, formally instituted in the third quarter of 2011, yielded a 43.8% screening rate in 2011 compared to a rate of 13.3% in 2010. Inpatient screening rates improved to 62.5% in 2012, and 77.8% in 2013 (Figures 3 and 4).
In 2011, 10 patients with CFRD were identified: 6 patients were diagnosed retrospectively based on a review of glucose levels collected in 2010 prior to the release of the new guidelines; 1 was diagnosed via inpatient screening; 3 were diagnosed via outpatient screening. In 2012, 5 patients with CFRD were identified: 4 were diagnosed via inpatient screening and one was diagnosed via outpatient screening. In 2013, 5 patients were identified: 1 diagnosed via inpatient screening and 4 diagnosed via outpatient screening. All diagnosed patients met the criteria of having a fasting blood sugar ≥ 126 mg/dL or a 2-hour postprandial ≥ 200 mg/dL that persisted for more than 48 hours.
Discussion
This collaborative initiative resulted in the development of structured screening protocols leading to overall screening improvement. A standardized screening protocol is fundamental to the identification and subsequent treatment of a patient with CFRD. Patients with CFRD may have decreased mortality when diagnosed early and treated aggressively, underscoring the necessity for CF centers to improve screening protocols [1,6].
Determining the best methods for implementation may vary from center to center, but the key factors we have identified from this project include strong leadership and team commitment—elements that have previously been identified as being predictive of positive quality improvement outcomes [14].
Education of staff, families, and patients is also an important factor. Education played a significant role during the inpatient screening PDSA cycles. For example, a time intensive but worthwhile training was conducted for the physicians and nurses on the order sets. Education for the patients and families during inpatient stays led to a better understanding of the rationale for, and support of, the additional blood draws.
The importance of family involvement was evident as we refined our outpatient protocol. Input from the phone surveys allowed us to identify barriers in our process that led us to establish more efficient clinic visits with reduced time in the lab, improved clinic flow and increased patient satisfaction. Other important factors were co-location of the endocrine and pulmonary clinics and flexibility of scheduling to facilitate seeing patients in both clinics on the same day.
Although we uncovered an error in oral glucose dosing, we were able to appropriately screen the majority of these patients within the next year. At the conclusion of 2013, our outpatient protocol had facilitated a screening rate of 95%, surpassing the 2012 rate of 77%. Interestingly, 2 of the patients diagnosed with CFRD in 2011 were diagnosed by HbA1c criteria, with normal OGTT results, perhaps due to the substandard glucose dose. Only 5 additional patients screened positive for CFRD at the end of 2013, which is the same number identified in 2012. It is unclear whether they would have been diagnosed earlier with the recommended dose of oral glucose or if they developed CFRD due to the progression of their disease.
A limitation of this project may be its reproducibility at other institutions. It is likely that the success of this project was due to the strong relationship between the endocrinology and pulmonary teams, committed leadership, institutional support for co-location of the clinics, and the team’s competency in quality improvement techniques with guidance from the LLC. The process of developing, implementing, and sustaining screening protocols was time-intensive and may be difficult to replicate in another center without similar resources available.
A strong motivation behind this project was the LLC, and while other centers may not have the same opportunity, the CFRD Evidence-Based Practice and Smart Change Idea Compendium was published as result of the LLC to serve as a guide for other centers [8]. Kern et al successfully demonstrated that a process based on the ideas from this compendium can help achieve higher OGTT outpatient screening rates in a CF center [15]. Kern et al’s project was similar to ours although the duration was shorter (< 1 year) and it began with a 47% screening rate prior to implementation. Our center extended the efforts of Kern et al by implementing our initiative over the course of 3 years, but unlike Kern et al, we included patients with failed or rescheduled appointments. Kern et al defined, identified, and excluded patients with moderate or severe pulmonary exacerbation from their eligible screening pool. We included all patients over the course of our year-long screening periods, as we have created an opportunity to screen ill patients at subsequent visits or as inpatients.
