User login
Diagnosing coronary heart disease: When to use stress imaging studies
- Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting electrocardiogram (ECG) and without contraindications to exercise.
- Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test.
- Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to target level are candidates for nuclear or echocardiographic pharmacologic stress testing.
- Patients with suspected coronary heart disease and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Most men and women experience symptoms before myocardial infarction (MI).1 Early recognition of these symptoms and prompt treatment are essential for prevention of death and disability related to coronary heart disease (CHD).
Patients with multiple risk factors, chest pain typically suggestive of CHD, or a history of CHD are usually easy to identify and triage. However, many patients do not have obvious risks for CHD but experience occasional symptoms of cardiac ischemia.
Patients can be stratified into low-, intermediate-, and high-risk categories that will help determine appropriate work-up. Those at intermediate risk can be difficult to assess, and may particularly benefit from stress-imaging studies.
The algorithm (Figure 1) is based on current guidelines,2 and indicates how patients with chest pain/symptoms may be identified and treated according to an initial estimate of the probability of obstructive coronary artery disease. The choice of noninvasive diagnostic tests for individuals with stable chest pain and a lower risk for CHD is then outlined.
Case studies
Patient 1
A 64-year-old, nonsmoking, obese woman with degenerative osteoarthritis of the knees occasionally experiences chest discomfort that lasts for a few minutes, sometimes radiating to her back. The discomfort, which started 4 weeks ago, occasionally becomes worse after a brief walk, but is not usually related to exertion, or associated with nausea or diaphoresis. She sometimes becomes short of breath climbing stairs.
Physical examination: In no acute distress; body-mass index 31.2, waist circumference 42 inches, heart rate 70 beats/min, blood pressure 142/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: resting electrocardiogram (ECG)—sinus rhythm otherwise normal; creatinine 1.2 mg/dL; fasting glucose 122 mg/dL; glycosylated hemoglobin 6.4%. Lipids: total cholesterol 232 mg/dL; triglycerides 230 mg/dL; high-density lipoprotein (HDL) cholesterol 28 mg/dL; low-density lipoprotein (LDL) cholesterol 158 mg/dL.
Patient 2
A 58-year-old, nonsmoking, otherwise healthy man experiences tightness in the chest, usually at night. The pain began 4 to 8 weeks ago; it lasts as long as 1 to 2 hours but is “very mild.” It does not radiate to the arm or jaw and is unrelated to exertion. There is no diaphoresis or nausea. He sometimes feels a bit winded, which “might be due to anxiety.”
Physical examination: slightly overweight man in no acute distress; body-mass index 27.0, waist circumference 36 inches, heart rate 74 beats/min, blood pressure 138/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: ECG— sinus rhythm, otherwise normal; creatinine 1.0 mg/dL; fasting glucose 98 mg/dL. Lipids: total cholesterol 215 mg/dL; triglycerides 150 mg/dL; HDL 40 mg/dL; LDL 145 mg/dL.
Diagnostic approaches
Standard diagnostic techniques include history, physical examination, laboratory testing as indicated, resting ECG, and assessment of risk factors for CHD.
Evaluation of chest pain
A careful history and physical examination can often quickly exclude many noncardiac causes of chest discomfort or pain. Table 1 contrasts the characteristics of atypical (noncardiac) symptoms with those of typical (cardiac) symptoms. Its quality, location, and the factors that relieve or provoke it, duration, and any associated symptoms should be evaluated. If high-risk or unstable signs or symptoms are present that suggest acute coronary syndrome (unstable angina or MI), evaluation in the emergency department should be performed.
Patients exhibiting stable or atypical (noncardiac) symptoms with some, but not all, of the features of angina described above have a lower probability of coronary artery disease, and should be considered for diagnostic evaluation under the guidance of the primary care physician.3
TABLE 1
Characteristics of atypical (noncardiac) vs typical cardiac symptoms
| Characteristic | Atypical/noncardiac | Typical/cardiac |
|---|---|---|
| Quality | Sharp, stabbing, positional | Squeezing, ache, pressure, fullness, burning, heavy, suffocating, “discomfort” |
| Location | Highly localized, below the epigastrium, above the mandible | Diffuse area—substernal, chest, jaw, back, arms |
| Provoked by | “Nothing,” body movement, cough, deep inspiration, chest palpation | Exertion, emotional stress, cold air |
| Relieved by | “Nothing,” position change, analgesics, heat, antacids | Rest; nitroglycerin |
| Duration | “Seconds” (fleeting), or hours, days | 30 seconds to 5 minutes |
| Associated symptoms | Reflux/heartburn | Dyspnea, diaphoresis, nausea, fatigue |
Evaluating women
In women aged <55 years, noncardiac chest pain is common, but since the prevalence of CHD is increasing among younger women, their symptoms should not be dismissed as “noncardiac” without full evaluation.
Women are also more likely than men to report dyspnea or pain in the jaw or back instead of, or in addition to, chest symptoms. Further, since women are often older and less active when they develop CHD, they may not exhibit typical exertional symptoms. Diagnosis in women is also hampered by lower accuracy of standard stress ECG testing compared with men. False-positive and false-negative tests may occur more frequently in women due to hormonal effects on the ECG, and more frequent comorbidities that limit maximal exercise capacity.
Physical examination
Palpation and auscultation of the chest may detect the presence of a friction rub or significant murmur, and thus identify a nonischemic cause for the chest symptoms. Carotid bruits or reduced pedal pulses indicate the presence of other vascular diseases. Patients with xanthomas, hypertension, or signs of congestive heart failure are more likely to have CHD, while those whose pain can be reproduced by body movement or by palpating the chest are less likely to have CHD.
Risk factor assessment. The assessment of risk factors for CHD allows the identification of many patients at high risk for CHD and can be helpful in guiding the choice of additional tests. As evident in the Framingham Heart Study,4 independent risk factors such as cigarette smoking, hypertension, diabetes mellitus, and hyperlipidemia are direct causes of CHD.
Laboratory tests
In the patient at low risk of CHD, blood testing for cardiac markers is not indicated. A lipid profile and blood glucose level help to establish the risk level associated with hyperlipidemia and diabetes. A complete blood count (eg, for anemia), thyroid hormone studies (eg, for hyperthyroidism), arterial blood gases (eg, PCO2 for chronic obstructive pulmonary disease), and other tests may help in diagnosing contributory conditions.
Resting electrocardiogram
A routine resting 12-lead ECG is an inexpensive but critical test that can provide important diagnostic and prognostic information. Evidence of infarction, ischemia, hypertrophy arrhythmias, and conduction disturbances can be detected and, if present, substantially increase the likelihood of a cardiac cause of symptoms.
Even the presence of mild or nonspecific ST-T wave changes, while not diagnostic, can aid the clinician by suggesting a higher probability of a nondiagnostic stress ECG and the need for an imaging stress test.3 An abnormal resting ECG with ST-T wave changes associated with digoxin use, left bundle branch block, left ventricular hypertrophy, and so on, limit interpretation of an exercise ECG, and points to a need for exercise testing with imaging.
It is important to note that a normal ECG obtained when the patient is asymptomatic does not exclude CHD, and additional risk stratification with noninvasive diagnostic stress testing may be indicated.5
Chest x-ray
A chest x-ray is often appropriate for patients with cardiac or pulmonary signs/symptoms. It may show cardiac enlargement, ventricular aneurysm, or evidence of heart failure, which may support the diagnosis of CHD and help to assess the extent of cardiopulmonary involvement.
Noninvasive stress testing
Considering our 2 patients with occasional episodes of unexplained chest discomfort: Based on the ECG and clinical findings, their risk for CHD is considered low to intermediate.
Test selection
Diagnostic tests should be selected based on the clinician’s estimate of probability of CHD.2
Low probability. If the likelihood of CHD is low, stress testing is generally not indicated, as its specificity is extremely low, and test results do not improve diagnostic accuracy over the clinical impression alone.
Intermediate probability. If the patient is able to exercise to capacity, the choice is exercise testing. Patients who can exercise and have an interpretable ECG, with no evidence of left ventricular dysfunction and no prior revascularization procedure, should usually undergo standard stress ECG testing. If the ECG is not interpretable, (due to repolarization abnormalities, left bundle branch block, left ventricular hypertrophy, digoxin use, etc) an exercise test with imaging (nuclear or echocardiographic) is indicated.
For patients unable to exercise, pharmacologic stress testing with imaging is indicated.
High probability. If the probability of CHD is high, it is reasonable to proceed directly to coronary angiography.
Exercise stress test
Exercise testing is a cardiovascular stress that uses treadmill or bicycle exercise with ECG and blood pressure monitoring. Such testing is widely available and relatively inexpensive.2 It allows assessment of exercise capacity and correlation of symptoms with ECG changes typical of myocardial ischemia.
Exercise testing provides the highest level of incremental diagnostic and prognostic information for patients with an intermediate probability of CHD.2 An important objective of stress testing is to identify individuals with a high risk for severe (left main or 3-vessel) CHD. More invasive procedures, such as percutaneous cutaneous angioplasty (PCTA), are recommended for these high-risk individuals to improve their survival.
Candidates for exercise treadmill testing include patients with stable symptoms who can be expected to exercise to an adequate workload. Patients with repolarization abnormalities on the resting ECG, such as left bundle branch block, left ventricular hypertrophy, or digoxin use, frequently have noninterpretable stress ECGs and may benefit from imaging techniques.
Limitations. Some patients referred for exercise treadmill testing are unable to achieve either adequate exercise levels or the target heart rate due to comorbid conditions,6 such as degenerative joint disease, obesity, pulmonary disease, peripheral vascular disease, central nervous system disorders, physical deconditioning, chronotropic incompetence, and medications such as beta blockers. More subtle factors, such as an unwillingness to exercise, may also affect a patient’s suitability for stress testing. These patients should be considered for pharmacologic stress testing.
Additionally, stress-induced ST-T wave changes do not accurately localize the site of myocardial ischemia and provide no direct information on left ventricular function and other clinically important variables. The sensitivity and specificity of exercise ECG testing ranges from approximately 67% to 72%, which is below that of stress imaging techniques, whose average sensitivity ranges from 80% to 85%.7-9
Stress imaging modalities
For a patient with an abnormal resting ECG, evidence of left ventricular dysfunction, or a prior coronary revascularization, stress imaging with either echocardiography or nuclear perfusion scanning is appropriate. Both techniques show higher specificity than the stress ECG alone.
Nuclear imaging. Nuclear imaging uses radiotracers (thallium-201, technetium-99m tetrofosmin [Myoview], or technetium-99m sestamibi [Cardiolite]) to evaluate myocardial perfusion and function, and has greatly advanced the ability to detect and assess the extent of CHD. Stress myocardial perfusion imaging has a sensitivity of >90% for detecting patients at risk of cardiac death or MI.6
To detect ischemia or infarction, a radioisotope is injected at rest and after stress to produce images of myocardial regional uptake, which is proportional to regional blood flow. Normally, with maximal exercise or pharmacologic stress, myocardial blood flow is greatly increased above the resting condition. If a fixed coronary stenosis is present, myocardial perfusion in the territory supplied by the stenosis cannot be increased, which will create a flow differential and uneven distribution of the tracer.
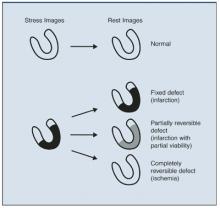
As illustrated in Figure 2, a normal myocardial perfusion image shows homogenous accumulation of radiotracer on both the stress and rest images. A perfusion defect appears as an area of reduced tracer uptake.
Nuclear perfusion studies can also provide a measure of left ventricular function and wall motion utilizing a bolus injection of radiotracer. While images can be obtained in most patients utilizing current techniques, artifacts due to breast and diaphragmatic tissue attenuation can lead to false-positive interpretation, particularly when examining women and when using thallium.
Echocardiography. Echocardiography visualizes the heart directly in real time using ultrasound, providing convenient assessment of the cardiac chambers, myocardium, valves, pericardium, and great vessels. The test can also identify mechanical complications of acute myocardial infarction, differentiate causes of reduced cardiac output and blood pressure, and help guide therapy. Stress echocardiography (exercise or pharmacologic stress) can be used to detect the presence, location, and severity of inducible myocardial ischemia as well as for risk stratification and prognosis.
During stress-induced ischemia, decrements in contractile function are directly related to decreases in regional subendocardial blood flow. Wall-motion changes precede ischemic ECG changes, accounting for the increased sensitivity of echocardiography versus ECG stress testing.
Interpretation of stress echocardiograms is based on analysis of segmental wall motion before and soon after stress. Normally, with exercise, or dobutamine infusion, left ventricular wall motion becomes hyperdynamic. The hallmark of ischemia is the development with stress of new, or the worsening of preexisting, wall motion abnormalities. The lack of improvement with stress in an already hypokinetic segment indicates infarction. Stress-induced left ventricular cavity enlargement, systolic dysfunction, or mitral regurgitation may also suggest CHD. Accuracy of stress echocardiography is similar to that of nuclear stress testing.
Considerable expertise in echocardiography is needed to rapidly acquire diagnostic images, so that its selection is limited by the skill of the technician. Image quality can be compromised by obesity and other factors, but the widespread use of intravenous contrast agents has significantly reduced the proportion of patients with uninterpretable images.
FIGURE 2
Accumulation of radiotracer in nuclear imaging (stress and rest images)
Patients who are not expected to achieve an adequate exercise capacity (as in our patient with osteoarthritis) should undergo pharmacologic stress testing with adenosine, dipyridamole, or dobutamine. Atrial pacing utilizing a swallowed esophageal electrode is also used in some cases. These agents, combined with echocardiographic or nuclear imaging, are particularly useful in patients who are unable to exercise adequately.
Pharmacologic stress agents are sometimes combined with low-level exercise protocols which may reduce the noncardiac side effects and improve image quality.10
Adenosine is the pharmacologic agent used most commonly in nuclear perfusion stress testing. An intravenous infusion of adenosine produces coronary vasodilation which is quickly attenuated when the infusion is terminated. Side effects, which are short-lived, include flushing, palpitations, and chest pain.
Dipyridamole is used less commonly due to its prolonged side effects and reports of lower specificity.11 Dobutamine, a beta-adrenergic agonist, increases heart rate and contractility in a dose-related fashion when infused intravenously. This agent is most commonly used in echocardiographic imaging. It can also be utilized with nuclear imaging when adenosine is contraindicated due to severe pulmonary or cerebrovascular disease. Side effects include transient arrhythmias, hypertension or hypotension, tremor, and chest pain.
Referral to a cardiologist
Referral to a cardiologist should be considered when the suspicion for cardiac disease is high, there is substantial diagnostic uncertainty after initial evaluation, or if symptoms persist, despite treatment of a noncardiac cause. Further evaluation and treatment often includes coronary angiography.
Coronary angiography
Most outpatients, such as the 2 presented, can be diagnosed with clinical and noninvasive measures. Coronary angiography is most commonly used to determine the presence and extent of obstructive CHD, and to guide decisions about revascularization in high-risk patients, or in patients with an abnormal stress test.
Cardiac catheterization presents a small but real risk to the patient, involves discomfort and substantial cost, and can challenge effective resource utilization. Risks and benefits to individual patients should be discussed between primary care physician and cardiologist.
Summary
Findings in our 2 patients are summarized below. Diagnostic decisions reflect the algorithm in Figure 1and are based on current guidelines.2
Patient 1. After initial assessment, our 64-year-old asymptomatic woman still falls into “intermediate probability of CHD” due to her multiple CHD risks. Stress testing was therefore indicated. Due to her inability to exercise because of an orthopedic limitation, she underwent pharmacologic stress testing with an adenosine sestamibi study. A small inferior reversible defect was identified, suggestive of myocardial ischemia.
Aggressive medical therapy aimed at minimizing symptoms and reducing risk was selected: aspirin, a beta-blocker for ischemia and hypertension, and a statin for hyperlipidemia. Longacting nitrates or calcium-channel blockers would have been reasonable alternatives. Consideration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is also indicated in light of her new CHD diagnosis and glucose intolerance. She was advised to initiate a low-fat, low-carbohydrate diet and to exercise (swim) regularly to lower risk. She will be seen in 6 weeks to reevaluate her symptoms, blood pressure, and lipid and glycemic control.
