User login
Antimicrobial Stewardship in an Outpatient Parenteral Antibiotic Therapy Program
Antimicrobial stewardship activities have been in place at the Edward Hines, Jr. VA Hospital in Hines, Illinois, since 1988. Initial activities, including antimicrobial restriction and the start of an outpatient-infusion program justified and led to dedicated funding for hiring the first infectious diseases (ID) clinical pharmacist. This position was initiated in 1992 and has been maintained since then. The committed multidisciplinary team, including ID physicians, ID clinical pharmacists, venous access nurses (VAN), microbiologists, infection control practitioners, and an outpatient-infusion coordinator have led stewardship activities at this VA.
One of the first efforts of the team was the development of the outpatient parenteral antibiotic therapy (OPAT) program.1 The program began in 1989 and has served more than 1,200 veterans. Outpatient parenteral antibiotic therapy is only one component of the stewardship program, which provides safe, effective, and cost-minimizing care for veterans, and is the focus of this article.
Background
Complex medical care and escalating costs have pushed all but the most seriously ill patients out of the hospital setting for care delivery. The reality is that patients who might have received care for non–life-threatening problems in a hospital bed are now relegated to an outpatient status. Beginning in the 1970s, OPAT has been used to facilitate the cost-effective, safe administration of antibiotics as an alternative to an extensive, expensive hospital stay.2 Initially developed for use in a nonhospital health care setting, the administration of antibiotics under the guidance of a health care provider (HCP) has now been extended to a self-administered infusion program.3,4 Under the latter, patients and caregivers are educated to safely administer IV antibiotics for extended periods at home.
This program uses elements of both health care–associated OPAT and self-administered OPAT (S-OPAT) to accomplish its goals: (1) safe, effective administration of antibiotic therapy to a variety of patients; (2) reduction in bed days of care (BDOC); (3) reduction of the economic burden to the hospitals’ global budgets; and (4) reduction in the incidence of common nosocomially-acquired infections, including those caused by Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococcus.3
The advantages of S-OPAT have been fully realized in a variety of countries, enabling patients to receive necessary therapy in the comfort of their homes and providing them with the ability to lead normal lives without the confinement of a protracted hospital stay.5-7
Description of OPAT
The outpatient-infusion team provides specialized care for patients in accordance with the OPAT national guidelines from patient screening to program discharge.8 The dedicated staff include the OPAT nurse coordinator, VAN, pharmacists, and ID consultants. The VAN places the venous access device (VAD), educates the patient and caregiver in the care and safety of the catheter, aseptic technique, and infusion of the selected antimicrobial agent, and monitors the laboratory work. The VA may contract an outside nursing agency to provide support and reinforcement of IV administration for the patient and caregiver.
The pharmacists oversee the pharmacokinetics and pharmacodynamics of the antimicrobials as well as monitor for any toxicities that could potentially arise during and after therapy. The ID consultants identify the infection, collaborate with the pharmacists to select the most appropriate antimicrobial regimen, and determine the duration of required therapy. The team then regularly monitors the patient in the ID clinic until there is evidence of infection resolution.
Primary care providers who want to enroll patients in the OPAT program place a formal electronic consult to the ID team for antibiotic recommendation, to the outpatient infusion team for assessment of potential outpatient therapy, and to the venous access team for insertion of the VAD. The consults are completed after receiving consent from the patient, developing a patient-centered treatment plan, and determining the patient’s ability to comprehend and adhere to the program requirements. The patient or caregiver must be able to competently demonstrate aseptic technique for IV administration prior to discharge. The pharmacist educates the patient or caregiver about the stability, storage requirements, and potential adverse drug reactions of the antimicrobial.
Eligible patients must have resolved their acute medical problems and require > 1 week of therapy to treat their infection. Patients chosen for OPAT or S-OPAT must have a suitable living environment with access to a refrigerator, a telephone, and transportation to return to the hospital for follow-up. Most patients and caregivers are eager to learn and recognize the advantages of home-based care.
The VANs are a central component of the program. They maintain open communication with the patient during the entire treatment course and help triage issues to the appropriate HCP. In addition, they are responsible for submitting catheter-related bloodstream infection (CRBSI) information to the hospital administration, which then gets reported to the National Healthcare Safety Network (NHSN).
Not all patients qualify for S-OPAT. Other options include returning to the hospital daily for infusions, being discharged to a skilled care facility, or arranging for a VA-contracted agency to provide nursing care while the VA provides all required medications and supplies.
On completion of OPAT, patients are asked to evaluate the program. The anonymous survey includes open-ended questions for patients to better express their experience with the program and staff. Patients are given the opportunity to suggest improvements and provide overall feedback. The team for quality assurance and patient satisfaction reviews every survey, which is used as a tool to improve team functions.