As with any quality improvement project, there is the risk of not sustaining improvements. In 2012 our outpatient screening rates were lower than the previous year. However, the coordination of the outpatient and inpatient protocols helped us achieve an improved overall screening rate of 92% in 2012. One possible explanation for the decrease in 2012 outpatient screening is that several patients eligible for screening in 2012 were less adherent with attending clinic visits. Five of these patients transitioned to an adult center or were diagnosed with CFRD in 2013. With this shift in the eligibility pool, our outpatient screening rate for 2013 surpassed our rate from 2012. In an attempt to sustain our gains, members of our team continue to review patient screening and endocrine referrals at monthly meetings. The decrease in outpatient screening demonstrates the importance of ongoing evaluation and monitoring of quality improvement projects after the initial objectives have been achieved.
Typically, our less adherent patients are only seen in clinic when experiencing an exacerbation when we cannot administer the OGTT. As a result, these patients are generally admitted for treatment of their exacerbation, and we are able to screen them during their hospitalization. Of the 9 patients not screened as outpatients in 2012, 7 were screened as inpatients. Three patients were unscreened that year: 1 patient failed to fast, another refused the test, and the last patient was inadvertently missed. While our intention is to screen all patients as an outpatient with OGTT, we have found that the only opportunity we have for screening less adherent patients is often while hospitalized, emphasizing the importance of a dual screening approach. This approach has allowed us to screen the majority of our patients as reflected in our overall screening rate.
One of our major challenges moving forward is to help the patients diagnosed with CFRD and their families accept yet another diagnosis and the burden of care associated with it. Our focus has shifted now to determine the best methods to motivate patients with CFRD to regularly attend endocrine clinic appointments, recognizing the challenges of additional clinic visits, monitoring, and medications. It is interesting to speculate whether improved CFRD clinical outcomes may correlate with improved screening rates. In 2012 our patients had a median HbA1c of 5.7 when the national average is 6.6 [16]. Further research is needed to delineate a possible relationship between an effective screening protocol and favorable clinical outcome measures.
Conclusion
The use of a structured process developed by a multidisciplinary team resulted in improved CFRD screening rates. In addition to outpatient protocols, it is critical to develop inpatient glucose testing protocols in order to capture patients who are only seen at times of exacerbation. Even in this era of treatment at a cellular level with correctors and potentiators, early detection and treatment of CFRD is essential for optimal clinical outcomes [1,5,17]. The next step for us is to sustain our gains and to improve endocrine care facilitation. We hope this report may guide other teams and institutional leadership in their efforts to improve identification of individuals with CFRD.
Acknowledgments: We would like to acknowledge Gautham Suresh, MD, Jennifer Abuzzahab, MD, Robert Payne, MD, and Andrew Flood, PhD, for their assistance in the preparation of our manuscript. We also acknowledge John Nash, MSW, LMSW, who provided us tools and coaching throughout the Learning and Leadership Collaborative.
Corresponding author: Lisa Read, MPH, 2525 Chicago Ave. South, MS 17-750, Minneapolis, MN 55404, lisa.read@childrensmn.org.
Funding/support. This work was supported by a grant from the Cystic Fibrosis Foundation for the Learning and Leadership Collaborative: Cystic Fibrosis-Related Diabetes Care (MCNAMA11Q10, to Dr. McNamara).
From Children’s Hospitals and Clinics of Minnesota, and Children’s Respiratory and Critical Care Specialists, Minneapolis, MN.
Abstract
- Objective: In an effort to improve our pediatric center’s processes for screening, identifying, and treating cystic fibrosis–related diabetes (CFRD), we aimed to create outpatient and inpatient CFRD screening protocols.
- Methods: We identified barriers in our existing screening processes. The lab protocol for outpatients receiving oral glucose tolerance tests was streamlined. Inpatient screening order sets were developed. Interdisciplinary communication between pulmonary and endocrine care teams was improved. A protocol was developed for endocrinology consultation and follow-up for CFRD patients. Staff and families received additional education.
- Results: Outpatient screening was 85% in 2010, 90% in 2011, 77% in 2012, and 95% in 2013 (P = 0.29). Inpatient screening was 13% in 2010, 44% in 2011, 63% in 2012, and 78% 2013 (P = 0.11). Therefore, the combined screening protocols improved overall screening from 87% in 2010 to 90% in 2011, 92% in 2012, and 93% in 2013 (P = 0.57).
- Conclusion: Development of screening protocols improved identification of patients with CFRD.