Patient 2. Our male patient also warranted stress testing. He was referred for a standard stress ECG due to his normal resting ECG, and the expectation that he would be able to exercise adequately. He satisfactorily completed 10.5 minutes (10 METS) of a Bruce protocol on a treadmill exercise stress test, which was entirely normal.
This admittedly anxious individual was reassured that his chest symptoms are not due to heart disease. An empiric trial with a proton pump inhibitor could be initiated if gastro-esophageal reflux is suspected.
Conclusions
Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting ECG and without contraindications to exercise, as in our male patient. Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test. Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to the target level are candidates for nuclear or echocardiographic pharmacologic stress testing, as was indicated for our female patient. Patients with suspected CHD and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Finally, when selecting a specific stress imaging technique, physicians should consider the local expertise with the various techniques available, together with their strengths and limitations in the individual patient.19
1. Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction. Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 2003;107:1681-1691.
2. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883-1892.
3. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092-2197.
4. Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the American Heart Association Task Force on Risk Reduction. Circulation 1998;97:1876-1887.
5. Zanger DR, Solomon AJ, Gersh BJ. Contemporary management of angina: part I: risk assessment. Am Fam Physician 1999;60:2543-2552.
6. Bar Harbor Invitation Meeting 2000. Panel 9: Nuclear imaging in coronary artery disease. J Nucl Cardiol 2001;8:305-316.
7. Kotler TS, Diamond GA. Exercise thallium-201 scintigraphy in the diagnosis and prognosis of coronary artery disease. Ann Intern Med 1990;113:684-702.
8. Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation 1991;84 (3 Suppl):I93-I99.
9. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc 1995;70:5-15.
10. Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-446.
11. Li T, Ahlberg A, Hachamovitch R, et al. Comparison of adenosine and dipyridamole in detecting coronary artery disease using Tc-99m sestamibi single-photon emission computed tomography imaging: a randomized, prospective clinical study. J Am Coll Cardiol 2003;41(suppl A):461A. Abstract 858-3.
12. American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, Tex: American Heart Association; 2003.
This special section of The Journal of Family Practiceis provided by an unrestricted grant fromFujisawa Healthcare, Inc. Disclosures: Dr McBride has served on the speakers’ bureau for the following: Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Glaxo SmithKline, KOS Pharmaceuticals, Merck & Co Inc, Pfizer Inc, Reliant Pharmaceuticals, and Sankyo. He has served as consultant to KOS Pharmaceuticals, Merck & Co Inc, and Pfizer Inc. He has received grant/research support from Merck & Co Inc and Pfizer Inc. Dr Hayes had no commercial interests to report. Corresponding author: Sharonne N. Hayes, MD, Mayo Clinic Women’s Heart Clinic, Rochester MN, 55905. E-mail: hayes.sharonne@mayo.edu.
- Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting electrocardiogram (ECG) and without contraindications to exercise.
- Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test.
- Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to target level are candidates for nuclear or echocardiographic pharmacologic stress testing.
- Patients with suspected coronary heart disease and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Most men and women experience symptoms before myocardial infarction (MI).1 Early recognition of these symptoms and prompt treatment are essential for prevention of death and disability related to coronary heart disease (CHD).
Patients with multiple risk factors, chest pain typically suggestive of CHD, or a history of CHD are usually easy to identify and triage. However, many patients do not have obvious risks for CHD but experience occasional symptoms of cardiac ischemia.
Patients can be stratified into low-, intermediate-, and high-risk categories that will help determine appropriate work-up. Those at intermediate risk can be difficult to assess, and may particularly benefit from stress-imaging studies.
The algorithm (Figure 1) is based on current guidelines,2 and indicates how patients with chest pain/symptoms may be identified and treated according to an initial estimate of the probability of obstructive coronary artery disease. The choice of noninvasive diagnostic tests for individuals with stable chest pain and a lower risk for CHD is then outlined.
Case studies
Patient 1
A 64-year-old, nonsmoking, obese woman with degenerative osteoarthritis of the knees occasionally experiences chest discomfort that lasts for a few minutes, sometimes radiating to her back. The discomfort, which started 4 weeks ago, occasionally becomes worse after a brief walk, but is not usually related to exertion, or associated with nausea or diaphoresis. She sometimes becomes short of breath climbing stairs.
Physical examination: In no acute distress; body-mass index 31.2, waist circumference 42 inches, heart rate 70 beats/min, blood pressure 142/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: resting electrocardiogram (ECG)—sinus rhythm otherwise normal; creatinine 1.2 mg/dL; fasting glucose 122 mg/dL; glycosylated hemoglobin 6.4%. Lipids: total cholesterol 232 mg/dL; triglycerides 230 mg/dL; high-density lipoprotein (HDL) cholesterol 28 mg/dL; low-density lipoprotein (LDL) cholesterol 158 mg/dL.
Patient 2
A 58-year-old, nonsmoking, otherwise healthy man experiences tightness in the chest, usually at night. The pain began 4 to 8 weeks ago; it lasts as long as 1 to 2 hours but is “very mild.” It does not radiate to the arm or jaw and is unrelated to exertion. There is no diaphoresis or nausea. He sometimes feels a bit winded, which “might be due to anxiety.”
Physical examination: slightly overweight man in no acute distress; body-mass index 27.0, waist circumference 36 inches, heart rate 74 beats/min, blood pressure 138/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: ECG— sinus rhythm, otherwise normal; creatinine 1.0 mg/dL; fasting glucose 98 mg/dL. Lipids: total cholesterol 215 mg/dL; triglycerides 150 mg/dL; HDL 40 mg/dL; LDL 145 mg/dL.
Diagnostic approaches
Standard diagnostic techniques include history, physical examination, laboratory testing as indicated, resting ECG, and assessment of risk factors for CHD.
Evaluation of chest pain
A careful history and physical examination can often quickly exclude many noncardiac causes of chest discomfort or pain. Table 1 contrasts the characteristics of atypical (noncardiac) symptoms with those of typical (cardiac) symptoms. Its quality, location, and the factors that relieve or provoke it, duration, and any associated symptoms should be evaluated. If high-risk or unstable signs or symptoms are present that suggest acute coronary syndrome (unstable angina or MI), evaluation in the emergency department should be performed.
Patients exhibiting stable or atypical (noncardiac) symptoms with some, but not all, of the features of angina described above have a lower probability of coronary artery disease, and should be considered for diagnostic evaluation under the guidance of the primary care physician.3
TABLE 1
Characteristics of atypical (noncardiac) vs typical cardiac symptoms
| Characteristic | Atypical/noncardiac | Typical/cardiac |
|---|---|---|
| Quality | Sharp, stabbing, positional | Squeezing, ache, pressure, fullness, burning, heavy, suffocating, “discomfort” |
| Location | Highly localized, below the epigastrium, above the mandible | Diffuse area—substernal, chest, jaw, back, arms |
| Provoked by | “Nothing,” body movement, cough, deep inspiration, chest palpation | Exertion, emotional stress, cold air |
| Relieved by | “Nothing,” position change, analgesics, heat, antacids | Rest; nitroglycerin |
| Duration | “Seconds” (fleeting), or hours, days | 30 seconds to 5 minutes |
| Associated symptoms | Reflux/heartburn | Dyspnea, diaphoresis, nausea, fatigue |
Evaluating women
In women aged <55 years, noncardiac chest pain is common, but since the prevalence of CHD is increasing among younger women, their symptoms should not be dismissed as “noncardiac” without full evaluation.
Women are also more likely than men to report dyspnea or pain in the jaw or back instead of, or in addition to, chest symptoms. Further, since women are often older and less active when they develop CHD, they may not exhibit typical exertional symptoms. Diagnosis in women is also hampered by lower accuracy of standard stress ECG testing compared with men. False-positive and false-negative tests may occur more frequently in women due to hormonal effects on the ECG, and more frequent comorbidities that limit maximal exercise capacity.
Physical examination
Palpation and auscultation of the chest may detect the presence of a friction rub or significant murmur, and thus identify a nonischemic cause for the chest symptoms. Carotid bruits or reduced pedal pulses indicate the presence of other vascular diseases. Patients with xanthomas, hypertension, or signs of congestive heart failure are more likely to have CHD, while those whose pain can be reproduced by body movement or by palpating the chest are less likely to have CHD.
Risk factor assessment. The assessment of risk factors for CHD allows the identification of many patients at high risk for CHD and can be helpful in guiding the choice of additional tests. As evident in the Framingham Heart Study,4 independent risk factors such as cigarette smoking, hypertension, diabetes mellitus, and hyperlipidemia are direct causes of CHD.
Laboratory tests
In the patient at low risk of CHD, blood testing for cardiac markers is not indicated. A lipid profile and blood glucose level help to establish the risk level associated with hyperlipidemia and diabetes. A complete blood count (eg, for anemia), thyroid hormone studies (eg, for hyperthyroidism), arterial blood gases (eg, PCO2 for chronic obstructive pulmonary disease), and other tests may help in diagnosing contributory conditions.
Resting electrocardiogram
A routine resting 12-lead ECG is an inexpensive but critical test that can provide important diagnostic and prognostic information. Evidence of infarction, ischemia, hypertrophy arrhythmias, and conduction disturbances can be detected and, if present, substantially increase the likelihood of a cardiac cause of symptoms.
Even the presence of mild or nonspecific ST-T wave changes, while not diagnostic, can aid the clinician by suggesting a higher probability of a nondiagnostic stress ECG and the need for an imaging stress test.3 An abnormal resting ECG with ST-T wave changes associated with digoxin use, left bundle branch block, left ventricular hypertrophy, and so on, limit interpretation of an exercise ECG, and points to a need for exercise testing with imaging.
It is important to note that a normal ECG obtained when the patient is asymptomatic does not exclude CHD, and additional risk stratification with noninvasive diagnostic stress testing may be indicated.5
Chest x-ray
A chest x-ray is often appropriate for patients with cardiac or pulmonary signs/symptoms. It may show cardiac enlargement, ventricular aneurysm, or evidence of heart failure, which may support the diagnosis of CHD and help to assess the extent of cardiopulmonary involvement.
Noninvasive stress testing
Considering our 2 patients with occasional episodes of unexplained chest discomfort: Based on the ECG and clinical findings, their risk for CHD is considered low to intermediate.
Test selection
Diagnostic tests should be selected based on the clinician’s estimate of probability of CHD.2
Low probability. If the likelihood of CHD is low, stress testing is generally not indicated, as its specificity is extremely low, and test results do not improve diagnostic accuracy over the clinical impression alone.
Intermediate probability. If the patient is able to exercise to capacity, the choice is exercise testing. Patients who can exercise and have an interpretable ECG, with no evidence of left ventricular dysfunction and no prior revascularization procedure, should usually undergo standard stress ECG testing. If the ECG is not interpretable, (due to repolarization abnormalities, left bundle branch block, left ventricular hypertrophy, digoxin use, etc) an exercise test with imaging (nuclear or echocardiographic) is indicated.
For patients unable to exercise, pharmacologic stress testing with imaging is indicated.
High probability. If the probability of CHD is high, it is reasonable to proceed directly to coronary angiography.
Exercise stress test
Exercise testing is a cardiovascular stress that uses treadmill or bicycle exercise with ECG and blood pressure monitoring. Such testing is widely available and relatively inexpensive.2 It allows assessment of exercise capacity and correlation of symptoms with ECG changes typical of myocardial ischemia.
Exercise testing provides the highest level of incremental diagnostic and prognostic information for patients with an intermediate probability of CHD.2 An important objective of stress testing is to identify individuals with a high risk for severe (left main or 3-vessel) CHD. More invasive procedures, such as percutaneous cutaneous angioplasty (PCTA), are recommended for these high-risk individuals to improve their survival.
Candidates for exercise treadmill testing include patients with stable symptoms who can be expected to exercise to an adequate workload. Patients with repolarization abnormalities on the resting ECG, such as left bundle branch block, left ventricular hypertrophy, or digoxin use, frequently have noninterpretable stress ECGs and may benefit from imaging techniques.
Limitations. Some patients referred for exercise treadmill testing are unable to achieve either adequate exercise levels or the target heart rate due to comorbid conditions,6 such as degenerative joint disease, obesity, pulmonary disease, peripheral vascular disease, central nervous system disorders, physical deconditioning, chronotropic incompetence, and medications such as beta blockers. More subtle factors, such as an unwillingness to exercise, may also affect a patient’s suitability for stress testing. These patients should be considered for pharmacologic stress testing.
Additionally, stress-induced ST-T wave changes do not accurately localize the site of myocardial ischemia and provide no direct information on left ventricular function and other clinically important variables. The sensitivity and specificity of exercise ECG testing ranges from approximately 67% to 72%, which is below that of stress imaging techniques, whose average sensitivity ranges from 80% to 85%.7-9
Stress imaging modalities
For a patient with an abnormal resting ECG, evidence of left ventricular dysfunction, or a prior coronary revascularization, stress imaging with either echocardiography or nuclear perfusion scanning is appropriate. Both techniques show higher specificity than the stress ECG alone.
Nuclear imaging. Nuclear imaging uses radiotracers (thallium-201, technetium-99m tetrofosmin [Myoview], or technetium-99m sestamibi [Cardiolite]) to evaluate myocardial perfusion and function, and has greatly advanced the ability to detect and assess the extent of CHD. Stress myocardial perfusion imaging has a sensitivity of >90% for detecting patients at risk of cardiac death or MI.6
To detect ischemia or infarction, a radioisotope is injected at rest and after stress to produce images of myocardial regional uptake, which is proportional to regional blood flow. Normally, with maximal exercise or pharmacologic stress, myocardial blood flow is greatly increased above the resting condition. If a fixed coronary stenosis is present, myocardial perfusion in the territory supplied by the stenosis cannot be increased, which will create a flow differential and uneven distribution of the tracer.
As illustrated in Figure 2, a normal myocardial perfusion image shows homogenous accumulation of radiotracer on both the stress and rest images. A perfusion defect appears as an area of reduced tracer uptake.
Nuclear perfusion studies can also provide a measure of left ventricular function and wall motion utilizing a bolus injection of radiotracer. While images can be obtained in most patients utilizing current techniques, artifacts due to breast and diaphragmatic tissue attenuation can lead to false-positive interpretation, particularly when examining women and when using thallium.
Echocardiography. Echocardiography visualizes the heart directly in real time using ultrasound, providing convenient assessment of the cardiac chambers, myocardium, valves, pericardium, and great vessels. The test can also identify mechanical complications of acute myocardial infarction, differentiate causes of reduced cardiac output and blood pressure, and help guide therapy. Stress echocardiography (exercise or pharmacologic stress) can be used to detect the presence, location, and severity of inducible myocardial ischemia as well as for risk stratification and prognosis.
During stress-induced ischemia, decrements in contractile function are directly related to decreases in regional subendocardial blood flow. Wall-motion changes precede ischemic ECG changes, accounting for the increased sensitivity of echocardiography versus ECG stress testing.
Interpretation of stress echocardiograms is based on analysis of segmental wall motion before and soon after stress. Normally, with exercise, or dobutamine infusion, left ventricular wall motion becomes hyperdynamic. The hallmark of ischemia is the development with stress of new, or the worsening of preexisting, wall motion abnormalities. The lack of improvement with stress in an already hypokinetic segment indicates infarction. Stress-induced left ventricular cavity enlargement, systolic dysfunction, or mitral regurgitation may also suggest CHD. Accuracy of stress echocardiography is similar to that of nuclear stress testing.
Considerable expertise in echocardiography is needed to rapidly acquire diagnostic images, so that its selection is limited by the skill of the technician. Image quality can be compromised by obesity and other factors, but the widespread use of intravenous contrast agents has significantly reduced the proportion of patients with uninterpretable images.