Data are also collected in the OPAT program to measure efficacy and monitor for safety. Data obtained from the start of the program in February 1989 through fiscal year (FY) 2011 include the number of patients who were candidates for outpatient-infusion therapy, type of infection, antibiotic selection, CRBSIs, hospital readmission rates, cost savings, and patient satisfaction.
Results
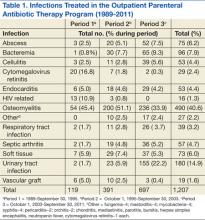
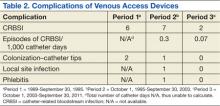
The Edward Hines, Jr. VA Hospital has a proven, successful OPAT program. Most of the patients in the program during the study period were men, which reflects a typical VA population. Patients with spinal cord injury comprised a large portion of those treated. Table 1 provides the number of patients treated and lists the frequency of infections. The data are divided into 3 periods. From 1989 to September 30, 1995, OPAT used other VADs before using peripherally inserted central catheters. During the second period (October 1, 1995-September 30, 2003), patients remained with a VAD for an average of 48.6 days; whereas in Period 3 (October 1, 2003-September 30, 2011), the patients had a VAD average of 34.7 days. Consequently, with fewer VAD days, there was a decreased incidence of complications (Table 2).
Osteomyelitis accounted for the majority of the infections (40.6%), which required ≥ 6 weeks of therapy. Complicated urinary tract infection (UTI), including pyelonephritis, perinephric abscess, and complicated cystitis, was the next most common (14.9%). Bacteremia was the third most common infection (7.9%), whereas abscesses of a diverse variety affected 6.2%, including brain, liver, intra-abdominal, soft tissue, and epidural abscesses. Endocarditis and septic arthritis accounted for 4.4% and 4.7%, respectively, of infected patients.
Three periods of the OPAT program were selected at random (1996, 2003, and 2011) to examine trends in antimicrobial selection. Overall, ceftriaxone was the most commonly used antibiotic (35%). Vancomycin was the next most commonly prescribed (27%). Since its 2001 FDA approval, ertapenem has become the third most commonly prescribed antibiotic for the OPAT program (11%). As expected, antimicrobial agents that have to be dosed more frequently than twice a day were rarely used for OPAT. In addition, there was low usage of aminoglycosides due to the need for the close monitoring of levels and potential toxicity.
Outcomes
Catheter Complications
The majority of catheter complications occurring in the first period were multifactorial, relating to nursing education, product selection, program development, insertion techniques, and a less comprehensive infection control program.
Hospital Readmissions
A snapshot of FY 2011 data was used to evaluate hospital readmissions. One hundred one patients were reviewed. Of these patients, 9 (9%) were readmitted to the hospital at some point after being discharged from OPAT. Readmission due to complications of OPAT was found in 2 of the 9 patients. One was due to an adverse drug reaction from the antibiotic; the other was due to a possible relapse of a hip osteomyelitis.
Cost Analysis
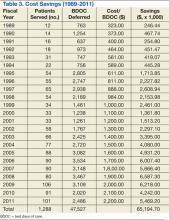
The OPAT program has resulted in a total savings to the global hospital budget from the deferred BDOC of more than $65 million (Table 3) since 1989. The OPAT program eliminated > 47,000 days of inpatient care. In FY 2009 the program cost the hospital $691.35 for each of the 106 patients enrolled (total cost: $73,283.10). This included all IV supplies, antimicrobials, visiting nurse costs when applicable, as well as nursing and pharmacy time dedicated to training the patient and making therapeutic decisions. Expenses for 3,109 BDOC would have cost about $6,218,000. The outpatient-infusion program saved the hospital nearly $6 million in 2009 alone.
Patient Satisfaction
About 60% of the patients discharged from the OPAT program responded to an evaluation survey. The feedback was overwhelmingly positive with about 99% of respondents reporting satisfaction relating to an improved quality of life. Most of the positive comments were directed toward the outpatient-infusion coordinator for resolving issues, being easily accessible, and acting as a patient advocate.
Discussion
The number and types of reasons for OPAT have grown with the knowledge that it is a safe, cost-effective method for the delivery of parenteral antimicrobials. In the early years of the program, before effective antiretroviral therapy was available, cytomegalovirus retinitis was the second most commonly treated infection of the OPAT program. In recent years, the rise of multidrug-resistant organisms has led to limited oral treatment options for UTIs, which are now the second most commonly treated infection of OPAT. Osteomyelitis clearly remains the top indication for OPAT because it requires long-term therapy. Ceftriaxone remains the drug of choice due to once-daily dosing, spectrum of activity, overall safety, and cost-effectiveness.
