User login
Effect of High-Dose Ergocalciferol on Rate of Falls in a Community-Dwelling, Home-Based Primary Care Veteran Population: A Case-Crossover Study
Annually, about 1 in 4 individuals aged ≥ 65 years will experience at least 1 fall, resulting in nearly 2.8 million cases of emergently treated injuries and more than 800,000 hospitalizations.1-3 Therefore, fall prevention has garnered heightened attention as the population ages. Many factors are at play in fall risk, including vitamin D levels.
Although vitamin D is essential for a multitude of physiologic processes, evidence suggests that serum concentrations of 25-hydroxy vitamin D (25[OH]D) < 30 ng/mL are associated with decreased bone mineral density, muscle weakness, impaired lower extremity function, balance problems, and high fall rates.4-12 Through a meta-analysis published in 2009 that included 8 randomized controlled trials of 2,426 participants aged ≥ 65 years, Bischoff-Ferrari and colleagues found that a dose of 700 to 1,000 IU/d significantly reduced the risk of falling compared with doses of 200 to 600 IU/d.13 A subsequent meta-analysis published in 2012 including 14 randomized trials across 28,135 participants aged ≥ 65 years evaluated the efficacy of supplementation with vitamin D with or without calcium cosupplement on fall prevention.14 Although no difference was found in falls across the total sample, a subgroup analysis exploring the effect in participants with lower vitamin D levels demonstrated a statistically significant benefit of vitamin D supplementation. To decrease the risk of fractures and falls, the American Geriatric Society (AGS) recommends vitamin D supplementation of at least 1,000 IU/d in combination with calcium supplementation in older adults, with a minimum goal 25(OH)D level of 30 ng/mL.15
Alarmingly, Bischoff-Ferrari and colleagues published a double-blind, randomized trial that described an association between higher monthly doses of vitamin D3 (cholecalciferol) and an increased risk of falls compared with 24,000 IU/mo. Particularly at higher achieved levels of 25(OH)D, with no difference in benefit was noted on the primary endpoint of lower extremity function.16
Although there exists limited representation of high-dose vitamin D2 and its resultant effects on falls in those aged ≥ 65 years, once weekly prescribing of vitamin D2 in the form of ergocalciferol 50,000 IU remains a commonly used option for repletion of low 25(OH)D. In this study, the authors evaluated the effect of high-dose ergocalciferol on rate of falls in a community-dwelling veteran population ≥ 65 years with low 25(OH)D.
Methods
Following approval from the Lexington Veteran Affairs Medical Center (Lexington VAMC) Institutional Review Board and Research and Development Committee, a retrospective chart review was conducted. Subjects were identified through use of Microsoft SQL (Redmond, WA). Veterans included were those enrolled in home-based primary care (HBPC), a primary care assignment for those individuals requiring skilled services and case management within the home and for whom falls are documented within the electronic health record (EHR). As fall data in a community-dwelling population are difficult to obtain in a retrospective analysis, the HBPC population offered a viable pool of data for evaluation. Some patients eligible for HBPC at the Lexington VAMC may be more dependent on specialized services offered through HBPC or have a reduced ability to perform activities of daily living (ADLs). Other patients can ambulate but may have difficulty traveling great distances to Lexington VAMC.
In addition to HBPC enrollment, veterans were included in the study if they were aged ≥ 65 years and had a 25(OH)D level < 20 ng/mL with subsequent prescribing of high-dose vitamin D2 for repletion, namely, ergocalciferol 50,000 IU once weekly, between March 1, 2005, and September 30, 2016.
Veterans were excluded if they had been enrolled in HBPC for less than 60 days before ergocalciferol initiation, if they were deceased or had been discharged from HBPC within 60 days of ergocalciferol initiation, if they had comorbid conditions that inherently increase the risk of falls (eg, Lewy body dementia, Parkinson disease, bilateral below-the-hip amputation, and hemi- or quadriplegia), or if they had been dispensed a previous prescription of ergocalciferol in the preceding 9 months.
A case-crossover study design was used, which compared the 60-day period prior to initiation of ergocalciferol supplementation with the 60-day period following initiation of supplementation. A 7-day period between these 2 periods was allotted to allow time for mailing of the new prescription and initiation of the supplement.
Data Collection
Data collected included age, sex, levels of 25(OH)D, ergocalciferol prescription data (dose, administration frequency, quantity, day supply, and fill date), falls documented during the 60 days preceding and during supplementation, and the number of medications that posed an increased risk of falls actively prescribed prior to and during supplementation. Those medications considered to increase risk of falls were determined according to the medications listed in the AGS 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.17
Endpoints
The primary endpoint assessed was the change in rate of falls between the time preceding and during supplementation. The number of falls during the 60 days preceding ergocalciferol supplementation was standardized to falls per person per 30 days and compared with the same parameter during the 60-day period following initiation of ergocalciferol.