FIGURE 2
Accumulation of radiotracer in nuclear imaging (stress and rest images)
Patients who are not expected to achieve an adequate exercise capacity (as in our patient with osteoarthritis) should undergo pharmacologic stress testing with adenosine, dipyridamole, or dobutamine. Atrial pacing utilizing a swallowed esophageal electrode is also used in some cases. These agents, combined with echocardiographic or nuclear imaging, are particularly useful in patients who are unable to exercise adequately.
Pharmacologic stress agents are sometimes combined with low-level exercise protocols which may reduce the noncardiac side effects and improve image quality.10
Adenosine is the pharmacologic agent used most commonly in nuclear perfusion stress testing. An intravenous infusion of adenosine produces coronary vasodilation which is quickly attenuated when the infusion is terminated. Side effects, which are short-lived, include flushing, palpitations, and chest pain.
Dipyridamole is used less commonly due to its prolonged side effects and reports of lower specificity.11 Dobutamine, a beta-adrenergic agonist, increases heart rate and contractility in a dose-related fashion when infused intravenously. This agent is most commonly used in echocardiographic imaging. It can also be utilized with nuclear imaging when adenosine is contraindicated due to severe pulmonary or cerebrovascular disease. Side effects include transient arrhythmias, hypertension or hypotension, tremor, and chest pain.
Referral to a cardiologist
Referral to a cardiologist should be considered when the suspicion for cardiac disease is high, there is substantial diagnostic uncertainty after initial evaluation, or if symptoms persist, despite treatment of a noncardiac cause. Further evaluation and treatment often includes coronary angiography.
Coronary angiography
Most outpatients, such as the 2 presented, can be diagnosed with clinical and noninvasive measures. Coronary angiography is most commonly used to determine the presence and extent of obstructive CHD, and to guide decisions about revascularization in high-risk patients, or in patients with an abnormal stress test.
Cardiac catheterization presents a small but real risk to the patient, involves discomfort and substantial cost, and can challenge effective resource utilization. Risks and benefits to individual patients should be discussed between primary care physician and cardiologist.
Summary
Findings in our 2 patients are summarized below. Diagnostic decisions reflect the algorithm in Figure 1and are based on current guidelines.2
Patient 1. After initial assessment, our 64-year-old asymptomatic woman still falls into “intermediate probability of CHD” due to her multiple CHD risks. Stress testing was therefore indicated. Due to her inability to exercise because of an orthopedic limitation, she underwent pharmacologic stress testing with an adenosine sestamibi study. A small inferior reversible defect was identified, suggestive of myocardial ischemia.
Aggressive medical therapy aimed at minimizing symptoms and reducing risk was selected: aspirin, a beta-blocker for ischemia and hypertension, and a statin for hyperlipidemia. Longacting nitrates or calcium-channel blockers would have been reasonable alternatives. Consideration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is also indicated in light of her new CHD diagnosis and glucose intolerance. She was advised to initiate a low-fat, low-carbohydrate diet and to exercise (swim) regularly to lower risk. She will be seen in 6 weeks to reevaluate her symptoms, blood pressure, and lipid and glycemic control.
Patient 2. Our male patient also warranted stress testing. He was referred for a standard stress ECG due to his normal resting ECG, and the expectation that he would be able to exercise adequately. He satisfactorily completed 10.5 minutes (10 METS) of a Bruce protocol on a treadmill exercise stress test, which was entirely normal.
This admittedly anxious individual was reassured that his chest symptoms are not due to heart disease. An empiric trial with a proton pump inhibitor could be initiated if gastro-esophageal reflux is suspected.
Conclusions
Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting ECG and without contraindications to exercise, as in our male patient. Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test. Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to the target level are candidates for nuclear or echocardiographic pharmacologic stress testing, as was indicated for our female patient. Patients with suspected CHD and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Finally, when selecting a specific stress imaging technique, physicians should consider the local expertise with the various techniques available, together with their strengths and limitations in the individual patient.19
- Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting electrocardiogram (ECG) and without contraindications to exercise.
- Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test.
- Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to target level are candidates for nuclear or echocardiographic pharmacologic stress testing.
- Patients with suspected coronary heart disease and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Most men and women experience symptoms before myocardial infarction (MI).1 Early recognition of these symptoms and prompt treatment are essential for prevention of death and disability related to coronary heart disease (CHD).
Patients with multiple risk factors, chest pain typically suggestive of CHD, or a history of CHD are usually easy to identify and triage. However, many patients do not have obvious risks for CHD but experience occasional symptoms of cardiac ischemia.
Patients can be stratified into low-, intermediate-, and high-risk categories that will help determine appropriate work-up. Those at intermediate risk can be difficult to assess, and may particularly benefit from stress-imaging studies.
The algorithm (Figure 1) is based on current guidelines,2 and indicates how patients with chest pain/symptoms may be identified and treated according to an initial estimate of the probability of obstructive coronary artery disease. The choice of noninvasive diagnostic tests for individuals with stable chest pain and a lower risk for CHD is then outlined.
Case studies
Patient 1
A 64-year-old, nonsmoking, obese woman with degenerative osteoarthritis of the knees occasionally experiences chest discomfort that lasts for a few minutes, sometimes radiating to her back. The discomfort, which started 4 weeks ago, occasionally becomes worse after a brief walk, but is not usually related to exertion, or associated with nausea or diaphoresis. She sometimes becomes short of breath climbing stairs.
Physical examination: In no acute distress; body-mass index 31.2, waist circumference 42 inches, heart rate 70 beats/min, blood pressure 142/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: resting electrocardiogram (ECG)—sinus rhythm otherwise normal; creatinine 1.2 mg/dL; fasting glucose 122 mg/dL; glycosylated hemoglobin 6.4%. Lipids: total cholesterol 232 mg/dL; triglycerides 230 mg/dL; high-density lipoprotein (HDL) cholesterol 28 mg/dL; low-density lipoprotein (LDL) cholesterol 158 mg/dL.
Patient 2
A 58-year-old, nonsmoking, otherwise healthy man experiences tightness in the chest, usually at night. The pain began 4 to 8 weeks ago; it lasts as long as 1 to 2 hours but is “very mild.” It does not radiate to the arm or jaw and is unrelated to exertion. There is no diaphoresis or nausea. He sometimes feels a bit winded, which “might be due to anxiety.”
Physical examination: slightly overweight man in no acute distress; body-mass index 27.0, waist circumference 36 inches, heart rate 74 beats/min, blood pressure 138/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: ECG— sinus rhythm, otherwise normal; creatinine 1.0 mg/dL; fasting glucose 98 mg/dL. Lipids: total cholesterol 215 mg/dL; triglycerides 150 mg/dL; HDL 40 mg/dL; LDL 145 mg/dL.
Diagnostic approaches
Standard diagnostic techniques include history, physical examination, laboratory testing as indicated, resting ECG, and assessment of risk factors for CHD.
Evaluation of chest pain
A careful history and physical examination can often quickly exclude many noncardiac causes of chest discomfort or pain. Table 1 contrasts the characteristics of atypical (noncardiac) symptoms with those of typical (cardiac) symptoms. Its quality, location, and the factors that relieve or provoke it, duration, and any associated symptoms should be evaluated. If high-risk or unstable signs or symptoms are present that suggest acute coronary syndrome (unstable angina or MI), evaluation in the emergency department should be performed.
Patients exhibiting stable or atypical (noncardiac) symptoms with some, but not all, of the features of angina described above have a lower probability of coronary artery disease, and should be considered for diagnostic evaluation under the guidance of the primary care physician.3
TABLE 1
Characteristics of atypical (noncardiac) vs typical cardiac symptoms
| Characteristic | Atypical/noncardiac | Typical/cardiac |
|---|---|---|
| Quality | Sharp, stabbing, positional | Squeezing, ache, pressure, fullness, burning, heavy, suffocating, “discomfort” |
| Location | Highly localized, below the epigastrium, above the mandible | Diffuse area—substernal, chest, jaw, back, arms |
| Provoked by | “Nothing,” body movement, cough, deep inspiration, chest palpation | Exertion, emotional stress, cold air |
| Relieved by | “Nothing,” position change, analgesics, heat, antacids | Rest; nitroglycerin |
| Duration | “Seconds” (fleeting), or hours, days | 30 seconds to 5 minutes |
| Associated symptoms | Reflux/heartburn | Dyspnea, diaphoresis, nausea, fatigue |
Evaluating women
In women aged <55 years, noncardiac chest pain is common, but since the prevalence of CHD is increasing among younger women, their symptoms should not be dismissed as “noncardiac” without full evaluation.
Women are also more likely than men to report dyspnea or pain in the jaw or back instead of, or in addition to, chest symptoms. Further, since women are often older and less active when they develop CHD, they may not exhibit typical exertional symptoms. Diagnosis in women is also hampered by lower accuracy of standard stress ECG testing compared with men. False-positive and false-negative tests may occur more frequently in women due to hormonal effects on the ECG, and more frequent comorbidities that limit maximal exercise capacity.
Physical examination
Palpation and auscultation of the chest may detect the presence of a friction rub or significant murmur, and thus identify a nonischemic cause for the chest symptoms. Carotid bruits or reduced pedal pulses indicate the presence of other vascular diseases. Patients with xanthomas, hypertension, or signs of congestive heart failure are more likely to have CHD, while those whose pain can be reproduced by body movement or by palpating the chest are less likely to have CHD.
Risk factor assessment. The assessment of risk factors for CHD allows the identification of many patients at high risk for CHD and can be helpful in guiding the choice of additional tests. As evident in the Framingham Heart Study,4 independent risk factors such as cigarette smoking, hypertension, diabetes mellitus, and hyperlipidemia are direct causes of CHD.
Laboratory tests
In the patient at low risk of CHD, blood testing for cardiac markers is not indicated. A lipid profile and blood glucose level help to establish the risk level associated with hyperlipidemia and diabetes. A complete blood count (eg, for anemia), thyroid hormone studies (eg, for hyperthyroidism), arterial blood gases (eg, PCO2 for chronic obstructive pulmonary disease), and other tests may help in diagnosing contributory conditions.
Resting electrocardiogram
A routine resting 12-lead ECG is an inexpensive but critical test that can provide important diagnostic and prognostic information. Evidence of infarction, ischemia, hypertrophy arrhythmias, and conduction disturbances can be detected and, if present, substantially increase the likelihood of a cardiac cause of symptoms.
Even the presence of mild or nonspecific ST-T wave changes, while not diagnostic, can aid the clinician by suggesting a higher probability of a nondiagnostic stress ECG and the need for an imaging stress test.3 An abnormal resting ECG with ST-T wave changes associated with digoxin use, left bundle branch block, left ventricular hypertrophy, and so on, limit interpretation of an exercise ECG, and points to a need for exercise testing with imaging.
It is important to note that a normal ECG obtained when the patient is asymptomatic does not exclude CHD, and additional risk stratification with noninvasive diagnostic stress testing may be indicated.5
Chest x-ray
A chest x-ray is often appropriate for patients with cardiac or pulmonary signs/symptoms. It may show cardiac enlargement, ventricular aneurysm, or evidence of heart failure, which may support the diagnosis of CHD and help to assess the extent of cardiopulmonary involvement.
Noninvasive stress testing
Considering our 2 patients with occasional episodes of unexplained chest discomfort: Based on the ECG and clinical findings, their risk for CHD is considered low to intermediate.
Test selection
Diagnostic tests should be selected based on the clinician’s estimate of probability of CHD.2
Low probability. If the likelihood of CHD is low, stress testing is generally not indicated, as its specificity is extremely low, and test results do not improve diagnostic accuracy over the clinical impression alone.
Intermediate probability. If the patient is able to exercise to capacity, the choice is exercise testing. Patients who can exercise and have an interpretable ECG, with no evidence of left ventricular dysfunction and no prior revascularization procedure, should usually undergo standard stress ECG testing. If the ECG is not interpretable, (due to repolarization abnormalities, left bundle branch block, left ventricular hypertrophy, digoxin use, etc) an exercise test with imaging (nuclear or echocardiographic) is indicated.
For patients unable to exercise, pharmacologic stress testing with imaging is indicated.
High probability. If the probability of CHD is high, it is reasonable to proceed directly to coronary angiography.
Exercise stress test
Exercise testing is a cardiovascular stress that uses treadmill or bicycle exercise with ECG and blood pressure monitoring. Such testing is widely available and relatively inexpensive.2 It allows assessment of exercise capacity and correlation of symptoms with ECG changes typical of myocardial ischemia.
Exercise testing provides the highest level of incremental diagnostic and prognostic information for patients with an intermediate probability of CHD.2 An important objective of stress testing is to identify individuals with a high risk for severe (left main or 3-vessel) CHD. More invasive procedures, such as percutaneous cutaneous angioplasty (PCTA), are recommended for these high-risk individuals to improve their survival.
Candidates for exercise treadmill testing include patients with stable symptoms who can be expected to exercise to an adequate workload. Patients with repolarization abnormalities on the resting ECG, such as left bundle branch block, left ventricular hypertrophy, or digoxin use, frequently have noninterpretable stress ECGs and may benefit from imaging techniques.
Limitations. Some patients referred for exercise treadmill testing are unable to achieve either adequate exercise levels or the target heart rate due to comorbid conditions,6 such as degenerative joint disease, obesity, pulmonary disease, peripheral vascular disease, central nervous system disorders, physical deconditioning, chronotropic incompetence, and medications such as beta blockers. More subtle factors, such as an unwillingness to exercise, may also affect a patient’s suitability for stress testing. These patients should be considered for pharmacologic stress testing.
Additionally, stress-induced ST-T wave changes do not accurately localize the site of myocardial ischemia and provide no direct information on left ventricular function and other clinically important variables. The sensitivity and specificity of exercise ECG testing ranges from approximately 67% to 72%, which is below that of stress imaging techniques, whose average sensitivity ranges from 80% to 85%.7-9
Stress imaging modalities
For a patient with an abnormal resting ECG, evidence of left ventricular dysfunction, or a prior coronary revascularization, stress imaging with either echocardiography or nuclear perfusion scanning is appropriate. Both techniques show higher specificity than the stress ECG alone.
Nuclear imaging. Nuclear imaging uses radiotracers (thallium-201, technetium-99m tetrofosmin [Myoview], or technetium-99m sestamibi [Cardiolite]) to evaluate myocardial perfusion and function, and has greatly advanced the ability to detect and assess the extent of CHD. Stress myocardial perfusion imaging has a sensitivity of >90% for detecting patients at risk of cardiac death or MI.6
To detect ischemia or infarction, a radioisotope is injected at rest and after stress to produce images of myocardial regional uptake, which is proportional to regional blood flow. Normally, with maximal exercise or pharmacologic stress, myocardial blood flow is greatly increased above the resting condition. If a fixed coronary stenosis is present, myocardial perfusion in the territory supplied by the stenosis cannot be increased, which will create a flow differential and uneven distribution of the tracer.
As illustrated in Figure 2, a normal myocardial perfusion image shows homogenous accumulation of radiotracer on both the stress and rest images. A perfusion defect appears as an area of reduced tracer uptake.
Nuclear perfusion studies can also provide a measure of left ventricular function and wall motion utilizing a bolus injection of radiotracer. While images can be obtained in most patients utilizing current techniques, artifacts due to breast and diaphragmatic tissue attenuation can lead to false-positive interpretation, particularly when examining women and when using thallium.
Echocardiography. Echocardiography visualizes the heart directly in real time using ultrasound, providing convenient assessment of the cardiac chambers, myocardium, valves, pericardium, and great vessels. The test can also identify mechanical complications of acute myocardial infarction, differentiate causes of reduced cardiac output and blood pressure, and help guide therapy. Stress echocardiography (exercise or pharmacologic stress) can be used to detect the presence, location, and severity of inducible myocardial ischemia as well as for risk stratification and prognosis.
During stress-induced ischemia, decrements in contractile function are directly related to decreases in regional subendocardial blood flow. Wall-motion changes precede ischemic ECG changes, accounting for the increased sensitivity of echocardiography versus ECG stress testing.