The most prevalent comorbidity of cystic fibrosis (CF) is cystic fibrosis–related diabetes (CFRD) [1]. The incidence of CFRD increases with age and disease progression. In 2009, Moran et al noted a CFRD prevalence of approximately 20% in adolescents and 40% to 50% in adults [1]. An early diagnosis is especially important due to the correlation of an insulin-deficient state with pulmonary decline, increased pulmonary exacerbations, nutritional impairment, and increased mortality [2–6].
In 2009, the CF Foundation (CFF), the American Diabetes Association, and the Pediatric Endocrine Society updated the clinical care guidelines for the screening, diagnosis, and medical management of CFRD [7]. Soon after, the CFF, the Pediatric Endocrine Society, and the Dartmouth Institute Microsystem Academy sponsored a Learning and Leadership Collaborative (LLC) focusing on CFRD with the purpose of standardizing evidence-based clinical care processes to improve outcomes for patients with CF [8]. Our institution was selected to participate in this endeavor along with 6 other accredited CF centers in the United States.
We report how our pediatric institution established CFRD screening processes in the areas of outpatient care and inpatient care, thereby increasing screening rates.
Methods
Context
Our center cares for approximately 140 pediatric patients with CF. Our CF multidisciplinary program provides outpatient care within a private practice as well as inpatient hospital care within an independent, not-for-profit health care system. The clinic and hospital collaboratively provide services for our patients. Our center has a comprehensive annual clinic day for each child including annual laboratory tests, x-rays, pulmonary function tests, and interaction with the multidisciplinary CF team.
This year-long CFRD screening project was conducted from 2011 to 2012. We began this project with an outpatient screening rate of 85% in 2010. While this rate is high, we identified room for improvement in our screening processes for both outpatients and inpatients. The LLC provided tools and coaching during the project. Locally, we received support and leadership from our institution.
Reflecting on our initial high outpatient screening rate, we identified 2 pre-existing elements:
- As the affiliate center of the University of Minnesota, we have been influenced by their leadership in the field of CFRD. Accordingly, we have made screening for CFRD a priority, which included integrating an endocrinologist into our CF team.
- A review of our established annual patient standard-of-care laboratory results demonstrated 75% of patients in 2010 were completing laboratory testing. Therefore, the timing of the oral glucose tolerance test (OGTT) was changed from an unscheduled basis to becoming part of the outpatient annual lab protocol in an effort to screen the majority of patients.
Target Patient Population
The 2010 CFF guidelines recommend screening patients for CFRD beginning at age 10 years; however, it has been our practice to initiate screening beginning at age 8 years. For the purpose of this project and to increase generalizability for other centers, our results were adapted to include only patients 10 years of age and older.
Definitions
Successful outpatient screening was defined as completion of a 2-hour OGTT obtained by the following process: 1) The patient fasted for 8 hours, 2) a venous blood sample was drawn for a fasting serum glucose, 3) the patient consumed an oral glucose dose of 75 g within 5 minutes of the fasting blood draw, 4) a venous blood sample was drawn 2 hours after glucose administration for the postprandial serum glucose [9]. Patients weighing less than 43 kg received 1.75 g/kg of oral glucose. Outpatients eligible for screening were 10 years of age or older at their first quarterly visit of the year when annual laboratory tests including serum glucose are drawn.
Inpatient screening parameters were determined with guidance from the clinical care guidelines for cystic fibrosis-related diabetes [7], which recommends “monitoring fasting and 2-hour postprandial plasma glucose levels for the first 48 hours.” As this recommendation leaves room for interpretation as far as the quantity and interval of testing, we elected to define a successful screening as completion of one 2-hour postprandial plasma glucose level within 24 hours of admission plus one fasting glucose level within 48 hours of admission. If a patient was identified as having a fasting level ≥ 125 mg/dL or a 2-hour postprandial ≥ 200 mg/dL, additional glucose testing continued beyond 48 hours. However, for the purpose of identifying a successful screening, only the criteria of completing one 2-hour postprandial glucose plus one fasting glucose was considered. Successful screening for inpatients who received a course of steroids was defined as completion of three 2-hour postprandial plasma glucose levels within 24, 48, and 72 hours of admission or initiation of steroids. Inpatients eligible for screening were ten years of age or older at the time of admission.
Patients were not included if they were lost to follow-up for longer than 1 year or were seen for 2 or more quarterly visits at another CF center. Additionally, patients were not eligible if they had been previously diagnosed with CFRD.