Catheter complication rates in the OPAT program were lower than those reported in the literature. According to the 2009 NHSN report, the catheter complication rate in the inpatient long-term care units was 1.0 CRBSI/1,000 catheter days.9 Moreover, this program has been instrumental in providing care that otherwise would be administered through the use of home health agencies.
In the private sector, OPAT is frequently contracted to agencies that provide the same type of service to outpatients who have insurance. These agencies charge for the antimicrobials, IV supplies, nursing visits, and laboratory costs for patient-safety monitoring. Use of an agency could raise expenses by a factor of 8-fold or more above the cost of a hospital-based OPAT program, an estimate based on a comparison with a local federally contracted home-infusion agency that provides specialized home-infusion services at a cost.
Although costs related to hospital readmissions were not factored in to the cost savings calculations, the rate of readmission was low in the snapshot analysis that was conducted at the Edward Hines, Jr. VA Hospital. It is believed that this is the result of the close follow-up and continuity of care that the patients in this OPAT program received.
In addition to cost containment, the data reflect the safe, effective care that resulted from treatment outside the hospital setting. One of the key attributes that has made the Edward Hines, Jr. VA Hospital OPAT program unique is that it is recognized in the community as the only VA facility in the area to provide OPAT as an option for the veteran patient. Other VA facilities in the area contract with home-infusion agencies, which are responsible for supplying the antibiotics and nursing care. The Edward Hines, Jr. VA Hospital is the only VA hospital in VISN 12 that has a facility-supported program that provides all supplies and antimicrobials from the VA—a major contributing factor to the cost savings. Continuity of care is provided to the patient who transitions from inpatient to outpatient status with the same team of providers contributing to the significant patient satisfaction that the program has engendered.
Conclusions
One of the main benefits realized with this transition of antibiotic therapy to the home setting is the avoidance of newly acquired nosocomial infections, including C difficile infection, fungal, and multidrug-resistant bacterial infections. Other benefits include early IV to oral switch in therapy when the patient is deemed a candidate, the ability to go back to work sooner, and the ability to receive treatment in the comfort of the patient’s home. Plans for data collection may include a more in-depth review of repeat admissions due to unresolved infections and the number of patients who are unable to complete OPAT at home.
The Edward Hines, Jr. VA Hospital OPAT program has shown that in a large, federally-funded hospital, OPAT is safe, cost-effective, convenient and leads to increased patient satisfaction in a diverse group of veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Lentino JR, Pachucki CT, Byrne R, Lau MT, Bayer D. Parenteral antibiotic therapy: A home-based program. Fed Pract. 2000;17(4):10-15.
2. Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51(suppl 2):S198-S208.
3. Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007;60(2):356-362.
4. Ingram PR, Sulaiman Z, Chua A, Fisher DA. Comment on: Outpatient parenteral antibiotic therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2008;61(1):226-227.
5. Bernard L, El-Hajj, Pron B, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: Evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26(6):445-451.
6. Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of outpatient parenteral antibiotic therapy (OPAT): An Asian perspective. Int J Antimicrob Agents. 2009;33(1):46-51.
7. Tice AD, Hoaglund PA, Nolet B, McKinnon PS, Mozaffari E. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy. 2002;22(2 Pt 2):63S-70S.
8. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651-1672.
9. Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783-805.
Antimicrobial stewardship activities have been in place at the Edward Hines, Jr. VA Hospital in Hines, Illinois, since 1988. Initial activities, including antimicrobial restriction and the start of an outpatient-infusion program justified and led to dedicated funding for hiring the first infectious diseases (ID) clinical pharmacist. This position was initiated in 1992 and has been maintained since then. The committed multidisciplinary team, including ID physicians, ID clinical pharmacists, venous access nurses (VAN), microbiologists, infection control practitioners, and an outpatient-infusion coordinator have led stewardship activities at this VA.
One of the first efforts of the team was the development of the outpatient parenteral antibiotic therapy (OPAT) program.1 The program began in 1989 and has served more than 1,200 veterans. Outpatient parenteral antibiotic therapy is only one component of the stewardship program, which provides safe, effective, and cost-minimizing care for veterans, and is the focus of this article.
Background
Complex medical care and escalating costs have pushed all but the most seriously ill patients out of the hospital setting for care delivery. The reality is that patients who might have received care for non–life-threatening problems in a hospital bed are now relegated to an outpatient status. Beginning in the 1970s, OPAT has been used to facilitate the cost-effective, safe administration of antibiotics as an alternative to an extensive, expensive hospital stay.2 Initially developed for use in a nonhospital health care setting, the administration of antibiotics under the guidance of a health care provider (HCP) has now been extended to a self-administered infusion program.3,4 Under the latter, patients and caregivers are educated to safely administer IV antibiotics for extended periods at home.
