User login
Multidisciplinary Diabetes Care in a Safety Net Clinic: Lessons Learned from a Quality Improvement Initiative
From the Department of Family and Community Medicine, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Zare, Ms Klawans, Dr. Moreno), Department of Family Medicine and Community Medicine, Baylor College of Medicine, Houston, TX (Dr. Mejia de Grubb, Dr. Juneja, Dr. Zoorob), Department of Psychiatry, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Suchting), andHarris Health System, Houston TX (Ms. Mathis).
Abstract
- Objective: To describe a pilot project to improve care for patients with uncontrolled diabetes in a safety net clinic.
- Methods: One of 3 clinical teams was designated the intervention team. Changes implemented by the intervention team included patient referral to a dietician and/or clinical pharmacist, provision of patient education, and assignment of a case manager. We compared outcomes of patients in the intervention group (n = 71), vs those receiving care from the other 2 teams (usual care) (n = 188).
- Results: HbA1c significantly decreased over time for patients in the intervention group as well as the usual care group. Within the intervention group, visits to clinical pharmacist (P = 0.034) and education (P = 0.004) predicted significantly greater decreases in HbA1c over time.
- Conclusions: Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Results support the need for further pragmatic research to weigh the impact of unblinded designs, outcome measurement, and real-world behaviors on evidence-based interventions.
Key words: diabetes; safety net; multidisciplinary diabetes care; primary care; diffusion of treatment.
The prevalence of type 2 diabetes in the United States is significantly higher among Hispanics and African Americans than in the general population (13% vs. 9.3%) [1]. Similarly, diabetes is highly prevalent among the uninsured, and many patients delay or forgo treatment due to cost [2]. Subsequently, the rates of comorbidities, including stroke, hypertension, and CVD, are elevated in these groups [3].
Association between elevated HbA1c and morbidity and mortality is well-documented, and an HbA1c reduction of just 1% has been shown to reduce mortality and improve quality of life [4]. Uncontrolled diabetes also results in increased medical costs. Reducing HbA1c from 9.0 to 7.5 reduces annual expenditures by as much as 73% [5].
Metropolitan Houston and Harris County, Texas, has one of the largest uninsured metro populations in the United States (over 3.6 million) [6]. Harris Health System serves this uninsured population and is the fourth largest safety net health system in the nation. Approximately 40,000 patients with diabetes receive care within the health system, and 34% of them have an HbA1c value greater than 9.
Developing novel, cost-efficient treatment and management models is crucial when providing care for patients with uncontrolled diabetes. However, the study of implementation strategies to successfully integrate evidence-based interventions in primary care using pragmatic approaches that aim to determine the effectiveness of interventions in “the real world” remain a challenge [7,8]. To address this issue, a quality improvement project was instituted at one of the system’s health centers to improve the care of patients with uncontrolled diabetes (known HbA1c above 9).
Methods
Setting
The pilot project was conducted from 1 Oct 2015 to 31 Dec 2015 in a primary care community health center within Harris Health System in Houston, Texas. This pilot was the first step of an institutional effort to introduce a multidisciplinary model of care across all clinics [9]. Our health center has 6 family medicine providers and 1 advanced practice nurse practitioner, organized into 3 pods with 2 physicians each. We randomly selected 1 pod (team) and designated it the intervention group.
The Standards for Quality Improvement Reporting Excellence guidelines [10] were followed and institutional review board approval was obtained.
Intervention
Practice changes introduced in the intervention team were assignment of a case manager to all patients, referral to a dietician and clinical pharmacist as needed, and patient education sessions. The team’s nurse assumed the role of case manager. The case manager was responsible for reviewing a patient checklist based on the America Diabetes Association guideline for comprehensive diabetes medical evaluation at initial and follow-up visits. Referrals were based on ADA guideline recommendations. Onsite brief patient education was provided to all patients. In addition, patients were enrolled in a “Diabetes 101” class, which follows an evidence-based curriculum that includes participation in at least 2 monthly sessions. Patients were asked to return to the clinic for a follow-up visit after 3 months in order to monitor medication compliance, re-evaluate their care plan, and measure HbA1c The usual care group patients were managed based on the current Standards of Medical Care in Diabetes [11]. The usual care group included patients from the same clinic under the care of providers in the teams that were not included in the multidisciplinary intervention.