Ethical Considerations
Ethical approval for this project was provided by Children’s Hospitals and Clinics of Minnesota Institutional Review Board.
Strategy for Change
We applied the principles of clinical microsystems and conducted numerous tests of change following the Plan-Do-Study-Act (PDSA) technique [10,11]. We organized a core team consisting of 6 individuals: our CF center director (pulmonologist); a hospital-employed pediatric endocrinologist; an outpatient CF clinic nurse coordinator; the hospital’s CF coordinator (pediatric nurse practitioner); a hospital-employed, certified diabetes educator (nurse); and the hospital’s CF dietitian. The core team met weekly throughout the year-long project and ensured other CF care providers were kept up-to-date on the changes implemented with the project.
Interventions
Outpatient Screening
The OGTT was added to a previously established protocol for all patients to complete their annual labs at their first visit of the year. We stressed the importance and rationale for the OGTT in an annual clinic letter sent to families. The family was also sent a reminder letter with fasting instructions, a copy of the annual lab orders, and a reminder telephone call before the appointment. Based on family input regarding delays in the turn-around time for venous blood samples, a point of care (POC) glucose protocol was implemented. Laboratory personnel drew annual blood work, completed a POC blood glucose (in addition to the serum glucose), and administered the oral glucose load if the POC glucose was < 200 mg/dL.
Subsequent to completion of our project, we learned that our lab had inadvertently administered a 37.5-g dose for the OGTT instead of the recommended 75-g dose. To identify patients who may have had CFRD but were not diagnosed due to the low oral glucose dose, we have screened all patients with the corrected dose since January 2013.
To improve communication between the pulmonary and endocrine teams, weekly meetings were scheduled. The teams reviewed patients who had recently completed their annual laboratory tests or recently saw an endocrinology provider. After review of lab values, the patient families were mailed letters informing them of their child’s glucose test results which were categorized as normal, impaired, or abnormal suggesting CFRD. If the patient did not complete the OGTT, the family was mailed a letter reiterating the importance of screening. In the event of an abnormal OGTT suggesting CFRD, the results were discussed with the family during a clinic appointment. The endocrinologist and diabetes educator were subsequently notified, and an endocrine clinic appointment was arranged with the family to discuss the results and care plan. An electronic dashboard (spreadsheet) was created to track lab values and clinic visits for patients with impaired blood glucose tolerance as well as CFRD. The dashboard was reviewed and updated on a quarterly basis.
Inpatient Screening
To improve screening of hospitalized patients with CF not known to have CFRD, 4 standardized order sets detailing blood glucose testing schedules were developed in our electronic medical record as listed below. Our team educated physicians and nurses on the standardized order sets as well as patient families about the additional testing needed during hospitalization. An endocrine nurse practitioner conducted daily rounds on weekdays. All glucose results were verified by the laboratory. If a patient was identified to have a fasting blood glucose ≥ 125 mg/dL or 2-hour postprandial ≥ 200 mg/dL, the endocrine service was notified, and additional glucose testing was ordered. If the patient’s glucose levels persisted at high levels, meeting the criteria for a diagnosis of CFRD, further education was provided for the family and follow-up care was arranged.
Order Sets
1) Not receiving G-tube feedings
Day 1: 2-hour postprandial
Day 2: Fasting
2) Receiving G-tube feedings
Day 1: 2-hour postprandial, 2-hours after start of
G-tube feeds and at end of G-tube feeds
3) Receiving low-dose steroid therapy (IV methylprednisolone up to 4 mg/kg per day, administered every 6 hours and oral prednisolone 1 mg/kg twice per day for 5 days) [12]
Day 1: 2-hour postprandial
Day 2: Fasting and 2-hour postprandial
Day 3: 2-hour postprandial
4) Receiving high-dose steroid therapy per the allergic bronchopulmonary aspergillosis protocol (IV methylprednisolone 10–15 mg/kg once per day for 3 days) [12]
Days 1-4: Point of care prior to every meal, at bedtime, and at 0200 hours
Analysis
All patients with CF who are seen at our clinic were included in this project. Approximately 96% of our patients have consented to be part of the CFF supported patient registry, PortCF [13]. Data are entered by the CF clinic nurse coordinator for these patients to document clinic visits, hospital admissions, medications, lab values, and other parameters. We retrieved data from PortCF documenting the number of patients eligible for the OGTT and the number of patients diagnosed with CFRD. Individual medical records were cross-referenced with these results and also reviewed for patients who do not participate in PortCF. We retrieved data reports from our hospital informatics department to identify the number of patients with CF who were hospitalized each year and to obtain all glucose levels obtained during hospitalization. From these data, we calculated screening rates and the annual number of patients diagnosed with CFRD. We used the Cochran’s Q test to compare the differences in frequency of screening across years.