The secondary outcome was the rate of falls according to the level of 25(OH)D achieved as a result of supplementation in those patients who achieved a minimum 25(OH)D level of 30 ng/mL according to AGS recommendations. Those patients who achieved a minimum 25(OH)D concentration of 30 ng/mL were separated into 2 equal groups according to their respective concentration relative to the median.
Statistical Analysis
Numerical variables were compared using a Student t test. For the primary outcome, 64 participants were required in order to achieve 80% power at a significance of .05 for a 2-tailed assessment, each serving as his or her own control in the case-crossover study design. For the secondary outcome of falls according to 25(OH)D level following supplementation in order to achieve 80% power at a significance of 0.05 for a 2-tailed assessment, a total of 128 participants who reach a minimum 25(OH)D level of 30 ng/mL were required.
Results
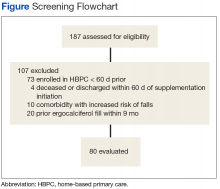
After screening 187 subjects who met the inclusion criteria, 107 subjects were excluded (Figure ).
Primary Endpoint
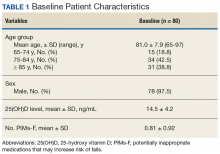
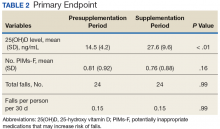
Following once weekly supplementation with ergocalciferol 50,000 IU, 25(OH)D levels increased from 14.5 ng/mL (SD 4.2) to 27.6 (SD 9.6) (P < .01). Of note, the timing of the 25(OH)D level obtained following initiation of supplementation ranged between 8 weeks and 24 weeks. The number of PIMs-F decreased marginally, although to a not statistically significant degree, from 0.81 PIMs-F per person (SD 0.92) to 0.76 PIMs-F per person (SD 0.88).
Secondary Endpoint
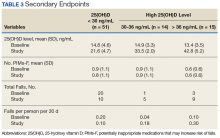
Although 51 of the subjects (63.8%) failed to achieve the target 25(OH)D level of ≥ 30 ng/mL, 29 were successful (Table 3).
In subjects whose achieved 25(OH)D level was 30 to 36 ng/mL, the rate of falls per person per 30 days increased from 0.036 to 0.18. Similarly, an increase in rate of falls per person per 30 days from 0.1 to 0.3 was noted in subjects whose attained 25(OH)D level was > 36.0 ng/mL. However, study enrollment was underpowered to claim statistical significance in these findings related to the secondary endpoint.
Discussion
In this retrospective chart review, individuals aged ≥ 65 years who were prescribed once weekly ergocalciferol 50,000 IU for increase of 25(OH)D levels < 20 ng/mL experienced no change in rate of falls across the entire study population. In those individuals whose achieved 25(OH)D level met the AGS recommendation of ≥ 30 ng/mL, there was a trend toward an increased rate of falls while the rate of falls decreased for subjects whose achieved 25(OH)D level was < 30 ng/mL.
High-dose vitamin D supplementation, albeit with vitamin D3, and its effect on falls have been evaluated in the geriatric population previously, most notably and recently, by Bischoff-Ferrari and colleagues.16 In a study comparing 24,000 IU vitamin D3 per month vs 60,000 IU vitamin D3 per month vs 24,000 IU vitamin D3 plus calcifediol 300 µg per month, lower extremity function did not differ in the 3 groups. However, an increased number of falls was noted in the second and third arm, respectively. Furthermore, after 12 months of treatment, those individuals who achieved the highest quartile of 25(OH)D level (44.7-98.9 ng/mL) had starkly increased odds of falling and number of falls compared with those achieving the lowest quartile (21.3-30.3 ng/mL).
The results of this study suggest that once-weekly high-dose vitamin D2 may carry a similar risk of increasing falls as found with high-dose vitamin D3, particularly at higher achieved levels of 25(OH)D. A possible explanation for a lower rate of falls in those individuals who did not achieve a 25(OH)D level of at least 30 ng/mL could be that these individuals may not have initiated the medication appropriately or administered it adherently, thereby avoiding a possible deleterious effect that the high-dose preparation may pose in this population.