This program uses elements of both health care–associated OPAT and self-administered OPAT (S-OPAT) to accomplish its goals: (1) safe, effective administration of antibiotic therapy to a variety of patients; (2) reduction in bed days of care (BDOC); (3) reduction of the economic burden to the hospitals’ global budgets; and (4) reduction in the incidence of common nosocomially-acquired infections, including those caused by Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococcus.3
The advantages of S-OPAT have been fully realized in a variety of countries, enabling patients to receive necessary therapy in the comfort of their homes and providing them with the ability to lead normal lives without the confinement of a protracted hospital stay.5-7
Description of OPAT
The outpatient-infusion team provides specialized care for patients in accordance with the OPAT national guidelines from patient screening to program discharge.8 The dedicated staff include the OPAT nurse coordinator, VAN, pharmacists, and ID consultants. The VAN places the venous access device (VAD), educates the patient and caregiver in the care and safety of the catheter, aseptic technique, and infusion of the selected antimicrobial agent, and monitors the laboratory work. The VA may contract an outside nursing agency to provide support and reinforcement of IV administration for the patient and caregiver.
The pharmacists oversee the pharmacokinetics and pharmacodynamics of the antimicrobials as well as monitor for any toxicities that could potentially arise during and after therapy. The ID consultants identify the infection, collaborate with the pharmacists to select the most appropriate antimicrobial regimen, and determine the duration of required therapy. The team then regularly monitors the patient in the ID clinic until there is evidence of infection resolution.
Primary care providers who want to enroll patients in the OPAT program place a formal electronic consult to the ID team for antibiotic recommendation, to the outpatient infusion team for assessment of potential outpatient therapy, and to the venous access team for insertion of the VAD. The consults are completed after receiving consent from the patient, developing a patient-centered treatment plan, and determining the patient’s ability to comprehend and adhere to the program requirements. The patient or caregiver must be able to competently demonstrate aseptic technique for IV administration prior to discharge. The pharmacist educates the patient or caregiver about the stability, storage requirements, and potential adverse drug reactions of the antimicrobial.
Eligible patients must have resolved their acute medical problems and require > 1 week of therapy to treat their infection. Patients chosen for OPAT or S-OPAT must have a suitable living environment with access to a refrigerator, a telephone, and transportation to return to the hospital for follow-up. Most patients and caregivers are eager to learn and recognize the advantages of home-based care.
The VANs are a central component of the program. They maintain open communication with the patient during the entire treatment course and help triage issues to the appropriate HCP. In addition, they are responsible for submitting catheter-related bloodstream infection (CRBSI) information to the hospital administration, which then gets reported to the National Healthcare Safety Network (NHSN).
Not all patients qualify for S-OPAT. Other options include returning to the hospital daily for infusions, being discharged to a skilled care facility, or arranging for a VA-contracted agency to provide nursing care while the VA provides all required medications and supplies.
On completion of OPAT, patients are asked to evaluate the program. The anonymous survey includes open-ended questions for patients to better express their experience with the program and staff. Patients are given the opportunity to suggest improvements and provide overall feedback. The team for quality assurance and patient satisfaction reviews every survey, which is used as a tool to improve team functions.
Data are also collected in the OPAT program to measure efficacy and monitor for safety. Data obtained from the start of the program in February 1989 through fiscal year (FY) 2011 include the number of patients who were candidates for outpatient-infusion therapy, type of infection, antibiotic selection, CRBSIs, hospital readmission rates, cost savings, and patient satisfaction.
Results
The Edward Hines, Jr. VA Hospital has a proven, successful OPAT program. Most of the patients in the program during the study period were men, which reflects a typical VA population. Patients with spinal cord injury comprised a large portion of those treated. Table 1 provides the number of patients treated and lists the frequency of infections. The data are divided into 3 periods. From 1989 to September 30, 1995, OPAT used other VADs before using peripherally inserted central catheters. During the second period (October 1, 1995-September 30, 2003), patients remained with a VAD for an average of 48.6 days; whereas in Period 3 (October 1, 2003-September 30, 2011), the patients had a VAD average of 34.7 days. Consequently, with fewer VAD days, there was a decreased incidence of complications (Table 2).