Results
The newly implemented inpatient protocol, formally instituted in the third quarter of 2011, yielded a 43.8% screening rate in 2011 compared to a rate of 13.3% in 2010. Inpatient screening rates improved to 62.5% in 2012, and 77.8% in 2013 (Figures 3 and 4).
In 2011, 10 patients with CFRD were identified: 6 patients were diagnosed retrospectively based on a review of glucose levels collected in 2010 prior to the release of the new guidelines; 1 was diagnosed via inpatient screening; 3 were diagnosed via outpatient screening. In 2012, 5 patients with CFRD were identified: 4 were diagnosed via inpatient screening and one was diagnosed via outpatient screening. In 2013, 5 patients were identified: 1 diagnosed via inpatient screening and 4 diagnosed via outpatient screening. All diagnosed patients met the criteria of having a fasting blood sugar ≥ 126 mg/dL or a 2-hour postprandial ≥ 200 mg/dL that persisted for more than 48 hours.
Discussion
This collaborative initiative resulted in the development of structured screening protocols leading to overall screening improvement. A standardized screening protocol is fundamental to the identification and subsequent treatment of a patient with CFRD. Patients with CFRD may have decreased mortality when diagnosed early and treated aggressively, underscoring the necessity for CF centers to improve screening protocols [1,6].
Determining the best methods for implementation may vary from center to center, but the key factors we have identified from this project include strong leadership and team commitment—elements that have previously been identified as being predictive of positive quality improvement outcomes [14].
Education of staff, families, and patients is also an important factor. Education played a significant role during the inpatient screening PDSA cycles. For example, a time intensive but worthwhile training was conducted for the physicians and nurses on the order sets. Education for the patients and families during inpatient stays led to a better understanding of the rationale for, and support of, the additional blood draws.
The importance of family involvement was evident as we refined our outpatient protocol. Input from the phone surveys allowed us to identify barriers in our process that led us to establish more efficient clinic visits with reduced time in the lab, improved clinic flow and increased patient satisfaction. Other important factors were co-location of the endocrine and pulmonary clinics and flexibility of scheduling to facilitate seeing patients in both clinics on the same day.
Although we uncovered an error in oral glucose dosing, we were able to appropriately screen the majority of these patients within the next year. At the conclusion of 2013, our outpatient protocol had facilitated a screening rate of 95%, surpassing the 2012 rate of 77%. Interestingly, 2 of the patients diagnosed with CFRD in 2011 were diagnosed by HbA1c criteria, with normal OGTT results, perhaps due to the substandard glucose dose. Only 5 additional patients screened positive for CFRD at the end of 2013, which is the same number identified in 2012. It is unclear whether they would have been diagnosed earlier with the recommended dose of oral glucose or if they developed CFRD due to the progression of their disease.
A limitation of this project may be its reproducibility at other institutions. It is likely that the success of this project was due to the strong relationship between the endocrinology and pulmonary teams, committed leadership, institutional support for co-location of the clinics, and the team’s competency in quality improvement techniques with guidance from the LLC. The process of developing, implementing, and sustaining screening protocols was time-intensive and may be difficult to replicate in another center without similar resources available.
A strong motivation behind this project was the LLC, and while other centers may not have the same opportunity, the CFRD Evidence-Based Practice and Smart Change Idea Compendium was published as result of the LLC to serve as a guide for other centers [8]. Kern et al successfully demonstrated that a process based on the ideas from this compendium can help achieve higher OGTT outpatient screening rates in a CF center [15]. Kern et al’s project was similar to ours although the duration was shorter (< 1 year) and it began with a 47% screening rate prior to implementation. Our center extended the efforts of Kern et al by implementing our initiative over the course of 3 years, but unlike Kern et al, we included patients with failed or rescheduled appointments. Kern et al defined, identified, and excluded patients with moderate or severe pulmonary exacerbation from their eligible screening pool. We included all patients over the course of our year-long screening periods, as we have created an opportunity to screen ill patients at subsequent visits or as inpatients.