Interpretation of stress echocardiograms is based on analysis of segmental wall motion before and soon after stress. Normally, with exercise, or dobutamine infusion, left ventricular wall motion becomes hyperdynamic. The hallmark of ischemia is the development with stress of new, or the worsening of preexisting, wall motion abnormalities. The lack of improvement with stress in an already hypokinetic segment indicates infarction. Stress-induced left ventricular cavity enlargement, systolic dysfunction, or mitral regurgitation may also suggest CHD. Accuracy of stress echocardiography is similar to that of nuclear stress testing.
Considerable expertise in echocardiography is needed to rapidly acquire diagnostic images, so that its selection is limited by the skill of the technician. Image quality can be compromised by obesity and other factors, but the widespread use of intravenous contrast agents has significantly reduced the proportion of patients with uninterpretable images.
FIGURE 2
Accumulation of radiotracer in nuclear imaging (stress and rest images)
Patients who are not expected to achieve an adequate exercise capacity (as in our patient with osteoarthritis) should undergo pharmacologic stress testing with adenosine, dipyridamole, or dobutamine. Atrial pacing utilizing a swallowed esophageal electrode is also used in some cases. These agents, combined with echocardiographic or nuclear imaging, are particularly useful in patients who are unable to exercise adequately.
Pharmacologic stress agents are sometimes combined with low-level exercise protocols which may reduce the noncardiac side effects and improve image quality.10
Adenosine is the pharmacologic agent used most commonly in nuclear perfusion stress testing. An intravenous infusion of adenosine produces coronary vasodilation which is quickly attenuated when the infusion is terminated. Side effects, which are short-lived, include flushing, palpitations, and chest pain.
Dipyridamole is used less commonly due to its prolonged side effects and reports of lower specificity.11 Dobutamine, a beta-adrenergic agonist, increases heart rate and contractility in a dose-related fashion when infused intravenously. This agent is most commonly used in echocardiographic imaging. It can also be utilized with nuclear imaging when adenosine is contraindicated due to severe pulmonary or cerebrovascular disease. Side effects include transient arrhythmias, hypertension or hypotension, tremor, and chest pain.
Referral to a cardiologist
Referral to a cardiologist should be considered when the suspicion for cardiac disease is high, there is substantial diagnostic uncertainty after initial evaluation, or if symptoms persist, despite treatment of a noncardiac cause. Further evaluation and treatment often includes coronary angiography.
Coronary angiography
Most outpatients, such as the 2 presented, can be diagnosed with clinical and noninvasive measures. Coronary angiography is most commonly used to determine the presence and extent of obstructive CHD, and to guide decisions about revascularization in high-risk patients, or in patients with an abnormal stress test.
Cardiac catheterization presents a small but real risk to the patient, involves discomfort and substantial cost, and can challenge effective resource utilization. Risks and benefits to individual patients should be discussed between primary care physician and cardiologist.
Summary
Findings in our 2 patients are summarized below. Diagnostic decisions reflect the algorithm in Figure 1and are based on current guidelines.2
Patient 1. After initial assessment, our 64-year-old asymptomatic woman still falls into “intermediate probability of CHD” due to her multiple CHD risks. Stress testing was therefore indicated. Due to her inability to exercise because of an orthopedic limitation, she underwent pharmacologic stress testing with an adenosine sestamibi study. A small inferior reversible defect was identified, suggestive of myocardial ischemia.
Aggressive medical therapy aimed at minimizing symptoms and reducing risk was selected: aspirin, a beta-blocker for ischemia and hypertension, and a statin for hyperlipidemia. Longacting nitrates or calcium-channel blockers would have been reasonable alternatives. Consideration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is also indicated in light of her new CHD diagnosis and glucose intolerance. She was advised to initiate a low-fat, low-carbohydrate diet and to exercise (swim) regularly to lower risk. She will be seen in 6 weeks to reevaluate her symptoms, blood pressure, and lipid and glycemic control.
Patient 2. Our male patient also warranted stress testing. He was referred for a standard stress ECG due to his normal resting ECG, and the expectation that he would be able to exercise adequately. He satisfactorily completed 10.5 minutes (10 METS) of a Bruce protocol on a treadmill exercise stress test, which was entirely normal.
This admittedly anxious individual was reassured that his chest symptoms are not due to heart disease. An empiric trial with a proton pump inhibitor could be initiated if gastro-esophageal reflux is suspected.
Conclusions
Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting ECG and without contraindications to exercise, as in our male patient. Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test. Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to the target level are candidates for nuclear or echocardiographic pharmacologic stress testing, as was indicated for our female patient. Patients with suspected CHD and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Finally, when selecting a specific stress imaging technique, physicians should consider the local expertise with the various techniques available, together with their strengths and limitations in the individual patient.19
1. Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction. Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 2003;107:1681-1691.
2. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883-1892.
3. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092-2197.
4. Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the American Heart Association Task Force on Risk Reduction. Circulation 1998;97:1876-1887.
5. Zanger DR, Solomon AJ, Gersh BJ. Contemporary management of angina: part I: risk assessment. Am Fam Physician 1999;60:2543-2552.
6. Bar Harbor Invitation Meeting 2000. Panel 9: Nuclear imaging in coronary artery disease. J Nucl Cardiol 2001;8:305-316.
7. Kotler TS, Diamond GA. Exercise thallium-201 scintigraphy in the diagnosis and prognosis of coronary artery disease. Ann Intern Med 1990;113:684-702.
8. Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation 1991;84 (3 Suppl):I93-I99.
9. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc 1995;70:5-15.
10. Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-446.
11. Li T, Ahlberg A, Hachamovitch R, et al. Comparison of adenosine and dipyridamole in detecting coronary artery disease using Tc-99m sestamibi single-photon emission computed tomography imaging: a randomized, prospective clinical study. J Am Coll Cardiol 2003;41(suppl A):461A. Abstract 858-3.
12. American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, Tex: American Heart Association; 2003.
This special section of The Journal of Family Practiceis provided by an unrestricted grant fromFujisawa Healthcare, Inc. Disclosures: Dr McBride has served on the speakers’ bureau for the following: Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Glaxo SmithKline, KOS Pharmaceuticals, Merck & Co Inc, Pfizer Inc, Reliant Pharmaceuticals, and Sankyo. He has served as consultant to KOS Pharmaceuticals, Merck & Co Inc, and Pfizer Inc. He has received grant/research support from Merck & Co Inc and Pfizer Inc. Dr Hayes had no commercial interests to report. Corresponding author: Sharonne N. Hayes, MD, Mayo Clinic Women’s Heart Clinic, Rochester MN, 55905. E-mail: hayes.sharonne@mayo.edu.
1. Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction. Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 2003;107:1681-1691.
2. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883-1892.
3. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092-2197.
4. Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the American Heart Association Task Force on Risk Reduction. Circulation 1998;97:1876-1887.
5. Zanger DR, Solomon AJ, Gersh BJ. Contemporary management of angina: part I: risk assessment. Am Fam Physician 1999;60:2543-2552.
6. Bar Harbor Invitation Meeting 2000. Panel 9: Nuclear imaging in coronary artery disease. J Nucl Cardiol 2001;8:305-316.
7. Kotler TS, Diamond GA. Exercise thallium-201 scintigraphy in the diagnosis and prognosis of coronary artery disease. Ann Intern Med 1990;113:684-702.
8. Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation 1991;84 (3 Suppl):I93-I99.
9. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc 1995;70:5-15.
10. Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-446.
11. Li T, Ahlberg A, Hachamovitch R, et al. Comparison of adenosine and dipyridamole in detecting coronary artery disease using Tc-99m sestamibi single-photon emission computed tomography imaging: a randomized, prospective clinical study. J Am Coll Cardiol 2003;41(suppl A):461A. Abstract 858-3.
12. American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, Tex: American Heart Association; 2003.
This special section of The Journal of Family Practiceis provided by an unrestricted grant fromFujisawa Healthcare, Inc. Disclosures: Dr McBride has served on the speakers’ bureau for the following: Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Glaxo SmithKline, KOS Pharmaceuticals, Merck & Co Inc, Pfizer Inc, Reliant Pharmaceuticals, and Sankyo. He has served as consultant to KOS Pharmaceuticals, Merck & Co Inc, and Pfizer Inc. He has received grant/research support from Merck & Co Inc and Pfizer Inc. Dr Hayes had no commercial interests to report. Corresponding author: Sharonne N. Hayes, MD, Mayo Clinic Women’s Heart Clinic, Rochester MN, 55905. E-mail: hayes.sharonne@mayo.edu.
Improving Prevention Systems in Primary Care Practices
METHODS: Primary care practices were recruited from 4 Midwestern states. The factorial design resulted in 4 study groups: conference only, conference and quality improvement consultations, conference and prevention coordinator, and all interventions combined. Medical record audits and physician, staff, and patient surveys assessed practice change in cardiovascular disease risk factor documentation.
RESULTS: Practices participated fully in this project, set goals to improve preventive services, and implemented recommended medical record tools. The number of goals set and the increase in the use of medical record tools were greatest in the combined intervention group, with improvements noted in all groups. The use of patient history questionnaires, problem lists, and flow sheets was significantly higher in the combined intervention group when compared with the conference-only group. Documentation of risk factor screening in a recommended medical record location improved in all intervention groups, with significant sustained improvements in the practices that received the combined intervention. Documented risk factor management significantly improved in all intervention groups compared with the conference-only control.
CONCLUSIONS: Primary care practices are interested in improving prevention systems and can change these systems in response to supportive external interventions. Promoting organizational change to produce sustained improvement in preventive service clinical outcomes is a complex process that requires further research.
Improving cardiovascular disease prevention services in primary care is increasingly recognized as a high priority, but self-reports, practice evaluations, and patient surveys indicate that physicians are not adequately identifying or treating people with cardiovascular disease risk factors.1-8 Organizational interventions and feedback systems can increase the delivery of preventive services.7,9-13 Several studies suggest that improving provider performance requires the interaction of personal factors, such as knowledge, attitudes, or training, and environmental factors, such as staff assistance, colleague support, referral resources, and office systems.7-10,14-16 Previous studies on improving cardiovascular disease preventive services had limited generalizability, because they studied academic or single practices, addressed a single risk factor, or evaluated only physician self-reports.7
The Health Education and Research Trial (HEART) used a factorial design to test 2 interventions to improve cardiovascular disease prevention services for adult patients in community primary care practices.17 Following an initial conference offered to all participating practices, the interventions were either a series of practice quality improvement consultations or the provision of a staff member called a prevention coordinator to assist with practice organization and patient education. The consultations involved relationship building, assessment, goal setting, intervention, and evaluation, similar to the quality improvement approaches currently used in managed care.18,19 The prevention coordinator intervention was developed on the basis of studies showing that staff can assist in the organization and provision of preventive services.14,19 It was hypothesized that either intervention alone would improve prevention practices but that the greatest effect would result from combining them, because they used complementary organizational strategies. Our initial report focuses on the outcomes of practice goal setting for preventive services and documentation of cardiovascular disease risk factor screening and management in private primary care practices.
Methods
Study Population
Primary care practices were recruited from a 100-mile radius of each of 4 regional centers: Madison and Eau Claire, Wisconsin; Minneapolis, Minnesota ; and Iowa City, Iowa. Forty-five practices were recruited sequentially during a 2-year period (1992-1994). We have previously described the recruitment protocols and outcomes.17
Interested physicians in practices meeting the criteria were required to consent to the following study activities: physician and staff attendance at a regional conference; provider completion of a series of 3 questionnaires; 3 random samplings and patient surveys; permission for 3 medical record audits of consenting patients’ charts; participation, if assigned, in the consultative process with their support staff; and acceptance, if assigned, of a prevention coordinator for 1 year. The exclusion criteria are provided in Table 1. Practices were compensated $250 per participating physician for completion of questionnaires and medical record handling.
Patient questionnaires and medical records provided information on physician and practice preventive services and were not used to assess patient outcomes. We took a sequential sample of equal numbers of men and women from appointment records with the goal of recruiting 35 patients per physician at baseline and 20 patients per physician at 12 and 18 months after the interventions began. The additional patients at baseline were used to obtain a risk cohort, which was not included in this report. The 3 patient samples were mutually exclusive; patients in earlier samples were excluded from any subsequent sample by reviewing patient logs. Eligible patients were aged 21 to 70 years; not pregnant; without a diagnosis of cancer, transplant, recent major surgery, or terminal illness; and had at least 2 practice visits in the past 2 years. Patients received a letter from their personal physician asking for their participation. The final sample included patients who consented within 4 weeks, completed baseline questionnaires, and were selected for a medical record review.
Intervention Groups
The interventions were delivered at the practice level, because physician behavior is influenced by practice organizational systems and practice support mechanisms are required for sustained improvements.10,11,14,15 Randomization of practices in each region was balanced across the 4 intervention conditions and occurred after the regional conference Figure 6. Physicians and staff could not be blinded to the interventions.
Conference-only group. A 1-day conference and HEART Kits were provided to the practices in each of the 4 groups. The conference was designed to serve as an incentive for practice participation and to efficiently impart concepts that were the basis for the other interventions. The conference-only group was considered a suitable control, because most of the physicians had been previously exposed to heart disease prevention continuing medical education (CME), and a conference was thought to be inadequate to initiate and sustain practice system change.7,15
The HEART Kit provided a workbook describing implementation of a practice prevention system, a patient education manual, medical record tools (patient questionnaire, problem lists, flow sheet, and chart labels), patient education materials (smoking, cholesterol, hypertension, weight reduction, and exercise), and professional references (articles, protocols, and flow charts). The kit provided tools and materials that practices could choose to implement. Those materials—simple, reproducible, and adaptable for a variety of practices—were presented during the conference to model and encourage their use. These materials are available on the Internet at www.fammed.wisc.edu/research.heart.
The theme of the conference and intervention protocols was “Screen, Manage, and Monitor,” representing an ongoing cycle of both preventive patient care and practice quality improvement strategies. Medical record tools, such as the problem list and patient questionnaire, were encouraged as efficient methods to screen and document health histories and risk factors. Medical record labels were encouraged as risk factor reminders, and flow sheets were suggested to improve risk management and monitoring. Changes in organizational systems and practice roles and routines to improve cardiovascular disease prevention services were recommended, and physician and staff teams from each practice met to evaluate their existing practice system and roles. Practices were encouraged to identify goals for improving prevention systems, choosing the risk factors they intended to address and the methods they planned to use.
Conference consistency in each region was ensured, because the conference was developed and primarily presented by the principal investigator and a coinvestigator with assistance from consultant faculty teams in the other regions.
Consultation group. These practices received a series of 3 consultation meetings and 2 reinforcement visits during a 1-year period. Figure 7 Consultations were held at the practice sites, initiated within 3 months of the conference, and designed to include all participating physicians and staff. The consultant faculty followed a specific protocol, and we observed a consultation in each region to ensure uniformity of consultation methodology and format.
At the first consultation, the HEART faculty presented data from the baseline medical record review and patient, physician, and staff questionnaires, describing the baseline practice prevention activity. This practice profile provided feedback for discussion of systems improvement. Each practice chose their own goals and action plans from the HEART target areas: screening, management, and monitoring of smoking, cholesterol, and hypertension. The practice was asked to identify 2 prevention leaders (one physician and one staff member) to lead meetings, encourage practice goals, and coordinate quality improvement activities.
At the second consultation approximately 2 months later, the practice prevention leaders presented goals and an implementation plan to the practice for discussion, modification, and endorsement. HEART consultants facilitated the second and third consultations to transition the leadership and responsibility to the practice. The third consultation emphasized ongoing monitoring and evaluation of practice goals and implementation efforts.
Nurse or dietitian HEART faculty held 2 reinforcement meetings with the leaders at the practice between consultations to review progress with goals, discuss barriers or problems, and provide advice or resources for further improvement.
Prevention coordinator group. These practices worked with HEART to select an individual who would devote 4.5 hours per week per participating physician to HEART activities for the intervention year. The role of the prevention coordinator was limited to cardiovascular disease activities, including assessment, facilitation, and coordination of prevention systems (one third of the prevention coordinator’s time) and coordination and provision of patient cardiovascular disease risk education (two thirds of the prevention coordinator’s time). The prevention coordinator was preferably a current practice staff member hired for extra time, but more commonly this person was someone new to the practice. The HEART grant funded prevention coordinator salaries, training, and evaluation.