Given the retrospective nature of the study and the evaluation of the change in the 25(OH)D level following approximately a 90-day supply of ergocalciferol, adherence was not addressed. In this case, although increased 25(OH)D level was the desired outcome of vitamin D supplementation, the increase in rate of falls may be attributable to the high-dose preparation itself. Alternatively, the 25(OH)D target of ≥ 30 ng/mL may be worth reconsidering in favor of a lower target with an upper limit.
The rate of falls in this study was collected over the 60 days following initiation of ergocalciferol. However, the achieved 25(OH)D level was not evaluated until between 8 and 24 weeks following initiation. In this context, it may be more likely that the increased rate of falls could be attributable to the high-dose nature of vitamin D2 supplementation or the rate of 25(OH)D repletion rather than the 25(OH)D level ultimately achieved.
Limitations
Given the study’s retrospective nature, at times there was difficulty in locating information in the EHR, including accurate reports of active medication use during study periods or documentation of all falls that had occurred in the appropriate format. This was further complicated by the reliance on self-reporting of falls, which may potentiate an underestimation of total falls.
The largely homogenous study population may limit extrapolating these results. Additionally, although some diseases and medications with an inherent risk on fall risk were incorporated into the exclusion criteria, on analysis, other diseases and medications were identified that also may pose a similar risk. These include legal blindness and a history of below-the-knee amputation as well as long-term opioid therapy and intensive antihypertensive therapy with multiple agents. Furthermore, other potential risk factors for falls were not addressed, such as functional status, use of assistive devices, or unsafe home environments.
For the secondary endpoint, sample size was not met for statistical significance, which limited the study’s ability to confirm the veracity of the trend of increased falls. Study duration posed an additional limitation. As most veterans enrolled in HBPC have vitamin D supplementation initiated soon after enrollment when the need for vitamin D repletion is routinely assessed, a 2-month duration for evaluation prior to and immediately following initiation of ergocalciferol was necessary to allow for adequate study enrollment for analysis of the primary endpoint. However, this may be resolved through conduction of a prospective study in the future.
Conclusion
There was no difference identified in the rate of falls immediately prior to and following initiation of ergocalciferol 50,000 IU self-administered once weekly. There was a trend of increased rate of falls in subjects with high levels of 25(OH)D achieved. In light of a similar finding of high-dose vitamin D3 associated with an increased rate of falls, particularly with higher achieved levels of 25(OH)D, it may be warranted to consider avoiding high-dose vitamin D2 supplementation. Future research including prospective, randomized clinical studies with a longer duration of follow-up would be recommended to confirm these findings and test the generalizability in the non-HBPC community-dwelling population.
1. Stevens JA, Ballesteros MF, Mack KA, Rudd RA, DeCaro E, Adler G. Gender differences in seeking care for falls in the aged Medicare population. Am J Prev Med. 2012;43(1):59-62.
2. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Welcome to WISQARS. https://www.cdc.gov/injury/wisqars/index.html. Updated February 5, 2018. Accessed April 10, 2018.
3. O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137(3):342-354.
4. Bischhoff-Ferrari HA, Dawson-Hughes B, Willet WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999-2006.
5. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
6. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
7. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
8. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-OH vitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > 60 years. Am J Clin Nutr. 2004;80(3):752-758.
9. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113-1118.
10. Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab. 2004;89(4):1572-1576.
11. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geratr Soc. 2003;51(11):1533-1538.
12. Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17(9):1318-1328.
13. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. BMJ. 2009;339:b3692.
14. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
15. American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for the prevention of falls and their consequences. J Am Geriatr Soc. 2014;62(1):147-152.
16. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175-183.
17. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
Annually, about 1 in 4 individuals aged ≥ 65 years will experience at least 1 fall, resulting in nearly 2.8 million cases of emergently treated injuries and more than 800,000 hospitalizations.1-3 Therefore, fall prevention has garnered heightened attention as the population ages. Many factors are at play in fall risk, including vitamin D levels.