Osteomyelitis accounted for the majority of the infections (40.6%), which required ≥ 6 weeks of therapy. Complicated urinary tract infection (UTI), including pyelonephritis, perinephric abscess, and complicated cystitis, was the next most common (14.9%). Bacteremia was the third most common infection (7.9%), whereas abscesses of a diverse variety affected 6.2%, including brain, liver, intra-abdominal, soft tissue, and epidural abscesses. Endocarditis and septic arthritis accounted for 4.4% and 4.7%, respectively, of infected patients.
Three periods of the OPAT program were selected at random (1996, 2003, and 2011) to examine trends in antimicrobial selection. Overall, ceftriaxone was the most commonly used antibiotic (35%). Vancomycin was the next most commonly prescribed (27%). Since its 2001 FDA approval, ertapenem has become the third most commonly prescribed antibiotic for the OPAT program (11%). As expected, antimicrobial agents that have to be dosed more frequently than twice a day were rarely used for OPAT. In addition, there was low usage of aminoglycosides due to the need for the close monitoring of levels and potential toxicity.
Outcomes
Catheter Complications
The majority of catheter complications occurring in the first period were multifactorial, relating to nursing education, product selection, program development, insertion techniques, and a less comprehensive infection control program.
Hospital Readmissions
A snapshot of FY 2011 data was used to evaluate hospital readmissions. One hundred one patients were reviewed. Of these patients, 9 (9%) were readmitted to the hospital at some point after being discharged from OPAT. Readmission due to complications of OPAT was found in 2 of the 9 patients. One was due to an adverse drug reaction from the antibiotic; the other was due to a possible relapse of a hip osteomyelitis.
Cost Analysis
The OPAT program has resulted in a total savings to the global hospital budget from the deferred BDOC of more than $65 million (Table 3) since 1989. The OPAT program eliminated > 47,000 days of inpatient care. In FY 2009 the program cost the hospital $691.35 for each of the 106 patients enrolled (total cost: $73,283.10). This included all IV supplies, antimicrobials, visiting nurse costs when applicable, as well as nursing and pharmacy time dedicated to training the patient and making therapeutic decisions. Expenses for 3,109 BDOC would have cost about $6,218,000. The outpatient-infusion program saved the hospital nearly $6 million in 2009 alone.
Patient Satisfaction
About 60% of the patients discharged from the OPAT program responded to an evaluation survey. The feedback was overwhelmingly positive with about 99% of respondents reporting satisfaction relating to an improved quality of life. Most of the positive comments were directed toward the outpatient-infusion coordinator for resolving issues, being easily accessible, and acting as a patient advocate.
Discussion
The number and types of reasons for OPAT have grown with the knowledge that it is a safe, cost-effective method for the delivery of parenteral antimicrobials. In the early years of the program, before effective antiretroviral therapy was available, cytomegalovirus retinitis was the second most commonly treated infection of the OPAT program. In recent years, the rise of multidrug-resistant organisms has led to limited oral treatment options for UTIs, which are now the second most commonly treated infection of OPAT. Osteomyelitis clearly remains the top indication for OPAT because it requires long-term therapy. Ceftriaxone remains the drug of choice due to once-daily dosing, spectrum of activity, overall safety, and cost-effectiveness.
Catheter complication rates in the OPAT program were lower than those reported in the literature. According to the 2009 NHSN report, the catheter complication rate in the inpatient long-term care units was 1.0 CRBSI/1,000 catheter days.9 Moreover, this program has been instrumental in providing care that otherwise would be administered through the use of home health agencies.
In the private sector, OPAT is frequently contracted to agencies that provide the same type of service to outpatients who have insurance. These agencies charge for the antimicrobials, IV supplies, nursing visits, and laboratory costs for patient-safety monitoring. Use of an agency could raise expenses by a factor of 8-fold or more above the cost of a hospital-based OPAT program, an estimate based on a comparison with a local federally contracted home-infusion agency that provides specialized home-infusion services at a cost.
Although costs related to hospital readmissions were not factored in to the cost savings calculations, the rate of readmission was low in the snapshot analysis that was conducted at the Edward Hines, Jr. VA Hospital. It is believed that this is the result of the close follow-up and continuity of care that the patients in this OPAT program received.
In addition to cost containment, the data reflect the safe, effective care that resulted from treatment outside the hospital setting. One of the key attributes that has made the Edward Hines, Jr. VA Hospital OPAT program unique is that it is recognized in the community as the only VA facility in the area to provide OPAT as an option for the veteran patient. Other VA facilities in the area contract with home-infusion agencies, which are responsible for supplying the antibiotics and nursing care. The Edward Hines, Jr. VA Hospital is the only VA hospital in VISN 12 that has a facility-supported program that provides all supplies and antimicrobials from the VA—a major contributing factor to the cost savings. Continuity of care is provided to the patient who transitions from inpatient to outpatient status with the same team of providers contributing to the significant patient satisfaction that the program has engendered.