A health educator and regional HEART faculty trained the prevention coordinators in an intensive 2-day skills-development workshop. The training included a study overview, definition of the systems approach and the prevention coordinator role, health education principles, and a detailed risk factor update. Regional nurse or dietitian HEART faculty provided ongoing prevention coordinator support through regular phone contacts (at least once per month), review of daily activity logs, and occasional practice visits (2 per prevention coordinator). HEART also provided support through regional conference calls (2 per prevention coordinator), newsletters (4 per prevention coordinator), and a toll-free telephone line.
Combined intervention. We hypothesized that the combination of the conference, consultation, and prevention coordinator interventions would produce the greatest improvement in cardiovascular disease preventive services, because it provided professional education through the conference; facilitated individual practice assessment, planning, and implementation through the consultation visits; and provided prevention coordinator time to coordinate and support system development. Prevention coordinator hiring occurred after the conference and the randomization of practices to intervention groups. Prevention coordinators in combined intervention practices attended all consultation and reinforcement meetings and often served as a prevention leader in the practice.
Sample Size and Power Analysis
Determination of sample size for this hierarchical analysis involved an estimation of the number of primary care practices, physicians, and patient medical records needed to approximate the screening and management of individual physicians, then a practice, and subsequently, the practices within an intervention group. The number of medical records needed to represent physician performance was based on an assessment of the changes in variance in the expected physician performance from pilot data for 2 outcome variables (documented screening of blood cholesterol and smoking history on the medical record), as a function of the number of records sampled. It was determined that 20 medical records would provide a stable estimate of physician performance. Power was based on practice group mean differences among physicians, and calculation of an appropriate sample size was conservatively based on the smallest effect size detected for a single outcome variable.
Data Collection and Management
Data was collected using physician and staff questionnaires, patient questionnaires, medical record reviews, and physician and staff phone interviews. The measures assessed the intervention effects at several levels and provided cross-validation.
Patient questionnaires included a consent form allowing a review of the medical record, patient demographics, family and personal cardiovascular disease history, attitudes toward and experience with practice preventive services, and personal cardiovascular disease risk factors including hypertension, smoking, diabetes, and lack of exercise. Physician and patient care staff working full time and at least 50% part time completed questionnaires that assessed attitudes, beliefs, and estimates of cardiovascular preventive services. Periodic telephone interviews with a sample (22%, n = 239/1075) of physician and patient care staff during the interventions assessed goal setting and validated practice cardiovascular disease screening and management activities.
The medical record review included the number of practice visits, cardiovascular disease status, family cardiovascular disease history, hypertension diagnosis and management, smoking status and management, diabetes diagnosis, height, weight, lipoprotein levels within 5 years, diet advice, cholesterol medication, and exercise information. We noted the record location of screening and management data. Information was entered directly into a customized Filemaker Pro computer database by HEART reviewers blinded to intervention group at baseline but not at 12 and 18 months. At baseline, 100% of the medical records were reviewed by 2 reviewers for data entry reliability. At 12 and 18 month there were random second reviews on 10% of the charts to assess consistency and ensure accuracy. Half of the second reviews showed no data differences, and the error rate for the other records was 1.3%.
Data Analysis Model
The study hypotheses were: An increase in documentation of patient heart disease risk factors on patient records would occur as a result of the interventions, and there would be an additional increase as a result of the combined intervention. Our hypotheses also stated that these changes would demonstrate durability at 18 months, 6 months after the end of the intervention. General descriptive statistics and testing of univariate associations between selected variables were done using the chi-square statistic on categorical variables and 1-way analysis of variance where appropriate. Since each physician worked within a practice and there were 10 to 11 practices per intervention group, we used a hierarchical analytic modeling strategy to test the original study hypotheses. The hierarchical statistical tests first assess the differences between each of the 3 intervention groups and the conference-only group at baseline. A separate hierarchical model was built for the 12-month and 18-month data to perform baseline adjusted contrasts of each intervention group with the conference-only group.
Results
All 45 practices completed the study. Practice, physician, and staff characteristics are described in Table 2. None of the characteristics show a significant difference between intervention groups, primarily because of wide standard deviations. Of the enrolled practices, 87% (n = 39) consisted of only or mainly family physicians, and 11% (n = 6) were only or mostly internists. Physician specialties were: family physicians 82% (n = 131), internists 14% (n = 23), general practitioners 2% (n = 5), and 1 geriatrician. Eleven (7%) participating physicians were from ethnic minority groups, and 19% were women. Seventy-one percent of the practices (n = 32) were autonomous entities at baseline, and 29% (n = 13) were administered by a larger organization (health maintenance organization [HMO], hospital, and so forth). Only 2 practices had a participating physician leave the practice during the interventions, and 4 practices had a physician depart between the 12- and 18-month data collections. All other physicians completed the study. The response rate for the 3 questionnaires sent to the 160 study physicians was 96%.
Patient care staff completing questionnaires included registered nurses (33%), licensed practical nurses (24%), medical or nursing assistants (27%), medical technicians (7%), physician assistants (4%), nurse practitioners (4%), and 1 pharmacist. Staff members were mostly white (97%) and women (98%). Overall, 76% of the patient-care staff participating at baseline were still in the practice at 18 months. The staff response rate to the 3 questionnaires was 96%.
Twenty-two prevention coordinators were hired. Five (23%) of the prevention coordinators were working in the practices and were hired for additional time, 6 were previously affiliated with the practice, and 11 were entirely new to the practice. Fifteen had nursing backgrounds, 4 were dietitians, and 3 were health educators.
The overall response rate to patient questionnaires was consistent with prestudy estimates of 50%. A total of 31,826 patients received an initial mailing consisting of a 9-item questionnaire and consent form. Fifty percent (n = 16,008) of the patients responded to this initial questionnaire mailing. Sixty-three percent (n = 10,158) of the responding patients were eligible, completed final questionnaires, consented, and had medical records reviewed. The resulting patient samples were predominantly women (56%), white (95%), married (77%), and had some college education (61%). The average age was 48 years. These figures were consistent across the 3 data collection points and are representative of census demographic data in the study regions. To address concerns about potential responder bias, we evaluated 4 practices in the same HMO by having them conduct an anonymous record review of a sample of eligible but nonconsenting patients. A comparison of the information from nonresponders’ records (n = 332) with the entire group of patients who were contacted indicated that nonresponders were less likely to have a documented cholesterol value, but no other significant differences related to study variables were found.
Intervention Outcomes
Goal setting. At the conference and during consultations, the practices were encouraged to follow a quality improvement process of first assessing current prevention services, then establishing goals for improvement. Physician and staff questionnaires and phone interviews revealed that nearly all practices (93%) reported setting goals to improve preventive services table 3. Practices in both the combined intervention and consultation groups set an average of 7 goals, while prevention coordinator practices averaged 5 goals, and conference-only practices averaged less than 3. The majority of the goals were related to implementing medical record tools, such as patient questionnaires, problem lists, flow sheet, or chart labels, or to increasing screening by making smoking a vital sign or routinely checking cholesterol levels. Risk factor management goals were set less often.
Practicewide meetings were recommended to develop consensus on prevention goals. On 12-month questionnaires, combined intervention practices reported a mean of 4 prevention meetings in the previous year, compared with 5 for prevention coordinator practices, 3 for consultation practices, and 1 meeting for conference-only practices.
Implementation of practice goals. To determine if practices followed through on their goals to use cardiovascular disease prevention record tools, we assessed the presence of cardiovascular disease risk information (a cholesterol level, hypertension diagnosis, or smoking status) on recommended tools in the medical record of each physician table 4 at baseline, 12, and 18 months, controlling for the baseline variables. Increases in cardiovascular disease risk documentation on the patient questionnaire (24%), problem list (35%), and medical record label (21%) were greatest in the combined intervention group at 12 months and were maintained at similar levels at 18 months. The prevention coordinator group showed the second-largest increases in use of the patient questionnaire (22%), problem list (13%), and chart label (10%) and had the largest increase in flow sheet use (22%), with most changes maintained at 18 months. Use of patient questionnaires, problem lists, and flow sheets increased to a lesser degree in the consultation group at 12 and 18 months. The conference-only group demonstrated a small increase in the use of patient questionnaires and flow sheets, used no chart labels, and did not improve their use of the problem list.
Changes in documentation of screening and management. Practices were encouraged to have a routine approach to screening, including a designated, easily accessible medical record location for risk factor documentation. For smoking screening, the problem list or a medical record label was defined as a recommended location, while the recommended location for hypercholesterolemia was the problem list, a medical record label, or a flow sheet. The percentage of all patients who had cardiovascular disease risk documented in a recommended location more than tripled in the combined intervention group, and this change was maintained at a significant level at 18 months. Screening documentation for at-risk patients in the prevention coordinator group significantly increased at 12 months and decreased slightly at 18 months. The consultation group increased screening rates, but these increases did reach significance table 5. There were no changes in the conference-only group.
Practices were encouraged to improve cardiovascular disease risk factor management by providing and documenting smoking cessation advice, quit dates, or nicotine replacement for smokers and diet advice or medication for patients with elevated cholesterol levels. When medical records of patients with risk factors (smoking, cholesterol >200 mg/dL, or hypertension) were reviewed, baseline values indicate that appropriate management of cardiovascular disease risk was documented for approximately 65% of the patients. Significant increases in risk management documentation were noted for the combined intervention group at 12 months compared with the conference-only group. Increases in other groups were not significant. However, at 18 months, all intervention groups had increased documentation of risk management compared with the control, with a significant difference in the prevention coordinator and consultation groups table 5.
Discussion
This study demonstrates that physicians, staff, and their practices will participate in health services research and make efforts to improve preventive services. More than 60% of the eligible practices contacted consented to participate in our trial.17 Practices were willing to evaluate their current preventive services, set goals, and develop improvement strategies. Practice members were receptive to the innovative conference and consultation formats and were especially positive about including all office staff. Many practices reported that the HEART conference was the first time they had ever met as an entire staff for any reason. Although there was initial resistance to the time involved in practicewide meetings, the vast majority of practices participated fully and were positive about their participation.
Our study also shows that practices can set goals, make changes in practice organization for prevention services, and increase risk factor screening and management documentation. Practice process change requires significant effort and is difficult to initiate without consensus and group support.10,14,20,21 Many physicians reported that before this study they had inadequate training or time for development and implementation of system changes and quality improvement. Physicians acknowledged that staff involvement is critical for office system change, and staff were enthusiastic about an expanded role in the prevention process. The greater number of practice meetings in the combined intervention group may have resulted in the larger preventive system changes. Group meetings facilitate communication and consensus development, which are important parts of organizational change.10,22,23
Medical records are appropriately viewed as tools to assist in optimal patient care. Practices were receptive to medical record changes that were designed to efficiently improve preventive care. Before this study, many practices were not routinely using problem lists, patient questionnaires, and flow sheets. Consultant faculty emphasized the benefits of these tools for organizing a prevention system, and as a result, combined intervention and prevention coordinator practices were more likely to use these tools for risk factor screening and management. Other studies support the need to make changes in practice systems to increase preventive services.11 In a randomized controlled trial in a staff model HMO, physician training and office systems changes increased physician counseling rates and resulted in significant decreases in patient cholesterol levels and body weight. The use of physician training alone did not produce an increase in counseling or change in patient outcomes, indicating that training is not adequate, and systems changes are needed to create a supportive office environment that will improve services.11
The study design allowed practices to set their own goals and timetable for change, as is recommended for quality improvement plans, and the resulting changes varied among the practices. The practices set screening goals more often than management goals, and smoking screening goals were set most often. This may have been due to physician and practice attitudes, exposure to previous practice guidelines, or because smoking screening requires fewer steps to accomplish than cholesterol screening. Screening goals may have been more common because screening is the first step in the prevention process. Screening, in general, is less complex than the provider and patient behavior change required for management services, and therefore, screening goals may be easier to achieve. Screening provides the foundation for management services, and our study results show that the combined intervention, consultation, and prevention coordinator practices achieved significant improvements not only in their documentation of screening but also in documenting management for patients with cardiovascular disease risk factors.
We noted differences in the effects of the consultation and prevention coordinator interventions. Consultation practices set more goals, but prevention coordinator practices achieved greater increases in the use of medical record tools and in the documentation of screening and management. The communication and collaboration involved in the consultations seemed to lead to more meetings and goal setting, while the additional dedicated prevention coordinator time appeared to improve implementation of the goals. Regarding documentation of cardiovascular disease screening after the 12-month intervention period, our 18-month results show screening rates continued to increase in the consultation group, but screening rates decreased in the prevention coordinator group. These results may indicate that the time and possibly leadership of the prevention coordinator was necessary to maintain changes in screening routines. The consultations produced smaller changes in screening during the intervention, but those changes seemed to be more durable.
Our study’s strengths include the participation of a variety of private practices in several regions, high completion and participation rates, and multiple data sources. The study methodology, including continuous monitoring, persistent reminders, modest financial incentives, and treatment of participating practices as “valued customers” throughout the study, helped achieve the high participation and completion rates. In addition, we demonstrated that this intervention model may be generalizable, as consultant faculty in 4 different regions implemented the same intervention and achieved similar results. This model could be used in managed care practice networks to improve preventive services.
Limitations
Selection bias from the volunteer practices is a possible limitation, although more than 60% of eligible practices participated, and this participation rate would have been higher if all practices that wanted to participate could have been accommodated.17 Nevertheless, our study results pertain only to the practices that participated. Although practices were randomized to interventions, each practice is different, and these differences may have affected the outcomes. For the practice variables we examined Table 2, no significant differences were noted. The intervention protocol encouraged practices to choose their own goals and allowed them to set multiple goals and change several practice systems simultaneously, providing the autonomy recommended in quality improvement projects. This flexibility may have diluted the effects of the inter- vention.,/p>
We designated the conference-only group as a control for secular changes, but the conference, the materials provided, the practices’ interest in prevention, and the knowledge that they were participating in a national trial may have influenced this group’s outcomes. On the basis of previous research, our initial hypothesis was that an educational intervention alone would have little effect.15 However, conference-only practices met with all staff and set goals at the 1-day conference, used system materials, and reported holding meetings and making system changes, which is more than expected with usual CME. There was no true control group in this study, and either secular change or the conference may explain the improvements in services noted in the conference-only group. It is also possible that because the conference-only practices set fewer goals, they were more likely to achieve them. Our results suggest that further research is needed on how a 1-day conference affects quality improvement, with protected time for group interaction and staff participation.
Conclusions
This trial demonstrates that private primary care practices will work to improve the quality of their prevention service systems. The interventions used were well received and were internalized by the practices to a significant degree. The practice system changes increased the provision and documentation of cardiovascular disease screening and management. Our study suggests that higher rates of screening are clearly related to more documentation of risk factor management, which is consistent with other studies.24 Further analysis is underway to assess practice characteristics associated with improvements, to further explain intervention effects, and to evaluate patient care outcomes. Although the practice efforts and improvements in this trial were positive, further research is needed to develop more effective methods and incentives to improve preventive services in community practices.