As with any quality improvement project, there is the risk of not sustaining improvements. In 2012 our outpatient screening rates were lower than the previous year. However, the coordination of the outpatient and inpatient protocols helped us achieve an improved overall screening rate of 92% in 2012. One possible explanation for the decrease in 2012 outpatient screening is that several patients eligible for screening in 2012 were less adherent with attending clinic visits. Five of these patients transitioned to an adult center or were diagnosed with CFRD in 2013. With this shift in the eligibility pool, our outpatient screening rate for 2013 surpassed our rate from 2012. In an attempt to sustain our gains, members of our team continue to review patient screening and endocrine referrals at monthly meetings. The decrease in outpatient screening demonstrates the importance of ongoing evaluation and monitoring of quality improvement projects after the initial objectives have been achieved.
Typically, our less adherent patients are only seen in clinic when experiencing an exacerbation when we cannot administer the OGTT. As a result, these patients are generally admitted for treatment of their exacerbation, and we are able to screen them during their hospitalization. Of the 9 patients not screened as outpatients in 2012, 7 were screened as inpatients. Three patients were unscreened that year: 1 patient failed to fast, another refused the test, and the last patient was inadvertently missed. While our intention is to screen all patients as an outpatient with OGTT, we have found that the only opportunity we have for screening less adherent patients is often while hospitalized, emphasizing the importance of a dual screening approach. This approach has allowed us to screen the majority of our patients as reflected in our overall screening rate.
One of our major challenges moving forward is to help the patients diagnosed with CFRD and their families accept yet another diagnosis and the burden of care associated with it. Our focus has shifted now to determine the best methods to motivate patients with CFRD to regularly attend endocrine clinic appointments, recognizing the challenges of additional clinic visits, monitoring, and medications. It is interesting to speculate whether improved CFRD clinical outcomes may correlate with improved screening rates. In 2012 our patients had a median HbA1c of 5.7 when the national average is 6.6 [16]. Further research is needed to delineate a possible relationship between an effective screening protocol and favorable clinical outcome measures.
Conclusion
The use of a structured process developed by a multidisciplinary team resulted in improved CFRD screening rates. In addition to outpatient protocols, it is critical to develop inpatient glucose testing protocols in order to capture patients who are only seen at times of exacerbation. Even in this era of treatment at a cellular level with correctors and potentiators, early detection and treatment of CFRD is essential for optimal clinical outcomes [1,5,17]. The next step for us is to sustain our gains and to improve endocrine care facilitation. We hope this report may guide other teams and institutional leadership in their efforts to improve identification of individuals with CFRD.
Acknowledgments: We would like to acknowledge Gautham Suresh, MD, Jennifer Abuzzahab, MD, Robert Payne, MD, and Andrew Flood, PhD, for their assistance in the preparation of our manuscript. We also acknowledge John Nash, MSW, LMSW, who provided us tools and coaching throughout the Learning and Leadership Collaborative.
Corresponding author: Lisa Read, MPH, 2525 Chicago Ave. South, MS 17-750, Minneapolis, MN 55404, lisa.read@childrensmn.org.
Funding/support. This work was supported by a grant from the Cystic Fibrosis Foundation for the Learning and Leadership Collaborative: Cystic Fibrosis-Related Diabetes Care (MCNAMA11Q10, to Dr. McNamara).
1. Moran A, Dunitz J, Nathan B, et al. Cystic fibrosis–related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–31.
2. Cawood TJ, McKenna MJ, Gallagher CG, et al. Cystic fibrosis-related diabetes in adults. Ir Med J 2006;99:83–6.
3. Koch C, Rainisio M, Madessani U, et al. Investigators of the European Epidemiologic Registry of Cystic Fibrosis. Presence of cystic fibrosis-related diabetes mellitus tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001;32:343–50.
4. Marshall BC, Butler SM, Stoddard M, et al. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–7.
5. Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141–4.
6. Lewis C, Blackman SM, Nelson A, et al. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med 2015;191:194–200.
7. Moran A, Brunzell C, Cohen, RC, et al. Clinical Care guidelines for cystic fibrosis–related diabetes. A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–708.