Conclusions
One of the main benefits realized with this transition of antibiotic therapy to the home setting is the avoidance of newly acquired nosocomial infections, including C difficile infection, fungal, and multidrug-resistant bacterial infections. Other benefits include early IV to oral switch in therapy when the patient is deemed a candidate, the ability to go back to work sooner, and the ability to receive treatment in the comfort of the patient’s home. Plans for data collection may include a more in-depth review of repeat admissions due to unresolved infections and the number of patients who are unable to complete OPAT at home.
The Edward Hines, Jr. VA Hospital OPAT program has shown that in a large, federally-funded hospital, OPAT is safe, cost-effective, convenient and leads to increased patient satisfaction in a diverse group of veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Antimicrobial stewardship activities have been in place at the Edward Hines, Jr. VA Hospital in Hines, Illinois, since 1988. Initial activities, including antimicrobial restriction and the start of an outpatient-infusion program justified and led to dedicated funding for hiring the first infectious diseases (ID) clinical pharmacist. This position was initiated in 1992 and has been maintained since then. The committed multidisciplinary team, including ID physicians, ID clinical pharmacists, venous access nurses (VAN), microbiologists, infection control practitioners, and an outpatient-infusion coordinator have led stewardship activities at this VA.
One of the first efforts of the team was the development of the outpatient parenteral antibiotic therapy (OPAT) program.1 The program began in 1989 and has served more than 1,200 veterans. Outpatient parenteral antibiotic therapy is only one component of the stewardship program, which provides safe, effective, and cost-minimizing care for veterans, and is the focus of this article.
Background
Complex medical care and escalating costs have pushed all but the most seriously ill patients out of the hospital setting for care delivery. The reality is that patients who might have received care for non–life-threatening problems in a hospital bed are now relegated to an outpatient status. Beginning in the 1970s, OPAT has been used to facilitate the cost-effective, safe administration of antibiotics as an alternative to an extensive, expensive hospital stay.2 Initially developed for use in a nonhospital health care setting, the administration of antibiotics under the guidance of a health care provider (HCP) has now been extended to a self-administered infusion program.3,4 Under the latter, patients and caregivers are educated to safely administer IV antibiotics for extended periods at home.
This program uses elements of both health care–associated OPAT and self-administered OPAT (S-OPAT) to accomplish its goals: (1) safe, effective administration of antibiotic therapy to a variety of patients; (2) reduction in bed days of care (BDOC); (3) reduction of the economic burden to the hospitals’ global budgets; and (4) reduction in the incidence of common nosocomially-acquired infections, including those caused by Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococcus.3
The advantages of S-OPAT have been fully realized in a variety of countries, enabling patients to receive necessary therapy in the comfort of their homes and providing them with the ability to lead normal lives without the confinement of a protracted hospital stay.5-7
Description of OPAT
The outpatient-infusion team provides specialized care for patients in accordance with the OPAT national guidelines from patient screening to program discharge.8 The dedicated staff include the OPAT nurse coordinator, VAN, pharmacists, and ID consultants. The VAN places the venous access device (VAD), educates the patient and caregiver in the care and safety of the catheter, aseptic technique, and infusion of the selected antimicrobial agent, and monitors the laboratory work. The VA may contract an outside nursing agency to provide support and reinforcement of IV administration for the patient and caregiver.
The pharmacists oversee the pharmacokinetics and pharmacodynamics of the antimicrobials as well as monitor for any toxicities that could potentially arise during and after therapy. The ID consultants identify the infection, collaborate with the pharmacists to select the most appropriate antimicrobial regimen, and determine the duration of required therapy. The team then regularly monitors the patient in the ID clinic until there is evidence of infection resolution.
Primary care providers who want to enroll patients in the OPAT program place a formal electronic consult to the ID team for antibiotic recommendation, to the outpatient infusion team for assessment of potential outpatient therapy, and to the venous access team for insertion of the VAD. The consults are completed after receiving consent from the patient, developing a patient-centered treatment plan, and determining the patient’s ability to comprehend and adhere to the program requirements. The patient or caregiver must be able to competently demonstrate aseptic technique for IV administration prior to discharge. The pharmacist educates the patient or caregiver about the stability, storage requirements, and potential adverse drug reactions of the antimicrobial.
Eligible patients must have resolved their acute medical problems and require > 1 week of therapy to treat their infection. Patients chosen for OPAT or S-OPAT must have a suitable living environment with access to a refrigerator, a telephone, and transportation to return to the hospital for follow-up. Most patients and caregivers are eager to learn and recognize the advantages of home-based care.
