User login
Do Probiotics Reduce C diff Risk in Hospitalized Patients?
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
Do probiotics reduce C diff risk in hospitalized patients?
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
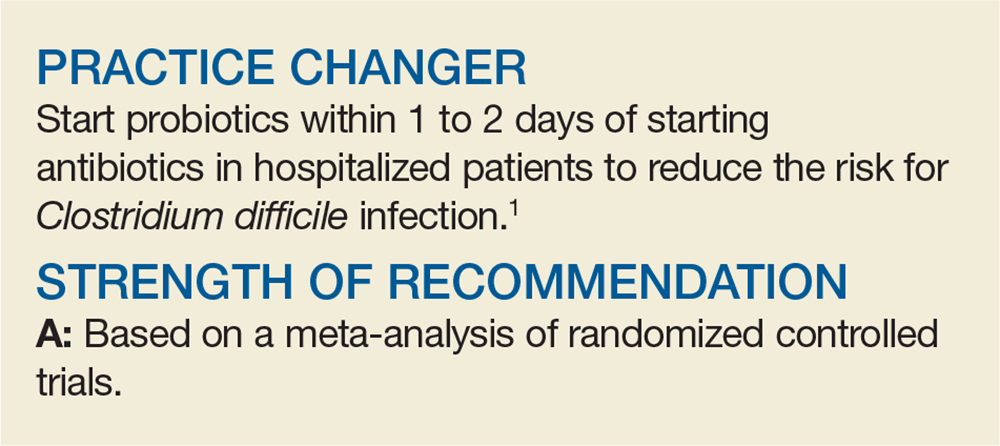
PRACTICE CHANGER
Start probiotics within 1 to 2 days of starting antibiotics in hospitalized patients to reduce the risk of Clostridium difficile infection.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900. e9.