Although vitamin D is essential for a multitude of physiologic processes, evidence suggests that serum concentrations of 25-hydroxy vitamin D (25[OH]D) < 30 ng/mL are associated with decreased bone mineral density, muscle weakness, impaired lower extremity function, balance problems, and high fall rates.4-12 Through a meta-analysis published in 2009 that included 8 randomized controlled trials of 2,426 participants aged ≥ 65 years, Bischoff-Ferrari and colleagues found that a dose of 700 to 1,000 IU/d significantly reduced the risk of falling compared with doses of 200 to 600 IU/d.13 A subsequent meta-analysis published in 2012 including 14 randomized trials across 28,135 participants aged ≥ 65 years evaluated the efficacy of supplementation with vitamin D with or without calcium cosupplement on fall prevention.14 Although no difference was found in falls across the total sample, a subgroup analysis exploring the effect in participants with lower vitamin D levels demonstrated a statistically significant benefit of vitamin D supplementation. To decrease the risk of fractures and falls, the American Geriatric Society (AGS) recommends vitamin D supplementation of at least 1,000 IU/d in combination with calcium supplementation in older adults, with a minimum goal 25(OH)D level of 30 ng/mL.15
Alarmingly, Bischoff-Ferrari and colleagues published a double-blind, randomized trial that described an association between higher monthly doses of vitamin D3 (cholecalciferol) and an increased risk of falls compared with 24,000 IU/mo. Particularly at higher achieved levels of 25(OH)D, with no difference in benefit was noted on the primary endpoint of lower extremity function.16
Although there exists limited representation of high-dose vitamin D2 and its resultant effects on falls in those aged ≥ 65 years, once weekly prescribing of vitamin D2 in the form of ergocalciferol 50,000 IU remains a commonly used option for repletion of low 25(OH)D. In this study, the authors evaluated the effect of high-dose ergocalciferol on rate of falls in a community-dwelling veteran population ≥ 65 years with low 25(OH)D.
Methods
Following approval from the Lexington Veteran Affairs Medical Center (Lexington VAMC) Institutional Review Board and Research and Development Committee, a retrospective chart review was conducted. Subjects were identified through use of Microsoft SQL (Redmond, WA). Veterans included were those enrolled in home-based primary care (HBPC), a primary care assignment for those individuals requiring skilled services and case management within the home and for whom falls are documented within the electronic health record (EHR). As fall data in a community-dwelling population are difficult to obtain in a retrospective analysis, the HBPC population offered a viable pool of data for evaluation. Some patients eligible for HBPC at the Lexington VAMC may be more dependent on specialized services offered through HBPC or have a reduced ability to perform activities of daily living (ADLs). Other patients can ambulate but may have difficulty traveling great distances to Lexington VAMC.
In addition to HBPC enrollment, veterans were included in the study if they were aged ≥ 65 years and had a 25(OH)D level < 20 ng/mL with subsequent prescribing of high-dose vitamin D2 for repletion, namely, ergocalciferol 50,000 IU once weekly, between March 1, 2005, and September 30, 2016.
Veterans were excluded if they had been enrolled in HBPC for less than 60 days before ergocalciferol initiation, if they were deceased or had been discharged from HBPC within 60 days of ergocalciferol initiation, if they had comorbid conditions that inherently increase the risk of falls (eg, Lewy body dementia, Parkinson disease, bilateral below-the-hip amputation, and hemi- or quadriplegia), or if they had been dispensed a previous prescription of ergocalciferol in the preceding 9 months.
A case-crossover study design was used, which compared the 60-day period prior to initiation of ergocalciferol supplementation with the 60-day period following initiation of supplementation. A 7-day period between these 2 periods was allotted to allow time for mailing of the new prescription and initiation of the supplement.
Data Collection
Data collected included age, sex, levels of 25(OH)D, ergocalciferol prescription data (dose, administration frequency, quantity, day supply, and fill date), falls documented during the 60 days preceding and during supplementation, and the number of medications that posed an increased risk of falls actively prescribed prior to and during supplementation. Those medications considered to increase risk of falls were determined according to the medications listed in the AGS 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.17
Endpoints
The primary endpoint assessed was the change in rate of falls between the time preceding and during supplementation. The number of falls during the 60 days preceding ergocalciferol supplementation was standardized to falls per person per 30 days and compared with the same parameter during the 60-day period following initiation of ergocalciferol.
The secondary outcome was the rate of falls according to the level of 25(OH)D achieved as a result of supplementation in those patients who achieved a minimum 25(OH)D level of 30 ng/mL according to AGS recommendations. Those patients who achieved a minimum 25(OH)D concentration of 30 ng/mL were separated into 2 equal groups according to their respective concentration relative to the median.
Statistical Analysis
Numerical variables were compared using a Student t test. For the primary outcome, 64 participants were required in order to achieve 80% power at a significance of .05 for a 2-tailed assessment, each serving as his or her own control in the case-crossover study design. For the secondary outcome of falls according to 25(OH)D level following supplementation in order to achieve 80% power at a significance of 0.05 for a 2-tailed assessment, a total of 128 participants who reach a minimum 25(OH)D level of 30 ng/mL were required.
Results
After screening 187 subjects who met the inclusion criteria, 107 subjects were excluded (Figure ).