Acknowledgments
This research is supported by a Public Health Services Grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (RO1 HL-47554). We thank the Wisconsin Research Network (Madison) and HealthPartners (Minneapolis, Minn) for their collaboration. We also express our gratitude to the HEART practice physicians, staff, and patients for their willingness to participate. Wisconsin: Blue Diamond Family Practice Center, Bloomer; Community Health Center, Union Grove; Family Health Associates, Chippewa Falls; Family Health Specialists (PrimeCare Centers, SC), Wausau; Family Practice Associates, Dodgeville; Franciscan Skemp Healthcare/Mayo Health System/Onalaska Clinic, Onalaska; Franciscan Skemp Healthcare/Mayo Health System/Sparta Campus, Sparta; Grant Community Clinic, Lancaster; Grantsburg Clinic, Grantsburg; Group Health Cooperative of Eau Claire-Riverview, Eau Claire; Health Directions Delafield, Delafield; Kickapoo Valley Medical Clinic-VMH, Soldiers Grove; LacCourte Oreilles Community Health Center, Hayward; Lodi Medical Clinic, Lodi; Marshfield Clinic-Colby/Abbotsford Center, Colby; Medical Associates of Watertown, Watertown; Mercy Whitewater Medical Center, Whitewater; Milwaukee Physicians and Therapists, SC, Mequon; North Woods Community Health Center, Minong; Roche-A-Cri Clinic, SC, Friendship; Sinai Samaritan-Johnston Primary Care Clinic, Milwaukee; and United Internists of Milwaukee, SC, New Berlin. Illinois: Drs Dorsey, Rone, and Savic, Rockford; and Family Care Affiliates, Silvis. Minnesota: Camden Physicians Ltd–Camden, Minneapolis; Camden Physicians Ltd–Grove Square, Maple Grove; Consultants-Internal Medicine, Edina; Eden Prairie Clinic, Ltd, Eden Prairie; Glencoe Medical Clinic, Glencoe; Health Partners Inver Grove Heights Clinic, Inver Grove Heights; Health Partners Ridgedale Clinic, Minnetonka; Health Partners West Medical and Dental Clinic, St. Louis Park; and Health Partners White Bear Lake Clinic, White Bear Lake. Iowa: Center for Family Medicine, McFarland Clinic, PC, Marshalltown; Dyersville Family Practice, Dyersville; Elkader Medical Associates, Elkader; Family Medical Center, PC, Marion; Family Medical Center, PC, Oskaloosa; Family Medicine of Mt. Pleasant, PC, Mt. Pleasant; Manchester Family Medical Associates, PC, Manchester; Maquoketa Family Clinic, Maquoketa; Marshalltown Family Medical Services, McFarland Clinic, PC, Marshalltown; and Monticello Medical Center, Monticello.
1. PE, Plane MB, Underbakke G, Brown RL, Solberg LI. Smoking screening and management in primary care practices. Arch Fam Med 1997;6:165-72.
2. McBride PE, Schrott HG, Plane MB, Underbakke G, Brown RL. Primary care practice adherence to National Cholesterol Education Program guidelines for patients with coronary heart disease. Arch Intern Med 1998;158:1238-44.
3. Pearson TA, McBride PE, Houston-Miller N, Smith S. Organization of preventive cardiology service (27th Bethesda Conference-Task Force 8). J Am Coll Cardiol 1996;27:1039-47.
4. Eaton CB, Monroe A, McQuade W, Eimer MJ. Cholesterol testing and management: a national comparison of family physicians, general internists, and cardiologists. J Am Board Fam Pract 1998;11:180-6.
5. Stafford RS, Blumenthal D. Specialty differences in cardiovascular disease prevention practices. J Am Coll Cardiol 1998;32:1238-43.
6. Stafford RS, Blumenthal D, Pasternak RC. Variations in cholesterol management practices of U.S. physicians. J Am Coll Cardiol 1997;29:139-46.
7. Ockene JK, McBride PE, Sallis JF, Bonollo DP, Ockene IS. Synthesis of lessons learned from cardiopulmonary preventive interventions in healthcare practice settings. Ann Epidemiol 1997;S7:S32-45.
8. Kottke TE, Solberg LI, Brekke ML, Cabrera A, Marquez M. Delivery rates for preventive services in 44 midwestern primary care clinics. Mayo Clinic Proc 1997;72:515-23.
9. Nutting PA. Health promotion in primary medical care: problems and potential. Prev Med 1986;15:537-48.
10. LI, Kottke TE, Brekke ML. Will primary care clinics organize themselves to improve the delivery of preventive service? A randomized controlled trial. Prev Med 1998;27:623-31.
11. IS, Herbert JR, Ockene JK, Merriam PA, Hurley TG, Saperia GM. Effect of training and a structured office practice on physician-delivered nutrition counseling: the Worcester-Area Trial for Counseling in Hyperlipoproteinemia. Am J Prev Med 1996;12:252-8.
12. AJ, O’Connor GT, Keller A, Carney PA, Levy D, Whaley FS. Cancer: improving early detection and prevention. A community practice randomized trial. BMJ 1992;304:687-91.
13. DM. From measuring to managing the improvement of prevention. Am J Prev Med 1995;11:385-7.
14. AJ, Woodruff CB, Carney PA. Changing office routines to enhance preventive care. The preventive GAPS approach. Arch Fam Med 1994;3:176-83.
15. D, Thomason MA, Oxman AD, Haynes B. Changing physician performance: a systematic review of the effect of continuing medical education strategies. JAMA 1995;274:700-5.
16. BC, Lyone TF, Neuhsus E, Kolton M, Dwarshius L. Method of evaluating and improving ambulatory medical care. Health Serv Res 1984;19:218-45.
17. PE, Massoth KM, Underbakke G, Solberg LI, Beasley JW, Plane MB. Recruitment of practices for primary care research: experiences in a preventive services clinical trial. J Fam Pract 1996;43:389-95.
18. D, Schulte AC. A social learning model of consultation. Prof Psych Res Pract 1987;18:283-7.
19. E, Fowler G, Gray M. Promoting prevention in primary care: controlled trial of low technology, low cost approach. BMJ 1987;294:1080-2.
METHODS: Primary care practices were recruited from 4 Midwestern states. The factorial design resulted in 4 study groups: conference only, conference and quality improvement consultations, conference and prevention coordinator, and all interventions combined. Medical record audits and physician, staff, and patient surveys assessed practice change in cardiovascular disease risk factor documentation.
RESULTS: Practices participated fully in this project, set goals to improve preventive services, and implemented recommended medical record tools. The number of goals set and the increase in the use of medical record tools were greatest in the combined intervention group, with improvements noted in all groups. The use of patient history questionnaires, problem lists, and flow sheets was significantly higher in the combined intervention group when compared with the conference-only group. Documentation of risk factor screening in a recommended medical record location improved in all intervention groups, with significant sustained improvements in the practices that received the combined intervention. Documented risk factor management significantly improved in all intervention groups compared with the conference-only control.
CONCLUSIONS: Primary care practices are interested in improving prevention systems and can change these systems in response to supportive external interventions. Promoting organizational change to produce sustained improvement in preventive service clinical outcomes is a complex process that requires further research.
Improving cardiovascular disease prevention services in primary care is increasingly recognized as a high priority, but self-reports, practice evaluations, and patient surveys indicate that physicians are not adequately identifying or treating people with cardiovascular disease risk factors.1-8 Organizational interventions and feedback systems can increase the delivery of preventive services.7,9-13 Several studies suggest that improving provider performance requires the interaction of personal factors, such as knowledge, attitudes, or training, and environmental factors, such as staff assistance, colleague support, referral resources, and office systems.7-10,14-16 Previous studies on improving cardiovascular disease preventive services had limited generalizability, because they studied academic or single practices, addressed a single risk factor, or evaluated only physician self-reports.7
The Health Education and Research Trial (HEART) used a factorial design to test 2 interventions to improve cardiovascular disease prevention services for adult patients in community primary care practices.17 Following an initial conference offered to all participating practices, the interventions were either a series of practice quality improvement consultations or the provision of a staff member called a prevention coordinator to assist with practice organization and patient education. The consultations involved relationship building, assessment, goal setting, intervention, and evaluation, similar to the quality improvement approaches currently used in managed care.18,19 The prevention coordinator intervention was developed on the basis of studies showing that staff can assist in the organization and provision of preventive services.14,19 It was hypothesized that either intervention alone would improve prevention practices but that the greatest effect would result from combining them, because they used complementary organizational strategies. Our initial report focuses on the outcomes of practice goal setting for preventive services and documentation of cardiovascular disease risk factor screening and management in private primary care practices.
Methods
Study Population
Primary care practices were recruited from a 100-mile radius of each of 4 regional centers: Madison and Eau Claire, Wisconsin; Minneapolis, Minnesota ; and Iowa City, Iowa. Forty-five practices were recruited sequentially during a 2-year period (1992-1994). We have previously described the recruitment protocols and outcomes.17
Interested physicians in practices meeting the criteria were required to consent to the following study activities: physician and staff attendance at a regional conference; provider completion of a series of 3 questionnaires; 3 random samplings and patient surveys; permission for 3 medical record audits of consenting patients’ charts; participation, if assigned, in the consultative process with their support staff; and acceptance, if assigned, of a prevention coordinator for 1 year. The exclusion criteria are provided in Table 1. Practices were compensated $250 per participating physician for completion of questionnaires and medical record handling.
Patient questionnaires and medical records provided information on physician and practice preventive services and were not used to assess patient outcomes. We took a sequential sample of equal numbers of men and women from appointment records with the goal of recruiting 35 patients per physician at baseline and 20 patients per physician at 12 and 18 months after the interventions began. The additional patients at baseline were used to obtain a risk cohort, which was not included in this report. The 3 patient samples were mutually exclusive; patients in earlier samples were excluded from any subsequent sample by reviewing patient logs. Eligible patients were aged 21 to 70 years; not pregnant; without a diagnosis of cancer, transplant, recent major surgery, or terminal illness; and had at least 2 practice visits in the past 2 years. Patients received a letter from their personal physician asking for their participation. The final sample included patients who consented within 4 weeks, completed baseline questionnaires, and were selected for a medical record review.
Intervention Groups
The interventions were delivered at the practice level, because physician behavior is influenced by practice organizational systems and practice support mechanisms are required for sustained improvements.10,11,14,15 Randomization of practices in each region was balanced across the 4 intervention conditions and occurred after the regional conference Figure 6. Physicians and staff could not be blinded to the interventions.
Conference-only group. A 1-day conference and HEART Kits were provided to the practices in each of the 4 groups. The conference was designed to serve as an incentive for practice participation and to efficiently impart concepts that were the basis for the other interventions. The conference-only group was considered a suitable control, because most of the physicians had been previously exposed to heart disease prevention continuing medical education (CME), and a conference was thought to be inadequate to initiate and sustain practice system change.7,15
The HEART Kit provided a workbook describing implementation of a practice prevention system, a patient education manual, medical record tools (patient questionnaire, problem lists, flow sheet, and chart labels), patient education materials (smoking, cholesterol, hypertension, weight reduction, and exercise), and professional references (articles, protocols, and flow charts). The kit provided tools and materials that practices could choose to implement. Those materials—simple, reproducible, and adaptable for a variety of practices—were presented during the conference to model and encourage their use. These materials are available on the Internet at www.fammed.wisc.edu/research.heart.
The theme of the conference and intervention protocols was “Screen, Manage, and Monitor,” representing an ongoing cycle of both preventive patient care and practice quality improvement strategies. Medical record tools, such as the problem list and patient questionnaire, were encouraged as efficient methods to screen and document health histories and risk factors. Medical record labels were encouraged as risk factor reminders, and flow sheets were suggested to improve risk management and monitoring. Changes in organizational systems and practice roles and routines to improve cardiovascular disease prevention services were recommended, and physician and staff teams from each practice met to evaluate their existing practice system and roles. Practices were encouraged to identify goals for improving prevention systems, choosing the risk factors they intended to address and the methods they planned to use.
Conference consistency in each region was ensured, because the conference was developed and primarily presented by the principal investigator and a coinvestigator with assistance from consultant faculty teams in the other regions.
Consultation group. These practices received a series of 3 consultation meetings and 2 reinforcement visits during a 1-year period. Figure 7 Consultations were held at the practice sites, initiated within 3 months of the conference, and designed to include all participating physicians and staff. The consultant faculty followed a specific protocol, and we observed a consultation in each region to ensure uniformity of consultation methodology and format.
At the first consultation, the HEART faculty presented data from the baseline medical record review and patient, physician, and staff questionnaires, describing the baseline practice prevention activity. This practice profile provided feedback for discussion of systems improvement. Each practice chose their own goals and action plans from the HEART target areas: screening, management, and monitoring of smoking, cholesterol, and hypertension. The practice was asked to identify 2 prevention leaders (one physician and one staff member) to lead meetings, encourage practice goals, and coordinate quality improvement activities.
At the second consultation approximately 2 months later, the practice prevention leaders presented goals and an implementation plan to the practice for discussion, modification, and endorsement. HEART consultants facilitated the second and third consultations to transition the leadership and responsibility to the practice. The third consultation emphasized ongoing monitoring and evaluation of practice goals and implementation efforts.
Nurse or dietitian HEART faculty held 2 reinforcement meetings with the leaders at the practice between consultations to review progress with goals, discuss barriers or problems, and provide advice or resources for further improvement.
Prevention coordinator group. These practices worked with HEART to select an individual who would devote 4.5 hours per week per participating physician to HEART activities for the intervention year. The role of the prevention coordinator was limited to cardiovascular disease activities, including assessment, facilitation, and coordination of prevention systems (one third of the prevention coordinator’s time) and coordination and provision of patient cardiovascular disease risk education (two thirds of the prevention coordinator’s time). The prevention coordinator was preferably a current practice staff member hired for extra time, but more commonly this person was someone new to the practice. The HEART grant funded prevention coordinator salaries, training, and evaluation.
A health educator and regional HEART faculty trained the prevention coordinators in an intensive 2-day skills-development workshop. The training included a study overview, definition of the systems approach and the prevention coordinator role, health education principles, and a detailed risk factor update. Regional nurse or dietitian HEART faculty provided ongoing prevention coordinator support through regular phone contacts (at least once per month), review of daily activity logs, and occasional practice visits (2 per prevention coordinator). HEART also provided support through regional conference calls (2 per prevention coordinator), newsletters (4 per prevention coordinator), and a toll-free telephone line.
Combined intervention. We hypothesized that the combination of the conference, consultation, and prevention coordinator interventions would produce the greatest improvement in cardiovascular disease preventive services, because it provided professional education through the conference; facilitated individual practice assessment, planning, and implementation through the consultation visits; and provided prevention coordinator time to coordinate and support system development. Prevention coordinator hiring occurred after the conference and the randomization of practices to intervention groups. Prevention coordinators in combined intervention practices attended all consultation and reinforcement meetings and often served as a prevention leader in the practice.
Sample Size and Power Analysis
Determination of sample size for this hierarchical analysis involved an estimation of the number of primary care practices, physicians, and patient medical records needed to approximate the screening and management of individual physicians, then a practice, and subsequently, the practices within an intervention group. The number of medical records needed to represent physician performance was based on an assessment of the changes in variance in the expected physician performance from pilot data for 2 outcome variables (documented screening of blood cholesterol and smoking history on the medical record), as a function of the number of records sampled. It was determined that 20 medical records would provide a stable estimate of physician performance. Power was based on practice group mean differences among physicians, and calculation of an appropriate sample size was conservatively based on the smallest effect size detected for a single outcome variable.
Data Collection and Management
Data was collected using physician and staff questionnaires, patient questionnaires, medical record reviews, and physician and staff phone interviews. The measures assessed the intervention effects at several levels and provided cross-validation.
Patient questionnaires included a consent form allowing a review of the medical record, patient demographics, family and personal cardiovascular disease history, attitudes toward and experience with practice preventive services, and personal cardiovascular disease risk factors including hypertension, smoking, diabetes, and lack of exercise. Physician and patient care staff working full time and at least 50% part time completed questionnaires that assessed attitudes, beliefs, and estimates of cardiovascular preventive services. Periodic telephone interviews with a sample (22%, n = 239/1075) of physician and patient care staff during the interventions assessed goal setting and validated practice cardiovascular disease screening and management activities.
The medical record review included the number of practice visits, cardiovascular disease status, family cardiovascular disease history, hypertension diagnosis and management, smoking status and management, diabetes diagnosis, height, weight, lipoprotein levels within 5 years, diet advice, cholesterol medication, and exercise information. We noted the record location of screening and management data. Information was entered directly into a customized Filemaker Pro computer database by HEART reviewers blinded to intervention group at baseline but not at 12 and 18 months. At baseline, 100% of the medical records were reviewed by 2 reviewers for data entry reliability. At 12 and 18 month there were random second reviews on 10% of the charts to assess consistency and ensure accuracy. Half of the second reviews showed no data differences, and the error rate for the other records was 1.3%.