Analysis
Data abstracted from de-identified patient records included HbA1c values, interventions received, and sociodemographic data. Generalized linear mixed modeling (GLMM) was used to examine changes in patient HbA1c levels over time [12]. All models included a random intercept to account for correlated observations within patient. All analyses were performed using Proc GLIMMIX in SAS v. 9.3 [13].
Results
A total 271 patients with HbA1c above 9 were included in the analysis: 71 in the intervention group and 188 in the usual care group. The intervention group was further differentiated by month of enrollment: October (n = 37), November (n = 27), and December (n = 9). Mean patient age in the overall sample was 51.6 years.
In the intervention group, most patients received patient education 56% (n = 40), almost half had a clinical pharmacy visit, but only 17% (n = 12) received a dietitian consultation. Overall, there was a 1.4% decrease in HbA1c in the intervention group, compared to a 1.3% HbA1c decrease in the usual care group.
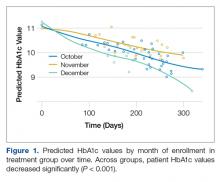
GLMM was used to examine differences in HbA1c levels according to month of intervention enrollment (October vs. November vs. December) in the intervention group over time. Figure 1 shows predicted HbA1c values over time with trend lines fit for each of the three subgroups.
Preliminary analysis showed that potential contamination (diffusion of the treatment) would be likely to attenuate differences in the outcomes between the intervention and usual care conditions. Further analysis by subgroups were conducted to describe the intervention potential “spillover” to the usual care group participants not intended to receive the intervention. GLMM also examined differences in HbA1c levels between the intervention and usual care groups over time. The interaction between each treatment group and time was not statistically significant (F(2,268) = 1.34, P = 0.26), indicating that changes in HbA1c over time were not related to treatment group. A statistically reliable main effect for time (F(1,268) = 44.33, P < 0.001) indicated that in all groups, HbA1c values significantly decreased over time.
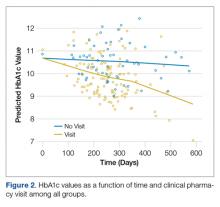
Follow-up analyses utilized GLMM to examine differences in HbA1c levels among patients in both groups who received at least one of the interventions (visiting a dietician, clinical pharmacist, education session, and clinical case manager). The interaction between intervention and time was not statistically significant for visiting the dietician, receiving education, or being assigned a case manager. The interaction between time and visiting a clinical pharmacist was statistically significant (F(1,204)= 7.78, P = 0.01) such that patients visiting the clinical pharmacist had lower HbA1c values over time relative to those that did not (Figure 2).
Discussion
HbA1c decreased significantly among intervention patients with uncontrolled diabetes over a 3-month period, regardless of which month they entered the study. However, there was no significant difference in HbA1c reduction between patients who received all 4 multidisciplinary interventions, one intervention, or those who received usual care. Patients in the intervention who attended clinical pharmacist visits had significantly greater HbA1c reduction than patients who did not, as did patients who attended a diabetes education session by a patient educator.
Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Diffusion refers to the unintended spread of a treatment effect when participants receive some or all treatments from an intervention to which they were not assigned, making outcomes descriptions of all study groups more challenging [14]. During the implementation period, other physicians and nurses in the clinic were aware of the multidisciplinary care model being piloted, and may have taken the initiative to connect their patients with clinical pharmacists, dieticians, certified diabetes educators, and clinical case managers. Pragmatic interventions are intended to maintain the internal validity of randomized control trials, yet they are meant to be implemented as close as possible to real-world settings in order to help patients, clinicians, and payers making informed health care decisions [8]. In this regard, participants in the control group could be exposed to the intervention through staff contact between the assigned groups implementing some of the intervention under study. In that case, the diffusion of treatment would be likely to attenuate differences in the outcomes between treatment and control groups [15].
This study has several limitations. We studied a small sample of patients that reflected the primary care population in one clinic in a safety net system with minority, underserved, and high-risk patients. Although attempts were made to keep the intervention limited to the intervention pod, diffusion of treatment might have impacted the internal validity of this intervention.
In summary, our results support the need for further systematic research work to weigh the impact of unblinded designs, simplified recruitment and outcome measurement, and real-world behaviors (such as noncompliance, cross over, and dropout) on evidence-based and multidisciplinary clinical interventions.