Primary Endpoint
Following once weekly supplementation with ergocalciferol 50,000 IU, 25(OH)D levels increased from 14.5 ng/mL (SD 4.2) to 27.6 (SD 9.6) (P < .01). Of note, the timing of the 25(OH)D level obtained following initiation of supplementation ranged between 8 weeks and 24 weeks. The number of PIMs-F decreased marginally, although to a not statistically significant degree, from 0.81 PIMs-F per person (SD 0.92) to 0.76 PIMs-F per person (SD 0.88).
Secondary Endpoint
Although 51 of the subjects (63.8%) failed to achieve the target 25(OH)D level of ≥ 30 ng/mL, 29 were successful (Table 3).
In subjects whose achieved 25(OH)D level was 30 to 36 ng/mL, the rate of falls per person per 30 days increased from 0.036 to 0.18. Similarly, an increase in rate of falls per person per 30 days from 0.1 to 0.3 was noted in subjects whose attained 25(OH)D level was > 36.0 ng/mL. However, study enrollment was underpowered to claim statistical significance in these findings related to the secondary endpoint.
Discussion
In this retrospective chart review, individuals aged ≥ 65 years who were prescribed once weekly ergocalciferol 50,000 IU for increase of 25(OH)D levels < 20 ng/mL experienced no change in rate of falls across the entire study population. In those individuals whose achieved 25(OH)D level met the AGS recommendation of ≥ 30 ng/mL, there was a trend toward an increased rate of falls while the rate of falls decreased for subjects whose achieved 25(OH)D level was < 30 ng/mL.
High-dose vitamin D supplementation, albeit with vitamin D3, and its effect on falls have been evaluated in the geriatric population previously, most notably and recently, by Bischoff-Ferrari and colleagues.16 In a study comparing 24,000 IU vitamin D3 per month vs 60,000 IU vitamin D3 per month vs 24,000 IU vitamin D3 plus calcifediol 300 µg per month, lower extremity function did not differ in the 3 groups. However, an increased number of falls was noted in the second and third arm, respectively. Furthermore, after 12 months of treatment, those individuals who achieved the highest quartile of 25(OH)D level (44.7-98.9 ng/mL) had starkly increased odds of falling and number of falls compared with those achieving the lowest quartile (21.3-30.3 ng/mL).
The results of this study suggest that once-weekly high-dose vitamin D2 may carry a similar risk of increasing falls as found with high-dose vitamin D3, particularly at higher achieved levels of 25(OH)D. A possible explanation for a lower rate of falls in those individuals who did not achieve a 25(OH)D level of at least 30 ng/mL could be that these individuals may not have initiated the medication appropriately or administered it adherently, thereby avoiding a possible deleterious effect that the high-dose preparation may pose in this population.
Given the retrospective nature of the study and the evaluation of the change in the 25(OH)D level following approximately a 90-day supply of ergocalciferol, adherence was not addressed. In this case, although increased 25(OH)D level was the desired outcome of vitamin D supplementation, the increase in rate of falls may be attributable to the high-dose preparation itself. Alternatively, the 25(OH)D target of ≥ 30 ng/mL may be worth reconsidering in favor of a lower target with an upper limit.
The rate of falls in this study was collected over the 60 days following initiation of ergocalciferol. However, the achieved 25(OH)D level was not evaluated until between 8 and 24 weeks following initiation. In this context, it may be more likely that the increased rate of falls could be attributable to the high-dose nature of vitamin D2 supplementation or the rate of 25(OH)D repletion rather than the 25(OH)D level ultimately achieved.
Limitations
Given the study’s retrospective nature, at times there was difficulty in locating information in the EHR, including accurate reports of active medication use during study periods or documentation of all falls that had occurred in the appropriate format. This was further complicated by the reliance on self-reporting of falls, which may potentiate an underestimation of total falls.
The largely homogenous study population may limit extrapolating these results. Additionally, although some diseases and medications with an inherent risk on fall risk were incorporated into the exclusion criteria, on analysis, other diseases and medications were identified that also may pose a similar risk. These include legal blindness and a history of below-the-knee amputation as well as long-term opioid therapy and intensive antihypertensive therapy with multiple agents. Furthermore, other potential risk factors for falls were not addressed, such as functional status, use of assistive devices, or unsafe home environments.
For the secondary endpoint, sample size was not met for statistical significance, which limited the study’s ability to confirm the veracity of the trend of increased falls. Study duration posed an additional limitation. As most veterans enrolled in HBPC have vitamin D supplementation initiated soon after enrollment when the need for vitamin D repletion is routinely assessed, a 2-month duration for evaluation prior to and immediately following initiation of ergocalciferol was necessary to allow for adequate study enrollment for analysis of the primary endpoint. However, this may be resolved through conduction of a prospective study in the future.