Data Analysis Model
The study hypotheses were: An increase in documentation of patient heart disease risk factors on patient records would occur as a result of the interventions, and there would be an additional increase as a result of the combined intervention. Our hypotheses also stated that these changes would demonstrate durability at 18 months, 6 months after the end of the intervention. General descriptive statistics and testing of univariate associations between selected variables were done using the chi-square statistic on categorical variables and 1-way analysis of variance where appropriate. Since each physician worked within a practice and there were 10 to 11 practices per intervention group, we used a hierarchical analytic modeling strategy to test the original study hypotheses. The hierarchical statistical tests first assess the differences between each of the 3 intervention groups and the conference-only group at baseline. A separate hierarchical model was built for the 12-month and 18-month data to perform baseline adjusted contrasts of each intervention group with the conference-only group.
Results
All 45 practices completed the study. Practice, physician, and staff characteristics are described in Table 2. None of the characteristics show a significant difference between intervention groups, primarily because of wide standard deviations. Of the enrolled practices, 87% (n = 39) consisted of only or mainly family physicians, and 11% (n = 6) were only or mostly internists. Physician specialties were: family physicians 82% (n = 131), internists 14% (n = 23), general practitioners 2% (n = 5), and 1 geriatrician. Eleven (7%) participating physicians were from ethnic minority groups, and 19% were women. Seventy-one percent of the practices (n = 32) were autonomous entities at baseline, and 29% (n = 13) were administered by a larger organization (health maintenance organization [HMO], hospital, and so forth). Only 2 practices had a participating physician leave the practice during the interventions, and 4 practices had a physician depart between the 12- and 18-month data collections. All other physicians completed the study. The response rate for the 3 questionnaires sent to the 160 study physicians was 96%.
Patient care staff completing questionnaires included registered nurses (33%), licensed practical nurses (24%), medical or nursing assistants (27%), medical technicians (7%), physician assistants (4%), nurse practitioners (4%), and 1 pharmacist. Staff members were mostly white (97%) and women (98%). Overall, 76% of the patient-care staff participating at baseline were still in the practice at 18 months. The staff response rate to the 3 questionnaires was 96%.
Twenty-two prevention coordinators were hired. Five (23%) of the prevention coordinators were working in the practices and were hired for additional time, 6 were previously affiliated with the practice, and 11 were entirely new to the practice. Fifteen had nursing backgrounds, 4 were dietitians, and 3 were health educators.
The overall response rate to patient questionnaires was consistent with prestudy estimates of 50%. A total of 31,826 patients received an initial mailing consisting of a 9-item questionnaire and consent form. Fifty percent (n = 16,008) of the patients responded to this initial questionnaire mailing. Sixty-three percent (n = 10,158) of the responding patients were eligible, completed final questionnaires, consented, and had medical records reviewed. The resulting patient samples were predominantly women (56%), white (95%), married (77%), and had some college education (61%). The average age was 48 years. These figures were consistent across the 3 data collection points and are representative of census demographic data in the study regions. To address concerns about potential responder bias, we evaluated 4 practices in the same HMO by having them conduct an anonymous record review of a sample of eligible but nonconsenting patients. A comparison of the information from nonresponders’ records (n = 332) with the entire group of patients who were contacted indicated that nonresponders were less likely to have a documented cholesterol value, but no other significant differences related to study variables were found.
Intervention Outcomes
Goal setting. At the conference and during consultations, the practices were encouraged to follow a quality improvement process of first assessing current prevention services, then establishing goals for improvement. Physician and staff questionnaires and phone interviews revealed that nearly all practices (93%) reported setting goals to improve preventive services table 3. Practices in both the combined intervention and consultation groups set an average of 7 goals, while prevention coordinator practices averaged 5 goals, and conference-only practices averaged less than 3. The majority of the goals were related to implementing medical record tools, such as patient questionnaires, problem lists, flow sheet, or chart labels, or to increasing screening by making smoking a vital sign or routinely checking cholesterol levels. Risk factor management goals were set less often.
Practicewide meetings were recommended to develop consensus on prevention goals. On 12-month questionnaires, combined intervention practices reported a mean of 4 prevention meetings in the previous year, compared with 5 for prevention coordinator practices, 3 for consultation practices, and 1 meeting for conference-only practices.
Implementation of practice goals. To determine if practices followed through on their goals to use cardiovascular disease prevention record tools, we assessed the presence of cardiovascular disease risk information (a cholesterol level, hypertension diagnosis, or smoking status) on recommended tools in the medical record of each physician table 4 at baseline, 12, and 18 months, controlling for the baseline variables. Increases in cardiovascular disease risk documentation on the patient questionnaire (24%), problem list (35%), and medical record label (21%) were greatest in the combined intervention group at 12 months and were maintained at similar levels at 18 months. The prevention coordinator group showed the second-largest increases in use of the patient questionnaire (22%), problem list (13%), and chart label (10%) and had the largest increase in flow sheet use (22%), with most changes maintained at 18 months. Use of patient questionnaires, problem lists, and flow sheets increased to a lesser degree in the consultation group at 12 and 18 months. The conference-only group demonstrated a small increase in the use of patient questionnaires and flow sheets, used no chart labels, and did not improve their use of the problem list.
Changes in documentation of screening and management. Practices were encouraged to have a routine approach to screening, including a designated, easily accessible medical record location for risk factor documentation. For smoking screening, the problem list or a medical record label was defined as a recommended location, while the recommended location for hypercholesterolemia was the problem list, a medical record label, or a flow sheet. The percentage of all patients who had cardiovascular disease risk documented in a recommended location more than tripled in the combined intervention group, and this change was maintained at a significant level at 18 months. Screening documentation for at-risk patients in the prevention coordinator group significantly increased at 12 months and decreased slightly at 18 months. The consultation group increased screening rates, but these increases did reach significance table 5. There were no changes in the conference-only group.
Practices were encouraged to improve cardiovascular disease risk factor management by providing and documenting smoking cessation advice, quit dates, or nicotine replacement for smokers and diet advice or medication for patients with elevated cholesterol levels. When medical records of patients with risk factors (smoking, cholesterol >200 mg/dL, or hypertension) were reviewed, baseline values indicate that appropriate management of cardiovascular disease risk was documented for approximately 65% of the patients. Significant increases in risk management documentation were noted for the combined intervention group at 12 months compared with the conference-only group. Increases in other groups were not significant. However, at 18 months, all intervention groups had increased documentation of risk management compared with the control, with a significant difference in the prevention coordinator and consultation groups table 5.
Discussion
This study demonstrates that physicians, staff, and their practices will participate in health services research and make efforts to improve preventive services. More than 60% of the eligible practices contacted consented to participate in our trial.17 Practices were willing to evaluate their current preventive services, set goals, and develop improvement strategies. Practice members were receptive to the innovative conference and consultation formats and were especially positive about including all office staff. Many practices reported that the HEART conference was the first time they had ever met as an entire staff for any reason. Although there was initial resistance to the time involved in practicewide meetings, the vast majority of practices participated fully and were positive about their participation.
Our study also shows that practices can set goals, make changes in practice organization for prevention services, and increase risk factor screening and management documentation. Practice process change requires significant effort and is difficult to initiate without consensus and group support.10,14,20,21 Many physicians reported that before this study they had inadequate training or time for development and implementation of system changes and quality improvement. Physicians acknowledged that staff involvement is critical for office system change, and staff were enthusiastic about an expanded role in the prevention process. The greater number of practice meetings in the combined intervention group may have resulted in the larger preventive system changes. Group meetings facilitate communication and consensus development, which are important parts of organizational change.10,22,23
Medical records are appropriately viewed as tools to assist in optimal patient care. Practices were receptive to medical record changes that were designed to efficiently improve preventive care. Before this study, many practices were not routinely using problem lists, patient questionnaires, and flow sheets. Consultant faculty emphasized the benefits of these tools for organizing a prevention system, and as a result, combined intervention and prevention coordinator practices were more likely to use these tools for risk factor screening and management. Other studies support the need to make changes in practice systems to increase preventive services.11 In a randomized controlled trial in a staff model HMO, physician training and office systems changes increased physician counseling rates and resulted in significant decreases in patient cholesterol levels and body weight. The use of physician training alone did not produce an increase in counseling or change in patient outcomes, indicating that training is not adequate, and systems changes are needed to create a supportive office environment that will improve services.11
The study design allowed practices to set their own goals and timetable for change, as is recommended for quality improvement plans, and the resulting changes varied among the practices. The practices set screening goals more often than management goals, and smoking screening goals were set most often. This may have been due to physician and practice attitudes, exposure to previous practice guidelines, or because smoking screening requires fewer steps to accomplish than cholesterol screening. Screening goals may have been more common because screening is the first step in the prevention process. Screening, in general, is less complex than the provider and patient behavior change required for management services, and therefore, screening goals may be easier to achieve. Screening provides the foundation for management services, and our study results show that the combined intervention, consultation, and prevention coordinator practices achieved significant improvements not only in their documentation of screening but also in documenting management for patients with cardiovascular disease risk factors.
We noted differences in the effects of the consultation and prevention coordinator interventions. Consultation practices set more goals, but prevention coordinator practices achieved greater increases in the use of medical record tools and in the documentation of screening and management. The communication and collaboration involved in the consultations seemed to lead to more meetings and goal setting, while the additional dedicated prevention coordinator time appeared to improve implementation of the goals. Regarding documentation of cardiovascular disease screening after the 12-month intervention period, our 18-month results show screening rates continued to increase in the consultation group, but screening rates decreased in the prevention coordinator group. These results may indicate that the time and possibly leadership of the prevention coordinator was necessary to maintain changes in screening routines. The consultations produced smaller changes in screening during the intervention, but those changes seemed to be more durable.
Our study’s strengths include the participation of a variety of private practices in several regions, high completion and participation rates, and multiple data sources. The study methodology, including continuous monitoring, persistent reminders, modest financial incentives, and treatment of participating practices as “valued customers” throughout the study, helped achieve the high participation and completion rates. In addition, we demonstrated that this intervention model may be generalizable, as consultant faculty in 4 different regions implemented the same intervention and achieved similar results. This model could be used in managed care practice networks to improve preventive services.
Limitations
Selection bias from the volunteer practices is a possible limitation, although more than 60% of eligible practices participated, and this participation rate would have been higher if all practices that wanted to participate could have been accommodated.17 Nevertheless, our study results pertain only to the practices that participated. Although practices were randomized to interventions, each practice is different, and these differences may have affected the outcomes. For the practice variables we examined Table 2, no significant differences were noted. The intervention protocol encouraged practices to choose their own goals and allowed them to set multiple goals and change several practice systems simultaneously, providing the autonomy recommended in quality improvement projects. This flexibility may have diluted the effects of the inter- vention.,/p>
We designated the conference-only group as a control for secular changes, but the conference, the materials provided, the practices’ interest in prevention, and the knowledge that they were participating in a national trial may have influenced this group’s outcomes. On the basis of previous research, our initial hypothesis was that an educational intervention alone would have little effect.15 However, conference-only practices met with all staff and set goals at the 1-day conference, used system materials, and reported holding meetings and making system changes, which is more than expected with usual CME. There was no true control group in this study, and either secular change or the conference may explain the improvements in services noted in the conference-only group. It is also possible that because the conference-only practices set fewer goals, they were more likely to achieve them. Our results suggest that further research is needed on how a 1-day conference affects quality improvement, with protected time for group interaction and staff participation.
Conclusions
This trial demonstrates that private primary care practices will work to improve the quality of their prevention service systems. The interventions used were well received and were internalized by the practices to a significant degree. The practice system changes increased the provision and documentation of cardiovascular disease screening and management. Our study suggests that higher rates of screening are clearly related to more documentation of risk factor management, which is consistent with other studies.24 Further analysis is underway to assess practice characteristics associated with improvements, to further explain intervention effects, and to evaluate patient care outcomes. Although the practice efforts and improvements in this trial were positive, further research is needed to develop more effective methods and incentives to improve preventive services in community practices.
Acknowledgments
This research is supported by a Public Health Services Grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (RO1 HL-47554). We thank the Wisconsin Research Network (Madison) and HealthPartners (Minneapolis, Minn) for their collaboration. We also express our gratitude to the HEART practice physicians, staff, and patients for their willingness to participate. Wisconsin: Blue Diamond Family Practice Center, Bloomer; Community Health Center, Union Grove; Family Health Associates, Chippewa Falls; Family Health Specialists (PrimeCare Centers, SC), Wausau; Family Practice Associates, Dodgeville; Franciscan Skemp Healthcare/Mayo Health System/Onalaska Clinic, Onalaska; Franciscan Skemp Healthcare/Mayo Health System/Sparta Campus, Sparta; Grant Community Clinic, Lancaster; Grantsburg Clinic, Grantsburg; Group Health Cooperative of Eau Claire-Riverview, Eau Claire; Health Directions Delafield, Delafield; Kickapoo Valley Medical Clinic-VMH, Soldiers Grove; LacCourte Oreilles Community Health Center, Hayward; Lodi Medical Clinic, Lodi; Marshfield Clinic-Colby/Abbotsford Center, Colby; Medical Associates of Watertown, Watertown; Mercy Whitewater Medical Center, Whitewater; Milwaukee Physicians and Therapists, SC, Mequon; North Woods Community Health Center, Minong; Roche-A-Cri Clinic, SC, Friendship; Sinai Samaritan-Johnston Primary Care Clinic, Milwaukee; and United Internists of Milwaukee, SC, New Berlin. Illinois: Drs Dorsey, Rone, and Savic, Rockford; and Family Care Affiliates, Silvis. Minnesota: Camden Physicians Ltd–Camden, Minneapolis; Camden Physicians Ltd–Grove Square, Maple Grove; Consultants-Internal Medicine, Edina; Eden Prairie Clinic, Ltd, Eden Prairie; Glencoe Medical Clinic, Glencoe; Health Partners Inver Grove Heights Clinic, Inver Grove Heights; Health Partners Ridgedale Clinic, Minnetonka; Health Partners West Medical and Dental Clinic, St. Louis Park; and Health Partners White Bear Lake Clinic, White Bear Lake. Iowa: Center for Family Medicine, McFarland Clinic, PC, Marshalltown; Dyersville Family Practice, Dyersville; Elkader Medical Associates, Elkader; Family Medical Center, PC, Marion; Family Medical Center, PC, Oskaloosa; Family Medicine of Mt. Pleasant, PC, Mt. Pleasant; Manchester Family Medical Associates, PC, Manchester; Maquoketa Family Clinic, Maquoketa; Marshalltown Family Medical Services, McFarland Clinic, PC, Marshalltown; and Monticello Medical Center, Monticello.
METHODS: Primary care practices were recruited from 4 Midwestern states. The factorial design resulted in 4 study groups: conference only, conference and quality improvement consultations, conference and prevention coordinator, and all interventions combined. Medical record audits and physician, staff, and patient surveys assessed practice change in cardiovascular disease risk factor documentation.
RESULTS: Practices participated fully in this project, set goals to improve preventive services, and implemented recommended medical record tools. The number of goals set and the increase in the use of medical record tools were greatest in the combined intervention group, with improvements noted in all groups. The use of patient history questionnaires, problem lists, and flow sheets was significantly higher in the combined intervention group when compared with the conference-only group. Documentation of risk factor screening in a recommended medical record location improved in all intervention groups, with significant sustained improvements in the practices that received the combined intervention. Documented risk factor management significantly improved in all intervention groups compared with the conference-only control.
CONCLUSIONS: Primary care practices are interested in improving prevention systems and can change these systems in response to supportive external interventions. Promoting organizational change to produce sustained improvement in preventive service clinical outcomes is a complex process that requires further research.