The VANs are a central component of the program. They maintain open communication with the patient during the entire treatment course and help triage issues to the appropriate HCP. In addition, they are responsible for submitting catheter-related bloodstream infection (CRBSI) information to the hospital administration, which then gets reported to the National Healthcare Safety Network (NHSN).
Not all patients qualify for S-OPAT. Other options include returning to the hospital daily for infusions, being discharged to a skilled care facility, or arranging for a VA-contracted agency to provide nursing care while the VA provides all required medications and supplies.
On completion of OPAT, patients are asked to evaluate the program. The anonymous survey includes open-ended questions for patients to better express their experience with the program and staff. Patients are given the opportunity to suggest improvements and provide overall feedback. The team for quality assurance and patient satisfaction reviews every survey, which is used as a tool to improve team functions.
Data are also collected in the OPAT program to measure efficacy and monitor for safety. Data obtained from the start of the program in February 1989 through fiscal year (FY) 2011 include the number of patients who were candidates for outpatient-infusion therapy, type of infection, antibiotic selection, CRBSIs, hospital readmission rates, cost savings, and patient satisfaction.
Results
The Edward Hines, Jr. VA Hospital has a proven, successful OPAT program. Most of the patients in the program during the study period were men, which reflects a typical VA population. Patients with spinal cord injury comprised a large portion of those treated. Table 1 provides the number of patients treated and lists the frequency of infections. The data are divided into 3 periods. From 1989 to September 30, 1995, OPAT used other VADs before using peripherally inserted central catheters. During the second period (October 1, 1995-September 30, 2003), patients remained with a VAD for an average of 48.6 days; whereas in Period 3 (October 1, 2003-September 30, 2011), the patients had a VAD average of 34.7 days. Consequently, with fewer VAD days, there was a decreased incidence of complications (Table 2).
Osteomyelitis accounted for the majority of the infections (40.6%), which required ≥ 6 weeks of therapy. Complicated urinary tract infection (UTI), including pyelonephritis, perinephric abscess, and complicated cystitis, was the next most common (14.9%). Bacteremia was the third most common infection (7.9%), whereas abscesses of a diverse variety affected 6.2%, including brain, liver, intra-abdominal, soft tissue, and epidural abscesses. Endocarditis and septic arthritis accounted for 4.4% and 4.7%, respectively, of infected patients.
Three periods of the OPAT program were selected at random (1996, 2003, and 2011) to examine trends in antimicrobial selection. Overall, ceftriaxone was the most commonly used antibiotic (35%). Vancomycin was the next most commonly prescribed (27%). Since its 2001 FDA approval, ertapenem has become the third most commonly prescribed antibiotic for the OPAT program (11%). As expected, antimicrobial agents that have to be dosed more frequently than twice a day were rarely used for OPAT. In addition, there was low usage of aminoglycosides due to the need for the close monitoring of levels and potential toxicity.
Outcomes
Catheter Complications
The majority of catheter complications occurring in the first period were multifactorial, relating to nursing education, product selection, program development, insertion techniques, and a less comprehensive infection control program.
Hospital Readmissions
A snapshot of FY 2011 data was used to evaluate hospital readmissions. One hundred one patients were reviewed. Of these patients, 9 (9%) were readmitted to the hospital at some point after being discharged from OPAT. Readmission due to complications of OPAT was found in 2 of the 9 patients. One was due to an adverse drug reaction from the antibiotic; the other was due to a possible relapse of a hip osteomyelitis.
Cost Analysis
The OPAT program has resulted in a total savings to the global hospital budget from the deferred BDOC of more than $65 million (Table 3) since 1989. The OPAT program eliminated > 47,000 days of inpatient care. In FY 2009 the program cost the hospital $691.35 for each of the 106 patients enrolled (total cost: $73,283.10). This included all IV supplies, antimicrobials, visiting nurse costs when applicable, as well as nursing and pharmacy time dedicated to training the patient and making therapeutic decisions. Expenses for 3,109 BDOC would have cost about $6,218,000. The outpatient-infusion program saved the hospital nearly $6 million in 2009 alone.
Patient Satisfaction
About 60% of the patients discharged from the OPAT program responded to an evaluation survey. The feedback was overwhelmingly positive with about 99% of respondents reporting satisfaction relating to an improved quality of life. Most of the positive comments were directed toward the outpatient-infusion coordinator for resolving issues, being easily accessible, and acting as a patient advocate.