Acknowledgements: The authors would like to thank Krystal Gamarra, MSW, LCSW, and Hope Galvan, MS, RN, CVRN-BC, CDE for assistance with project implementation and data collection throughout this process.
Corresponding author: Maria C. Mejia de Grubb, MD, MPH, 3751 Kirby Dr, Suite 600, Houston, TX 77098, mcgrubb@bcm.edu.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
2. Casagrande SS, Cowie CC. Health insurance coverage among people with and without diabetes in the US adult population. Diabetes Care 2012;35:2243–9.
3. American Diabetes Association. Statistics about diabetes. Arlington, VA; 2017.
4. Eeg-Olofsson K, Eliasson B, Zethelius B, et al. HbA1c reduction and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish NDR. Diabetes 2012.
5. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016;33:1575–81.
6. Harris County Healthcare Alliance. The State of Health in Houston/Harris County 2015-2016. Accessed 17 Mar 2015 at http://houstonstateofhealth.org/soh_doc/.
7. Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials (London, England) 2012;9:436–46.
8. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009;301:831–41.
9. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Qty 1996:511–44.
10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Safety 2016;25:986–92.
11. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. American Diabetes Association. Clin Diabetes 2015;33:97–111.
12. Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. New York: Cambridge University Press; 2007.
13. SAS Institute I. Base SAS Procedures Guide: Statistical procedures. In: SAS Institute I, editor. Cary, NC; 2011.
14. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin; 2002.
15. Kane R. Understanding health care outcomes research. 2nd ed. In: Learning JB, editor. Burlington, MA: Jones and Bartlett; 2006: 44–6.
From the Department of Family and Community Medicine, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Zare, Ms Klawans, Dr. Moreno), Department of Family Medicine and Community Medicine, Baylor College of Medicine, Houston, TX (Dr. Mejia de Grubb, Dr. Juneja, Dr. Zoorob), Department of Psychiatry, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Suchting), andHarris Health System, Houston TX (Ms. Mathis).
Abstract
- Objective: To describe a pilot project to improve care for patients with uncontrolled diabetes in a safety net clinic.
- Methods: One of 3 clinical teams was designated the intervention team. Changes implemented by the intervention team included patient referral to a dietician and/or clinical pharmacist, provision of patient education, and assignment of a case manager. We compared outcomes of patients in the intervention group (n = 71), vs those receiving care from the other 2 teams (usual care) (n = 188).
- Results: HbA1c significantly decreased over time for patients in the intervention group as well as the usual care group. Within the intervention group, visits to clinical pharmacist (P = 0.034) and education (P = 0.004) predicted significantly greater decreases in HbA1c over time.
- Conclusions: Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Results support the need for further pragmatic research to weigh the impact of unblinded designs, outcome measurement, and real-world behaviors on evidence-based interventions.
Key words: diabetes; safety net; multidisciplinary diabetes care; primary care; diffusion of treatment.
The prevalence of type 2 diabetes in the United States is significantly higher among Hispanics and African Americans than in the general population (13% vs. 9.3%) [1]. Similarly, diabetes is highly prevalent among the uninsured, and many patients delay or forgo treatment due to cost [2]. Subsequently, the rates of comorbidities, including stroke, hypertension, and CVD, are elevated in these groups [3].
Association between elevated HbA1c and morbidity and mortality is well-documented, and an HbA1c reduction of just 1% has been shown to reduce mortality and improve quality of life [4]. Uncontrolled diabetes also results in increased medical costs. Reducing HbA1c from 9.0 to 7.5 reduces annual expenditures by as much as 73% [5].
Metropolitan Houston and Harris County, Texas, has one of the largest uninsured metro populations in the United States (over 3.6 million) [6]. Harris Health System serves this uninsured population and is the fourth largest safety net health system in the nation. Approximately 40,000 patients with diabetes receive care within the health system, and 34% of them have an HbA1c value greater than 9.
Developing novel, cost-efficient treatment and management models is crucial when providing care for patients with uncontrolled diabetes. However, the study of implementation strategies to successfully integrate evidence-based interventions in primary care using pragmatic approaches that aim to determine the effectiveness of interventions in “the real world” remain a challenge [7,8]. To address this issue, a quality improvement project was instituted at one of the system’s health centers to improve the care of patients with uncontrolled diabetes (known HbA1c above 9).