8. Nash J, Messier R, Casella SJ, et al. Learning and Leadership Collaborative: CFRD. Cystic fibrosis related diabetes (CFRD) evidence-based practice and smart change idea compendium 2012. Bethesda, MD: Cystic Fibrosis Foundation; McLean, VA: Pediatric Endocrine Society; Lebanon, NH: Dartmouth Institute Microsystem Academy; May 2012.
9. WHO Expert Committee on Diabetes Mellitus: Second Report of the WHO Expert Committee on Diabetes Mellitus. Geneva: World Health Organization; 1980 (Tech. Rep. Ser no. 646).
10. Cystic Fibrosis Foundation, Dartmouth Medical School: Center for the Evaluative Clinical Sciences, Dartmouth/Hitchcock Medical Center. Action guide for accelerating improvement in cystic fibrosis care: clinical microsystems. 2006.
11. Langley GJ, Nolan KM, Nolan TW, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco, CA: Jossey-Bass; 1996.
12. Cohen-Cymberknoh M, Blau H, Shoseyov D, et al. Intravenous monthly pulse methylprednisolone treatment for ABPA in patients with cystic fibrosis. J Cyst Fibros 2009;8:253–7.
13. Center Specific Patient Registry Report for 2011. Bethesda, MD: Cystic Fibrosis Foundation; 2012.
14. Parker VA, Wubbenhorst WH, Young GJ, et al. Implementing quality improvement in hospitals: The role of leadership and culture. Am J Med Qual 1999; 14:64–9.
15. Kern AS, Prestridge AL. Improving screening for cystic fibrosis-related diabetes at a pediatric cystic fibrosis program. Pediatrics 2013;132:e512–8.
16. Center Specific Patient Registry Report for 2012. Bethesda, MD: Cystic Fibrosis Foundation; 2013.
17. Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–61.
1. Moran A, Dunitz J, Nathan B, et al. Cystic fibrosis–related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–31.
2. Cawood TJ, McKenna MJ, Gallagher CG, et al. Cystic fibrosis-related diabetes in adults. Ir Med J 2006;99:83–6.
3. Koch C, Rainisio M, Madessani U, et al. Investigators of the European Epidemiologic Registry of Cystic Fibrosis. Presence of cystic fibrosis-related diabetes mellitus tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001;32:343–50.
4. Marshall BC, Butler SM, Stoddard M, et al. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–7.
5. Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141–4.
6. Lewis C, Blackman SM, Nelson A, et al. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med 2015;191:194–200.
7. Moran A, Brunzell C, Cohen, RC, et al. Clinical Care guidelines for cystic fibrosis–related diabetes. A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–708.
8. Nash J, Messier R, Casella SJ, et al. Learning and Leadership Collaborative: CFRD. Cystic fibrosis related diabetes (CFRD) evidence-based practice and smart change idea compendium 2012. Bethesda, MD: Cystic Fibrosis Foundation; McLean, VA: Pediatric Endocrine Society; Lebanon, NH: Dartmouth Institute Microsystem Academy; May 2012.
9. WHO Expert Committee on Diabetes Mellitus: Second Report of the WHO Expert Committee on Diabetes Mellitus. Geneva: World Health Organization; 1980 (Tech. Rep. Ser no. 646).
10. Cystic Fibrosis Foundation, Dartmouth Medical School: Center for the Evaluative Clinical Sciences, Dartmouth/Hitchcock Medical Center. Action guide for accelerating improvement in cystic fibrosis care: clinical microsystems. 2006.
11. Langley GJ, Nolan KM, Nolan TW, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco, CA: Jossey-Bass; 1996.
12. Cohen-Cymberknoh M, Blau H, Shoseyov D, et al. Intravenous monthly pulse methylprednisolone treatment for ABPA in patients with cystic fibrosis. J Cyst Fibros 2009;8:253–7.
13. Center Specific Patient Registry Report for 2011. Bethesda, MD: Cystic Fibrosis Foundation; 2012.
14. Parker VA, Wubbenhorst WH, Young GJ, et al. Implementing quality improvement in hospitals: The role of leadership and culture. Am J Med Qual 1999; 14:64–9.
15. Kern AS, Prestridge AL. Improving screening for cystic fibrosis-related diabetes at a pediatric cystic fibrosis program. Pediatrics 2013;132:e512–8.
16. Center Specific Patient Registry Report for 2012. Bethesda, MD: Cystic Fibrosis Foundation; 2013.
17. Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–61.