Discussion
The number and types of reasons for OPAT have grown with the knowledge that it is a safe, cost-effective method for the delivery of parenteral antimicrobials. In the early years of the program, before effective antiretroviral therapy was available, cytomegalovirus retinitis was the second most commonly treated infection of the OPAT program. In recent years, the rise of multidrug-resistant organisms has led to limited oral treatment options for UTIs, which are now the second most commonly treated infection of OPAT. Osteomyelitis clearly remains the top indication for OPAT because it requires long-term therapy. Ceftriaxone remains the drug of choice due to once-daily dosing, spectrum of activity, overall safety, and cost-effectiveness.
Catheter complication rates in the OPAT program were lower than those reported in the literature. According to the 2009 NHSN report, the catheter complication rate in the inpatient long-term care units was 1.0 CRBSI/1,000 catheter days.9 Moreover, this program has been instrumental in providing care that otherwise would be administered through the use of home health agencies.
In the private sector, OPAT is frequently contracted to agencies that provide the same type of service to outpatients who have insurance. These agencies charge for the antimicrobials, IV supplies, nursing visits, and laboratory costs for patient-safety monitoring. Use of an agency could raise expenses by a factor of 8-fold or more above the cost of a hospital-based OPAT program, an estimate based on a comparison with a local federally contracted home-infusion agency that provides specialized home-infusion services at a cost.
Although costs related to hospital readmissions were not factored in to the cost savings calculations, the rate of readmission was low in the snapshot analysis that was conducted at the Edward Hines, Jr. VA Hospital. It is believed that this is the result of the close follow-up and continuity of care that the patients in this OPAT program received.
In addition to cost containment, the data reflect the safe, effective care that resulted from treatment outside the hospital setting. One of the key attributes that has made the Edward Hines, Jr. VA Hospital OPAT program unique is that it is recognized in the community as the only VA facility in the area to provide OPAT as an option for the veteran patient. Other VA facilities in the area contract with home-infusion agencies, which are responsible for supplying the antibiotics and nursing care. The Edward Hines, Jr. VA Hospital is the only VA hospital in VISN 12 that has a facility-supported program that provides all supplies and antimicrobials from the VA—a major contributing factor to the cost savings. Continuity of care is provided to the patient who transitions from inpatient to outpatient status with the same team of providers contributing to the significant patient satisfaction that the program has engendered.
Conclusions
One of the main benefits realized with this transition of antibiotic therapy to the home setting is the avoidance of newly acquired nosocomial infections, including C difficile infection, fungal, and multidrug-resistant bacterial infections. Other benefits include early IV to oral switch in therapy when the patient is deemed a candidate, the ability to go back to work sooner, and the ability to receive treatment in the comfort of the patient’s home. Plans for data collection may include a more in-depth review of repeat admissions due to unresolved infections and the number of patients who are unable to complete OPAT at home.
The Edward Hines, Jr. VA Hospital OPAT program has shown that in a large, federally-funded hospital, OPAT is safe, cost-effective, convenient and leads to increased patient satisfaction in a diverse group of veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Lentino JR, Pachucki CT, Byrne R, Lau MT, Bayer D. Parenteral antibiotic therapy: A home-based program. Fed Pract. 2000;17(4):10-15.
2. Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51(suppl 2):S198-S208.
3. Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007;60(2):356-362.
4. Ingram PR, Sulaiman Z, Chua A, Fisher DA. Comment on: Outpatient parenteral antibiotic therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2008;61(1):226-227.
5. Bernard L, El-Hajj, Pron B, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: Evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26(6):445-451.
6. Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of outpatient parenteral antibiotic therapy (OPAT): An Asian perspective. Int J Antimicrob Agents. 2009;33(1):46-51.
7. Tice AD, Hoaglund PA, Nolet B, McKinnon PS, Mozaffari E. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy. 2002;22(2 Pt 2):63S-70S.
8. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651-1672.
9. Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783-805.
1. Lentino JR, Pachucki CT, Byrne R, Lau MT, Bayer D. Parenteral antibiotic therapy: A home-based program. Fed Pract. 2000;17(4):10-15.
2. Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51(suppl 2):S198-S208.
3. Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007;60(2):356-362.
4. Ingram PR, Sulaiman Z, Chua A, Fisher DA. Comment on: Outpatient parenteral antibiotic therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2008;61(1):226-227.
5. Bernard L, El-Hajj, Pron B, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: Evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26(6):445-451.
6. Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of outpatient parenteral antibiotic therapy (OPAT): An Asian perspective. Int J Antimicrob Agents. 2009;33(1):46-51.
7. Tice AD, Hoaglund PA, Nolet B, McKinnon PS, Mozaffari E. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy. 2002;22(2 Pt 2):63S-70S.
8. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651-1672.
9. Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783-805.