Methods
Setting
The pilot project was conducted from 1 Oct 2015 to 31 Dec 2015 in a primary care community health center within Harris Health System in Houston, Texas. This pilot was the first step of an institutional effort to introduce a multidisciplinary model of care across all clinics [9]. Our health center has 6 family medicine providers and 1 advanced practice nurse practitioner, organized into 3 pods with 2 physicians each. We randomly selected 1 pod (team) and designated it the intervention group.
The Standards for Quality Improvement Reporting Excellence guidelines [10] were followed and institutional review board approval was obtained.
Intervention
Practice changes introduced in the intervention team were assignment of a case manager to all patients, referral to a dietician and clinical pharmacist as needed, and patient education sessions. The team’s nurse assumed the role of case manager. The case manager was responsible for reviewing a patient checklist based on the America Diabetes Association guideline for comprehensive diabetes medical evaluation at initial and follow-up visits. Referrals were based on ADA guideline recommendations. Onsite brief patient education was provided to all patients. In addition, patients were enrolled in a “Diabetes 101” class, which follows an evidence-based curriculum that includes participation in at least 2 monthly sessions. Patients were asked to return to the clinic for a follow-up visit after 3 months in order to monitor medication compliance, re-evaluate their care plan, and measure HbA1c The usual care group patients were managed based on the current Standards of Medical Care in Diabetes [11]. The usual care group included patients from the same clinic under the care of providers in the teams that were not included in the multidisciplinary intervention.
Analysis
Data abstracted from de-identified patient records included HbA1c values, interventions received, and sociodemographic data. Generalized linear mixed modeling (GLMM) was used to examine changes in patient HbA1c levels over time [12]. All models included a random intercept to account for correlated observations within patient. All analyses were performed using Proc GLIMMIX in SAS v. 9.3 [13].
Results
A total 271 patients with HbA1c above 9 were included in the analysis: 71 in the intervention group and 188 in the usual care group. The intervention group was further differentiated by month of enrollment: October (n = 37), November (n = 27), and December (n = 9). Mean patient age in the overall sample was 51.6 years.
In the intervention group, most patients received patient education 56% (n = 40), almost half had a clinical pharmacy visit, but only 17% (n = 12) received a dietitian consultation. Overall, there was a 1.4% decrease in HbA1c in the intervention group, compared to a 1.3% HbA1c decrease in the usual care group.
GLMM was used to examine differences in HbA1c levels according to month of intervention enrollment (October vs. November vs. December) in the intervention group over time. Figure 1 shows predicted HbA1c values over time with trend lines fit for each of the three subgroups.
Preliminary analysis showed that potential contamination (diffusion of the treatment) would be likely to attenuate differences in the outcomes between the intervention and usual care conditions. Further analysis by subgroups were conducted to describe the intervention potential “spillover” to the usual care group participants not intended to receive the intervention. GLMM also examined differences in HbA1c levels between the intervention and usual care groups over time. The interaction between each treatment group and time was not statistically significant (F(2,268) = 1.34, P = 0.26), indicating that changes in HbA1c over time were not related to treatment group. A statistically reliable main effect for time (F(1,268) = 44.33, P < 0.001) indicated that in all groups, HbA1c values significantly decreased over time.
Follow-up analyses utilized GLMM to examine differences in HbA1c levels among patients in both groups who received at least one of the interventions (visiting a dietician, clinical pharmacist, education session, and clinical case manager). The interaction between intervention and time was not statistically significant for visiting the dietician, receiving education, or being assigned a case manager. The interaction between time and visiting a clinical pharmacist was statistically significant (F(1,204)= 7.78, P = 0.01) such that patients visiting the clinical pharmacist had lower HbA1c values over time relative to those that did not (Figure 2).
Discussion
HbA1c decreased significantly among intervention patients with uncontrolled diabetes over a 3-month period, regardless of which month they entered the study. However, there was no significant difference in HbA1c reduction between patients who received all 4 multidisciplinary interventions, one intervention, or those who received usual care. Patients in the intervention who attended clinical pharmacist visits had significantly greater HbA1c reduction than patients who did not, as did patients who attended a diabetes education session by a patient educator.
Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Diffusion refers to the unintended spread of a treatment effect when participants receive some or all treatments from an intervention to which they were not assigned, making outcomes descriptions of all study groups more challenging [14]. During the implementation period, other physicians and nurses in the clinic were aware of the multidisciplinary care model being piloted, and may have taken the initiative to connect their patients with clinical pharmacists, dieticians, certified diabetes educators, and clinical case managers. Pragmatic interventions are intended to maintain the internal validity of randomized control trials, yet they are meant to be implemented as close as possible to real-world settings in order to help patients, clinicians, and payers making informed health care decisions [8]. In this regard, participants in the control group could be exposed to the intervention through staff contact between the assigned groups implementing some of the intervention under study. In that case, the diffusion of treatment would be likely to attenuate differences in the outcomes between treatment and control groups [15].
This study has several limitations. We studied a small sample of patients that reflected the primary care population in one clinic in a safety net system with minority, underserved, and high-risk patients. Although attempts were made to keep the intervention limited to the intervention pod, diffusion of treatment might have impacted the internal validity of this intervention.
In summary, our results support the need for further systematic research work to weigh the impact of unblinded designs, simplified recruitment and outcome measurement, and real-world behaviors (such as noncompliance, cross over, and dropout) on evidence-based and multidisciplinary clinical interventions.
Acknowledgements: The authors would like to thank Krystal Gamarra, MSW, LCSW, and Hope Galvan, MS, RN, CVRN-BC, CDE for assistance with project implementation and data collection throughout this process.
Corresponding author: Maria C. Mejia de Grubb, MD, MPH, 3751 Kirby Dr, Suite 600, Houston, TX 77098, mcgrubb@bcm.edu.
Financial disclosures: None.
From the Department of Family and Community Medicine, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Zare, Ms Klawans, Dr. Moreno), Department of Family Medicine and Community Medicine, Baylor College of Medicine, Houston, TX (Dr. Mejia de Grubb, Dr. Juneja, Dr. Zoorob), Department of Psychiatry, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Suchting), andHarris Health System, Houston TX (Ms. Mathis).
Abstract
- Objective: To describe a pilot project to improve care for patients with uncontrolled diabetes in a safety net clinic.
- Methods: One of 3 clinical teams was designated the intervention team. Changes implemented by the intervention team included patient referral to a dietician and/or clinical pharmacist, provision of patient education, and assignment of a case manager. We compared outcomes of patients in the intervention group (n = 71), vs those receiving care from the other 2 teams (usual care) (n = 188).
- Results: HbA1c significantly decreased over time for patients in the intervention group as well as the usual care group. Within the intervention group, visits to clinical pharmacist (P = 0.034) and education (P = 0.004) predicted significantly greater decreases in HbA1c over time.
- Conclusions: Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Results support the need for further pragmatic research to weigh the impact of unblinded designs, outcome measurement, and real-world behaviors on evidence-based interventions.
Key words: diabetes; safety net; multidisciplinary diabetes care; primary care; diffusion of treatment.
The prevalence of type 2 diabetes in the United States is significantly higher among Hispanics and African Americans than in the general population (13% vs. 9.3%) [1]. Similarly, diabetes is highly prevalent among the uninsured, and many patients delay or forgo treatment due to cost [2]. Subsequently, the rates of comorbidities, including stroke, hypertension, and CVD, are elevated in these groups [3].
Association between elevated HbA1c and morbidity and mortality is well-documented, and an HbA1c reduction of just 1% has been shown to reduce mortality and improve quality of life [4]. Uncontrolled diabetes also results in increased medical costs. Reducing HbA1c from 9.0 to 7.5 reduces annual expenditures by as much as 73% [5].
Metropolitan Houston and Harris County, Texas, has one of the largest uninsured metro populations in the United States (over 3.6 million) [6]. Harris Health System serves this uninsured population and is the fourth largest safety net health system in the nation. Approximately 40,000 patients with diabetes receive care within the health system, and 34% of them have an HbA1c value greater than 9.