Conclusion
There was no difference identified in the rate of falls immediately prior to and following initiation of ergocalciferol 50,000 IU self-administered once weekly. There was a trend of increased rate of falls in subjects with high levels of 25(OH)D achieved. In light of a similar finding of high-dose vitamin D3 associated with an increased rate of falls, particularly with higher achieved levels of 25(OH)D, it may be warranted to consider avoiding high-dose vitamin D2 supplementation. Future research including prospective, randomized clinical studies with a longer duration of follow-up would be recommended to confirm these findings and test the generalizability in the non-HBPC community-dwelling population.
Annually, about 1 in 4 individuals aged ≥ 65 years will experience at least 1 fall, resulting in nearly 2.8 million cases of emergently treated injuries and more than 800,000 hospitalizations.1-3 Therefore, fall prevention has garnered heightened attention as the population ages. Many factors are at play in fall risk, including vitamin D levels.
Although vitamin D is essential for a multitude of physiologic processes, evidence suggests that serum concentrations of 25-hydroxy vitamin D (25[OH]D) < 30 ng/mL are associated with decreased bone mineral density, muscle weakness, impaired lower extremity function, balance problems, and high fall rates.4-12 Through a meta-analysis published in 2009 that included 8 randomized controlled trials of 2,426 participants aged ≥ 65 years, Bischoff-Ferrari and colleagues found that a dose of 700 to 1,000 IU/d significantly reduced the risk of falling compared with doses of 200 to 600 IU/d.13 A subsequent meta-analysis published in 2012 including 14 randomized trials across 28,135 participants aged ≥ 65 years evaluated the efficacy of supplementation with vitamin D with or without calcium cosupplement on fall prevention.14 Although no difference was found in falls across the total sample, a subgroup analysis exploring the effect in participants with lower vitamin D levels demonstrated a statistically significant benefit of vitamin D supplementation. To decrease the risk of fractures and falls, the American Geriatric Society (AGS) recommends vitamin D supplementation of at least 1,000 IU/d in combination with calcium supplementation in older adults, with a minimum goal 25(OH)D level of 30 ng/mL.15
Alarmingly, Bischoff-Ferrari and colleagues published a double-blind, randomized trial that described an association between higher monthly doses of vitamin D3 (cholecalciferol) and an increased risk of falls compared with 24,000 IU/mo. Particularly at higher achieved levels of 25(OH)D, with no difference in benefit was noted on the primary endpoint of lower extremity function.16
Although there exists limited representation of high-dose vitamin D2 and its resultant effects on falls in those aged ≥ 65 years, once weekly prescribing of vitamin D2 in the form of ergocalciferol 50,000 IU remains a commonly used option for repletion of low 25(OH)D. In this study, the authors evaluated the effect of high-dose ergocalciferol on rate of falls in a community-dwelling veteran population ≥ 65 years with low 25(OH)D.
Methods
Following approval from the Lexington Veteran Affairs Medical Center (Lexington VAMC) Institutional Review Board and Research and Development Committee, a retrospective chart review was conducted. Subjects were identified through use of Microsoft SQL (Redmond, WA). Veterans included were those enrolled in home-based primary care (HBPC), a primary care assignment for those individuals requiring skilled services and case management within the home and for whom falls are documented within the electronic health record (EHR). As fall data in a community-dwelling population are difficult to obtain in a retrospective analysis, the HBPC population offered a viable pool of data for evaluation. Some patients eligible for HBPC at the Lexington VAMC may be more dependent on specialized services offered through HBPC or have a reduced ability to perform activities of daily living (ADLs). Other patients can ambulate but may have difficulty traveling great distances to Lexington VAMC.
In addition to HBPC enrollment, veterans were included in the study if they were aged ≥ 65 years and had a 25(OH)D level < 20 ng/mL with subsequent prescribing of high-dose vitamin D2 for repletion, namely, ergocalciferol 50,000 IU once weekly, between March 1, 2005, and September 30, 2016.
Veterans were excluded if they had been enrolled in HBPC for less than 60 days before ergocalciferol initiation, if they were deceased or had been discharged from HBPC within 60 days of ergocalciferol initiation, if they had comorbid conditions that inherently increase the risk of falls (eg, Lewy body dementia, Parkinson disease, bilateral below-the-hip amputation, and hemi- or quadriplegia), or if they had been dispensed a previous prescription of ergocalciferol in the preceding 9 months.