Developing novel, cost-efficient treatment and management models is crucial when providing care for patients with uncontrolled diabetes. However, the study of implementation strategies to successfully integrate evidence-based interventions in primary care using pragmatic approaches that aim to determine the effectiveness of interventions in “the real world” remain a challenge [7,8]. To address this issue, a quality improvement project was instituted at one of the system’s health centers to improve the care of patients with uncontrolled diabetes (known HbA1c above 9).
Methods
Setting
The pilot project was conducted from 1 Oct 2015 to 31 Dec 2015 in a primary care community health center within Harris Health System in Houston, Texas. This pilot was the first step of an institutional effort to introduce a multidisciplinary model of care across all clinics [9]. Our health center has 6 family medicine providers and 1 advanced practice nurse practitioner, organized into 3 pods with 2 physicians each. We randomly selected 1 pod (team) and designated it the intervention group.
The Standards for Quality Improvement Reporting Excellence guidelines [10] were followed and institutional review board approval was obtained.
Intervention
Practice changes introduced in the intervention team were assignment of a case manager to all patients, referral to a dietician and clinical pharmacist as needed, and patient education sessions. The team’s nurse assumed the role of case manager. The case manager was responsible for reviewing a patient checklist based on the America Diabetes Association guideline for comprehensive diabetes medical evaluation at initial and follow-up visits. Referrals were based on ADA guideline recommendations. Onsite brief patient education was provided to all patients. In addition, patients were enrolled in a “Diabetes 101” class, which follows an evidence-based curriculum that includes participation in at least 2 monthly sessions. Patients were asked to return to the clinic for a follow-up visit after 3 months in order to monitor medication compliance, re-evaluate their care plan, and measure HbA1c The usual care group patients were managed based on the current Standards of Medical Care in Diabetes [11]. The usual care group included patients from the same clinic under the care of providers in the teams that were not included in the multidisciplinary intervention.
Analysis
Data abstracted from de-identified patient records included HbA1c values, interventions received, and sociodemographic data. Generalized linear mixed modeling (GLMM) was used to examine changes in patient HbA1c levels over time [12]. All models included a random intercept to account for correlated observations within patient. All analyses were performed using Proc GLIMMIX in SAS v. 9.3 [13].
Results
A total 271 patients with HbA1c above 9 were included in the analysis: 71 in the intervention group and 188 in the usual care group. The intervention group was further differentiated by month of enrollment: October (n = 37), November (n = 27), and December (n = 9). Mean patient age in the overall sample was 51.6 years.
In the intervention group, most patients received patient education 56% (n = 40), almost half had a clinical pharmacy visit, but only 17% (n = 12) received a dietitian consultation. Overall, there was a 1.4% decrease in HbA1c in the intervention group, compared to a 1.3% HbA1c decrease in the usual care group.
GLMM was used to examine differences in HbA1c levels according to month of intervention enrollment (October vs. November vs. December) in the intervention group over time. Figure 1 shows predicted HbA1c values over time with trend lines fit for each of the three subgroups.
Preliminary analysis showed that potential contamination (diffusion of the treatment) would be likely to attenuate differences in the outcomes between the intervention and usual care conditions. Further analysis by subgroups were conducted to describe the intervention potential “spillover” to the usual care group participants not intended to receive the intervention. GLMM also examined differences in HbA1c levels between the intervention and usual care groups over time. The interaction between each treatment group and time was not statistically significant (F(2,268) = 1.34, P = 0.26), indicating that changes in HbA1c over time were not related to treatment group. A statistically reliable main effect for time (F(1,268) = 44.33, P < 0.001) indicated that in all groups, HbA1c values significantly decreased over time.
Follow-up analyses utilized GLMM to examine differences in HbA1c levels among patients in both groups who received at least one of the interventions (visiting a dietician, clinical pharmacist, education session, and clinical case manager). The interaction between intervention and time was not statistically significant for visiting the dietician, receiving education, or being assigned a case manager. The interaction between time and visiting a clinical pharmacist was statistically significant (F(1,204)= 7.78, P = 0.01) such that patients visiting the clinical pharmacist had lower HbA1c values over time relative to those that did not (Figure 2).
Discussion
HbA1c decreased significantly among intervention patients with uncontrolled diabetes over a 3-month period, regardless of which month they entered the study. However, there was no significant difference in HbA1c reduction between patients who received all 4 multidisciplinary interventions, one intervention, or those who received usual care. Patients in the intervention who attended clinical pharmacist visits had significantly greater HbA1c reduction than patients who did not, as did patients who attended a diabetes education session by a patient educator.
Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Diffusion refers to the unintended spread of a treatment effect when participants receive some or all treatments from an intervention to which they were not assigned, making outcomes descriptions of all study groups more challenging [14]. During the implementation period, other physicians and nurses in the clinic were aware of the multidisciplinary care model being piloted, and may have taken the initiative to connect their patients with clinical pharmacists, dieticians, certified diabetes educators, and clinical case managers. Pragmatic interventions are intended to maintain the internal validity of randomized control trials, yet they are meant to be implemented as close as possible to real-world settings in order to help patients, clinicians, and payers making informed health care decisions [8]. In this regard, participants in the control group could be exposed to the intervention through staff contact between the assigned groups implementing some of the intervention under study. In that case, the diffusion of treatment would be likely to attenuate differences in the outcomes between treatment and control groups [15].
This study has several limitations. We studied a small sample of patients that reflected the primary care population in one clinic in a safety net system with minority, underserved, and high-risk patients. Although attempts were made to keep the intervention limited to the intervention pod, diffusion of treatment might have impacted the internal validity of this intervention.
In summary, our results support the need for further systematic research work to weigh the impact of unblinded designs, simplified recruitment and outcome measurement, and real-world behaviors (such as noncompliance, cross over, and dropout) on evidence-based and multidisciplinary clinical interventions.
Acknowledgements: The authors would like to thank Krystal Gamarra, MSW, LCSW, and Hope Galvan, MS, RN, CVRN-BC, CDE for assistance with project implementation and data collection throughout this process.
Corresponding author: Maria C. Mejia de Grubb, MD, MPH, 3751 Kirby Dr, Suite 600, Houston, TX 77098, mcgrubb@bcm.edu.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
2. Casagrande SS, Cowie CC. Health insurance coverage among people with and without diabetes in the US adult population. Diabetes Care 2012;35:2243–9.
3. American Diabetes Association. Statistics about diabetes. Arlington, VA; 2017.
4. Eeg-Olofsson K, Eliasson B, Zethelius B, et al. HbA1c reduction and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish NDR. Diabetes 2012.
5. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016;33:1575–81.
6. Harris County Healthcare Alliance. The State of Health in Houston/Harris County 2015-2016. Accessed 17 Mar 2015 at http://houstonstateofhealth.org/soh_doc/.
7. Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials (London, England) 2012;9:436–46.
8. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009;301:831–41.
9. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Qty 1996:511–44.
10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Safety 2016;25:986–92.
11. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. American Diabetes Association. Clin Diabetes 2015;33:97–111.
12. Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. New York: Cambridge University Press; 2007.
13. SAS Institute I. Base SAS Procedures Guide: Statistical procedures. In: SAS Institute I, editor. Cary, NC; 2011.
14. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin; 2002.
15. Kane R. Understanding health care outcomes research. 2nd ed. In: Learning JB, editor. Burlington, MA: Jones and Bartlett; 2006: 44–6.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
2. Casagrande SS, Cowie CC. Health insurance coverage among people with and without diabetes in the US adult population. Diabetes Care 2012;35:2243–9.
3. American Diabetes Association. Statistics about diabetes. Arlington, VA; 2017.
4. Eeg-Olofsson K, Eliasson B, Zethelius B, et al. HbA1c reduction and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish NDR. Diabetes 2012.
5. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016;33:1575–81.
6. Harris County Healthcare Alliance. The State of Health in Houston/Harris County 2015-2016. Accessed 17 Mar 2015 at http://houstonstateofhealth.org/soh_doc/.
7. Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials (London, England) 2012;9:436–46.
8. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009;301:831–41.
9. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Qty 1996:511–44.
10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Safety 2016;25:986–92.
11. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. American Diabetes Association. Clin Diabetes 2015;33:97–111.
12. Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. New York: Cambridge University Press; 2007.
13. SAS Institute I. Base SAS Procedures Guide: Statistical procedures. In: SAS Institute I, editor. Cary, NC; 2011.
14. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin; 2002.
15. Kane R. Understanding health care outcomes research. 2nd ed. In: Learning JB, editor. Burlington, MA: Jones and Bartlett; 2006: 44–6.