A case-crossover study design was used, which compared the 60-day period prior to initiation of ergocalciferol supplementation with the 60-day period following initiation of supplementation. A 7-day period between these 2 periods was allotted to allow time for mailing of the new prescription and initiation of the supplement.
Data Collection
Data collected included age, sex, levels of 25(OH)D, ergocalciferol prescription data (dose, administration frequency, quantity, day supply, and fill date), falls documented during the 60 days preceding and during supplementation, and the number of medications that posed an increased risk of falls actively prescribed prior to and during supplementation. Those medications considered to increase risk of falls were determined according to the medications listed in the AGS 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.17
Endpoints
The primary endpoint assessed was the change in rate of falls between the time preceding and during supplementation. The number of falls during the 60 days preceding ergocalciferol supplementation was standardized to falls per person per 30 days and compared with the same parameter during the 60-day period following initiation of ergocalciferol.
The secondary outcome was the rate of falls according to the level of 25(OH)D achieved as a result of supplementation in those patients who achieved a minimum 25(OH)D level of 30 ng/mL according to AGS recommendations. Those patients who achieved a minimum 25(OH)D concentration of 30 ng/mL were separated into 2 equal groups according to their respective concentration relative to the median.
Statistical Analysis
Numerical variables were compared using a Student t test. For the primary outcome, 64 participants were required in order to achieve 80% power at a significance of .05 for a 2-tailed assessment, each serving as his or her own control in the case-crossover study design. For the secondary outcome of falls according to 25(OH)D level following supplementation in order to achieve 80% power at a significance of 0.05 for a 2-tailed assessment, a total of 128 participants who reach a minimum 25(OH)D level of 30 ng/mL were required.
Results
After screening 187 subjects who met the inclusion criteria, 107 subjects were excluded (Figure ).
Primary Endpoint
Following once weekly supplementation with ergocalciferol 50,000 IU, 25(OH)D levels increased from 14.5 ng/mL (SD 4.2) to 27.6 (SD 9.6) (P < .01). Of note, the timing of the 25(OH)D level obtained following initiation of supplementation ranged between 8 weeks and 24 weeks. The number of PIMs-F decreased marginally, although to a not statistically significant degree, from 0.81 PIMs-F per person (SD 0.92) to 0.76 PIMs-F per person (SD 0.88).
Secondary Endpoint
Although 51 of the subjects (63.8%) failed to achieve the target 25(OH)D level of ≥ 30 ng/mL, 29 were successful (Table 3).
In subjects whose achieved 25(OH)D level was 30 to 36 ng/mL, the rate of falls per person per 30 days increased from 0.036 to 0.18. Similarly, an increase in rate of falls per person per 30 days from 0.1 to 0.3 was noted in subjects whose attained 25(OH)D level was > 36.0 ng/mL. However, study enrollment was underpowered to claim statistical significance in these findings related to the secondary endpoint.
Discussion
In this retrospective chart review, individuals aged ≥ 65 years who were prescribed once weekly ergocalciferol 50,000 IU for increase of 25(OH)D levels < 20 ng/mL experienced no change in rate of falls across the entire study population. In those individuals whose achieved 25(OH)D level met the AGS recommendation of ≥ 30 ng/mL, there was a trend toward an increased rate of falls while the rate of falls decreased for subjects whose achieved 25(OH)D level was < 30 ng/mL.
High-dose vitamin D supplementation, albeit with vitamin D3, and its effect on falls have been evaluated in the geriatric population previously, most notably and recently, by Bischoff-Ferrari and colleagues.16 In a study comparing 24,000 IU vitamin D3 per month vs 60,000 IU vitamin D3 per month vs 24,000 IU vitamin D3 plus calcifediol 300 µg per month, lower extremity function did not differ in the 3 groups. However, an increased number of falls was noted in the second and third arm, respectively. Furthermore, after 12 months of treatment, those individuals who achieved the highest quartile of 25(OH)D level (44.7-98.9 ng/mL) had starkly increased odds of falling and number of falls compared with those achieving the lowest quartile (21.3-30.3 ng/mL).
The results of this study suggest that once-weekly high-dose vitamin D2 may carry a similar risk of increasing falls as found with high-dose vitamin D3, particularly at higher achieved levels of 25(OH)D. A possible explanation for a lower rate of falls in those individuals who did not achieve a 25(OH)D level of at least 30 ng/mL could be that these individuals may not have initiated the medication appropriately or administered it adherently, thereby avoiding a possible deleterious effect that the high-dose preparation may pose in this population.
Given the retrospective nature of the study and the evaluation of the change in the 25(OH)D level following approximately a 90-day supply of ergocalciferol, adherence was not addressed. In this case, although increased 25(OH)D level was the desired outcome of vitamin D supplementation, the increase in rate of falls may be attributable to the high-dose preparation itself. Alternatively, the 25(OH)D target of ≥ 30 ng/mL may be worth reconsidering in favor of a lower target with an upper limit.
The rate of falls in this study was collected over the 60 days following initiation of ergocalciferol. However, the achieved 25(OH)D level was not evaluated until between 8 and 24 weeks following initiation. In this context, it may be more likely that the increased rate of falls could be attributable to the high-dose nature of vitamin D2 supplementation or the rate of 25(OH)D repletion rather than the 25(OH)D level ultimately achieved.
Limitations
Given the study’s retrospective nature, at times there was difficulty in locating information in the EHR, including accurate reports of active medication use during study periods or documentation of all falls that had occurred in the appropriate format. This was further complicated by the reliance on self-reporting of falls, which may potentiate an underestimation of total falls.
The largely homogenous study population may limit extrapolating these results. Additionally, although some diseases and medications with an inherent risk on fall risk were incorporated into the exclusion criteria, on analysis, other diseases and medications were identified that also may pose a similar risk. These include legal blindness and a history of below-the-knee amputation as well as long-term opioid therapy and intensive antihypertensive therapy with multiple agents. Furthermore, other potential risk factors for falls were not addressed, such as functional status, use of assistive devices, or unsafe home environments.
For the secondary endpoint, sample size was not met for statistical significance, which limited the study’s ability to confirm the veracity of the trend of increased falls. Study duration posed an additional limitation. As most veterans enrolled in HBPC have vitamin D supplementation initiated soon after enrollment when the need for vitamin D repletion is routinely assessed, a 2-month duration for evaluation prior to and immediately following initiation of ergocalciferol was necessary to allow for adequate study enrollment for analysis of the primary endpoint. However, this may be resolved through conduction of a prospective study in the future.
Conclusion
There was no difference identified in the rate of falls immediately prior to and following initiation of ergocalciferol 50,000 IU self-administered once weekly. There was a trend of increased rate of falls in subjects with high levels of 25(OH)D achieved. In light of a similar finding of high-dose vitamin D3 associated with an increased rate of falls, particularly with higher achieved levels of 25(OH)D, it may be warranted to consider avoiding high-dose vitamin D2 supplementation. Future research including prospective, randomized clinical studies with a longer duration of follow-up would be recommended to confirm these findings and test the generalizability in the non-HBPC community-dwelling population.
1. Stevens JA, Ballesteros MF, Mack KA, Rudd RA, DeCaro E, Adler G. Gender differences in seeking care for falls in the aged Medicare population. Am J Prev Med. 2012;43(1):59-62.
2. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Welcome to WISQARS. https://www.cdc.gov/injury/wisqars/index.html. Updated February 5, 2018. Accessed April 10, 2018.
3. O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137(3):342-354.
4. Bischhoff-Ferrari HA, Dawson-Hughes B, Willet WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999-2006.
5. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
6. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
7. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
8. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-OH vitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > 60 years. Am J Clin Nutr. 2004;80(3):752-758.
9. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113-1118.
10. Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab. 2004;89(4):1572-1576.
11. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geratr Soc. 2003;51(11):1533-1538.
12. Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17(9):1318-1328.
13. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. BMJ. 2009;339:b3692.
14. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
15. American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for the prevention of falls and their consequences. J Am Geriatr Soc. 2014;62(1):147-152.
16. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175-183.
17. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
1. Stevens JA, Ballesteros MF, Mack KA, Rudd RA, DeCaro E, Adler G. Gender differences in seeking care for falls in the aged Medicare population. Am J Prev Med. 2012;43(1):59-62.
2. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Welcome to WISQARS. https://www.cdc.gov/injury/wisqars/index.html. Updated February 5, 2018. Accessed April 10, 2018.
3. O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137(3):342-354.
4. Bischhoff-Ferrari HA, Dawson-Hughes B, Willet WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999-2006.
5. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
6. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
7. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
8. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-OH vitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > 60 years. Am J Clin Nutr. 2004;80(3):752-758.
9. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113-1118.
10. Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab. 2004;89(4):1572-1576.
11. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geratr Soc. 2003;51(11):1533-1538.
12. Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17(9):1318-1328.
13. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. BMJ. 2009;339:b3692.
14. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
15. American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for the prevention of falls and their consequences. J Am Geriatr Soc. 2014;62(1):147-152.
16. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175-183.
17. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.