Improving cardiovascular disease prevention services in primary care is increasingly recognized as a high priority, but self-reports, practice evaluations, and patient surveys indicate that physicians are not adequately identifying or treating people with cardiovascular disease risk factors.1-8 Organizational interventions and feedback systems can increase the delivery of preventive services.7,9-13 Several studies suggest that improving provider performance requires the interaction of personal factors, such as knowledge, attitudes, or training, and environmental factors, such as staff assistance, colleague support, referral resources, and office systems.7-10,14-16 Previous studies on improving cardiovascular disease preventive services had limited generalizability, because they studied academic or single practices, addressed a single risk factor, or evaluated only physician self-reports.7
The Health Education and Research Trial (HEART) used a factorial design to test 2 interventions to improve cardiovascular disease prevention services for adult patients in community primary care practices.17 Following an initial conference offered to all participating practices, the interventions were either a series of practice quality improvement consultations or the provision of a staff member called a prevention coordinator to assist with practice organization and patient education. The consultations involved relationship building, assessment, goal setting, intervention, and evaluation, similar to the quality improvement approaches currently used in managed care.18,19 The prevention coordinator intervention was developed on the basis of studies showing that staff can assist in the organization and provision of preventive services.14,19 It was hypothesized that either intervention alone would improve prevention practices but that the greatest effect would result from combining them, because they used complementary organizational strategies. Our initial report focuses on the outcomes of practice goal setting for preventive services and documentation of cardiovascular disease risk factor screening and management in private primary care practices.
Methods
Study Population
Primary care practices were recruited from a 100-mile radius of each of 4 regional centers: Madison and Eau Claire, Wisconsin; Minneapolis, Minnesota ; and Iowa City, Iowa. Forty-five practices were recruited sequentially during a 2-year period (1992-1994). We have previously described the recruitment protocols and outcomes.17
Interested physicians in practices meeting the criteria were required to consent to the following study activities: physician and staff attendance at a regional conference; provider completion of a series of 3 questionnaires; 3 random samplings and patient surveys; permission for 3 medical record audits of consenting patients’ charts; participation, if assigned, in the consultative process with their support staff; and acceptance, if assigned, of a prevention coordinator for 1 year. The exclusion criteria are provided in Table 1. Practices were compensated $250 per participating physician for completion of questionnaires and medical record handling.
Patient questionnaires and medical records provided information on physician and practice preventive services and were not used to assess patient outcomes. We took a sequential sample of equal numbers of men and women from appointment records with the goal of recruiting 35 patients per physician at baseline and 20 patients per physician at 12 and 18 months after the interventions began. The additional patients at baseline were used to obtain a risk cohort, which was not included in this report. The 3 patient samples were mutually exclusive; patients in earlier samples were excluded from any subsequent sample by reviewing patient logs. Eligible patients were aged 21 to 70 years; not pregnant; without a diagnosis of cancer, transplant, recent major surgery, or terminal illness; and had at least 2 practice visits in the past 2 years. Patients received a letter from their personal physician asking for their participation. The final sample included patients who consented within 4 weeks, completed baseline questionnaires, and were selected for a medical record review.
Intervention Groups
The interventions were delivered at the practice level, because physician behavior is influenced by practice organizational systems and practice support mechanisms are required for sustained improvements.10,11,14,15 Randomization of practices in each region was balanced across the 4 intervention conditions and occurred after the regional conference Figure 6. Physicians and staff could not be blinded to the interventions.
Conference-only group. A 1-day conference and HEART Kits were provided to the practices in each of the 4 groups. The conference was designed to serve as an incentive for practice participation and to efficiently impart concepts that were the basis for the other interventions. The conference-only group was considered a suitable control, because most of the physicians had been previously exposed to heart disease prevention continuing medical education (CME), and a conference was thought to be inadequate to initiate and sustain practice system change.7,15
The HEART Kit provided a workbook describing implementation of a practice prevention system, a patient education manual, medical record tools (patient questionnaire, problem lists, flow sheet, and chart labels), patient education materials (smoking, cholesterol, hypertension, weight reduction, and exercise), and professional references (articles, protocols, and flow charts). The kit provided tools and materials that practices could choose to implement. Those materials—simple, reproducible, and adaptable for a variety of practices—were presented during the conference to model and encourage their use. These materials are available on the Internet at www.fammed.wisc.edu/research.heart.
The theme of the conference and intervention protocols was “Screen, Manage, and Monitor,” representing an ongoing cycle of both preventive patient care and practice quality improvement strategies. Medical record tools, such as the problem list and patient questionnaire, were encouraged as efficient methods to screen and document health histories and risk factors. Medical record labels were encouraged as risk factor reminders, and flow sheets were suggested to improve risk management and monitoring. Changes in organizational systems and practice roles and routines to improve cardiovascular disease prevention services were recommended, and physician and staff teams from each practice met to evaluate their existing practice system and roles. Practices were encouraged to identify goals for improving prevention systems, choosing the risk factors they intended to address and the methods they planned to use.
Conference consistency in each region was ensured, because the conference was developed and primarily presented by the principal investigator and a coinvestigator with assistance from consultant faculty teams in the other regions.
Consultation group. These practices received a series of 3 consultation meetings and 2 reinforcement visits during a 1-year period. Figure 7 Consultations were held at the practice sites, initiated within 3 months of the conference, and designed to include all participating physicians and staff. The consultant faculty followed a specific protocol, and we observed a consultation in each region to ensure uniformity of consultation methodology and format.
At the first consultation, the HEART faculty presented data from the baseline medical record review and patient, physician, and staff questionnaires, describing the baseline practice prevention activity. This practice profile provided feedback for discussion of systems improvement. Each practice chose their own goals and action plans from the HEART target areas: screening, management, and monitoring of smoking, cholesterol, and hypertension. The practice was asked to identify 2 prevention leaders (one physician and one staff member) to lead meetings, encourage practice goals, and coordinate quality improvement activities.
At the second consultation approximately 2 months later, the practice prevention leaders presented goals and an implementation plan to the practice for discussion, modification, and endorsement. HEART consultants facilitated the second and third consultations to transition the leadership and responsibility to the practice. The third consultation emphasized ongoing monitoring and evaluation of practice goals and implementation efforts.
Nurse or dietitian HEART faculty held 2 reinforcement meetings with the leaders at the practice between consultations to review progress with goals, discuss barriers or problems, and provide advice or resources for further improvement.
Prevention coordinator group. These practices worked with HEART to select an individual who would devote 4.5 hours per week per participating physician to HEART activities for the intervention year. The role of the prevention coordinator was limited to cardiovascular disease activities, including assessment, facilitation, and coordination of prevention systems (one third of the prevention coordinator’s time) and coordination and provision of patient cardiovascular disease risk education (two thirds of the prevention coordinator’s time). The prevention coordinator was preferably a current practice staff member hired for extra time, but more commonly this person was someone new to the practice. The HEART grant funded prevention coordinator salaries, training, and evaluation.
A health educator and regional HEART faculty trained the prevention coordinators in an intensive 2-day skills-development workshop. The training included a study overview, definition of the systems approach and the prevention coordinator role, health education principles, and a detailed risk factor update. Regional nurse or dietitian HEART faculty provided ongoing prevention coordinator support through regular phone contacts (at least once per month), review of daily activity logs, and occasional practice visits (2 per prevention coordinator). HEART also provided support through regional conference calls (2 per prevention coordinator), newsletters (4 per prevention coordinator), and a toll-free telephone line.
Combined intervention. We hypothesized that the combination of the conference, consultation, and prevention coordinator interventions would produce the greatest improvement in cardiovascular disease preventive services, because it provided professional education through the conference; facilitated individual practice assessment, planning, and implementation through the consultation visits; and provided prevention coordinator time to coordinate and support system development. Prevention coordinator hiring occurred after the conference and the randomization of practices to intervention groups. Prevention coordinators in combined intervention practices attended all consultation and reinforcement meetings and often served as a prevention leader in the practice.
Sample Size and Power Analysis
Determination of sample size for this hierarchical analysis involved an estimation of the number of primary care practices, physicians, and patient medical records needed to approximate the screening and management of individual physicians, then a practice, and subsequently, the practices within an intervention group. The number of medical records needed to represent physician performance was based on an assessment of the changes in variance in the expected physician performance from pilot data for 2 outcome variables (documented screening of blood cholesterol and smoking history on the medical record), as a function of the number of records sampled. It was determined that 20 medical records would provide a stable estimate of physician performance. Power was based on practice group mean differences among physicians, and calculation of an appropriate sample size was conservatively based on the smallest effect size detected for a single outcome variable.
Data Collection and Management
Data was collected using physician and staff questionnaires, patient questionnaires, medical record reviews, and physician and staff phone interviews. The measures assessed the intervention effects at several levels and provided cross-validation.
Patient questionnaires included a consent form allowing a review of the medical record, patient demographics, family and personal cardiovascular disease history, attitudes toward and experience with practice preventive services, and personal cardiovascular disease risk factors including hypertension, smoking, diabetes, and lack of exercise. Physician and patient care staff working full time and at least 50% part time completed questionnaires that assessed attitudes, beliefs, and estimates of cardiovascular preventive services. Periodic telephone interviews with a sample (22%, n = 239/1075) of physician and patient care staff during the interventions assessed goal setting and validated practice cardiovascular disease screening and management activities.
The medical record review included the number of practice visits, cardiovascular disease status, family cardiovascular disease history, hypertension diagnosis and management, smoking status and management, diabetes diagnosis, height, weight, lipoprotein levels within 5 years, diet advice, cholesterol medication, and exercise information. We noted the record location of screening and management data. Information was entered directly into a customized Filemaker Pro computer database by HEART reviewers blinded to intervention group at baseline but not at 12 and 18 months. At baseline, 100% of the medical records were reviewed by 2 reviewers for data entry reliability. At 12 and 18 month there were random second reviews on 10% of the charts to assess consistency and ensure accuracy. Half of the second reviews showed no data differences, and the error rate for the other records was 1.3%.
Data Analysis Model
The study hypotheses were: An increase in documentation of patient heart disease risk factors on patient records would occur as a result of the interventions, and there would be an additional increase as a result of the combined intervention. Our hypotheses also stated that these changes would demonstrate durability at 18 months, 6 months after the end of the intervention. General descriptive statistics and testing of univariate associations between selected variables were done using the chi-square statistic on categorical variables and 1-way analysis of variance where appropriate. Since each physician worked within a practice and there were 10 to 11 practices per intervention group, we used a hierarchical analytic modeling strategy to test the original study hypotheses. The hierarchical statistical tests first assess the differences between each of the 3 intervention groups and the conference-only group at baseline. A separate hierarchical model was built for the 12-month and 18-month data to perform baseline adjusted contrasts of each intervention group with the conference-only group.
Results
All 45 practices completed the study. Practice, physician, and staff characteristics are described in Table 2. None of the characteristics show a significant difference between intervention groups, primarily because of wide standard deviations. Of the enrolled practices, 87% (n = 39) consisted of only or mainly family physicians, and 11% (n = 6) were only or mostly internists. Physician specialties were: family physicians 82% (n = 131), internists 14% (n = 23), general practitioners 2% (n = 5), and 1 geriatrician. Eleven (7%) participating physicians were from ethnic minority groups, and 19% were women. Seventy-one percent of the practices (n = 32) were autonomous entities at baseline, and 29% (n = 13) were administered by a larger organization (health maintenance organization [HMO], hospital, and so forth). Only 2 practices had a participating physician leave the practice during the interventions, and 4 practices had a physician depart between the 12- and 18-month data collections. All other physicians completed the study. The response rate for the 3 questionnaires sent to the 160 study physicians was 96%.
Patient care staff completing questionnaires included registered nurses (33%), licensed practical nurses (24%), medical or nursing assistants (27%), medical technicians (7%), physician assistants (4%), nurse practitioners (4%), and 1 pharmacist. Staff members were mostly white (97%) and women (98%). Overall, 76% of the patient-care staff participating at baseline were still in the practice at 18 months. The staff response rate to the 3 questionnaires was 96%.
Twenty-two prevention coordinators were hired. Five (23%) of the prevention coordinators were working in the practices and were hired for additional time, 6 were previously affiliated with the practice, and 11 were entirely new to the practice. Fifteen had nursing backgrounds, 4 were dietitians, and 3 were health educators.
The overall response rate to patient questionnaires was consistent with prestudy estimates of 50%. A total of 31,826 patients received an initial mailing consisting of a 9-item questionnaire and consent form. Fifty percent (n = 16,008) of the patients responded to this initial questionnaire mailing. Sixty-three percent (n = 10,158) of the responding patients were eligible, completed final questionnaires, consented, and had medical records reviewed. The resulting patient samples were predominantly women (56%), white (95%), married (77%), and had some college education (61%). The average age was 48 years. These figures were consistent across the 3 data collection points and are representative of census demographic data in the study regions. To address concerns about potential responder bias, we evaluated 4 practices in the same HMO by having them conduct an anonymous record review of a sample of eligible but nonconsenting patients. A comparison of the information from nonresponders’ records (n = 332) with the entire group of patients who were contacted indicated that nonresponders were less likely to have a documented cholesterol value, but no other significant differences related to study variables were found.
Intervention Outcomes
Goal setting. At the conference and during consultations, the practices were encouraged to follow a quality improvement process of first assessing current prevention services, then establishing goals for improvement. Physician and staff questionnaires and phone interviews revealed that nearly all practices (93%) reported setting goals to improve preventive services table 3. Practices in both the combined intervention and consultation groups set an average of 7 goals, while prevention coordinator practices averaged 5 goals, and conference-only practices averaged less than 3. The majority of the goals were related to implementing medical record tools, such as patient questionnaires, problem lists, flow sheet, or chart labels, or to increasing screening by making smoking a vital sign or routinely checking cholesterol levels. Risk factor management goals were set less often.
Practicewide meetings were recommended to develop consensus on prevention goals. On 12-month questionnaires, combined intervention practices reported a mean of 4 prevention meetings in the previous year, compared with 5 for prevention coordinator practices, 3 for consultation practices, and 1 meeting for conference-only practices.
Implementation of practice goals. To determine if practices followed through on their goals to use cardiovascular disease prevention record tools, we assessed the presence of cardiovascular disease risk information (a cholesterol level, hypertension diagnosis, or smoking status) on recommended tools in the medical record of each physician table 4 at baseline, 12, and 18 months, controlling for the baseline variables. Increases in cardiovascular disease risk documentation on the patient questionnaire (24%), problem list (35%), and medical record label (21%) were greatest in the combined intervention group at 12 months and were maintained at similar levels at 18 months. The prevention coordinator group showed the second-largest increases in use of the patient questionnaire (22%), problem list (13%), and chart label (10%) and had the largest increase in flow sheet use (22%), with most changes maintained at 18 months. Use of patient questionnaires, problem lists, and flow sheets increased to a lesser degree in the consultation group at 12 and 18 months. The conference-only group demonstrated a small increase in the use of patient questionnaires and flow sheets, used no chart labels, and did not improve their use of the problem list.
Changes in documentation of screening and management. Practices were encouraged to have a routine approach to screening, including a designated, easily accessible medical record location for risk factor documentation. For smoking screening, the problem list or a medical record label was defined as a recommended location, while the recommended location for hypercholesterolemia was the problem list, a medical record label, or a flow sheet. The percentage of all patients who had cardiovascular disease risk documented in a recommended location more than tripled in the combined intervention group, and this change was maintained at a significant level at 18 months. Screening documentation for at-risk patients in the prevention coordinator group significantly increased at 12 months and decreased slightly at 18 months. The consultation group increased screening rates, but these increases did reach significance table 5. There were no changes in the conference-only group.
Practices were encouraged to improve cardiovascular disease risk factor management by providing and documenting smoking cessation advice, quit dates, or nicotine replacement for smokers and diet advice or medication for patients with elevated cholesterol levels. When medical records of patients with risk factors (smoking, cholesterol >200 mg/dL, or hypertension) were reviewed, baseline values indicate that appropriate management of cardiovascular disease risk was documented for approximately 65% of the patients. Significant increases in risk management documentation were noted for the combined intervention group at 12 months compared with the conference-only group. Increases in other groups were not significant. However, at 18 months, all intervention groups had increased documentation of risk management compared with the control, with a significant difference in the prevention coordinator and consultation groups table 5.
Discussion
This study demonstrates that physicians, staff, and their practices will participate in health services research and make efforts to improve preventive services. More than 60% of the eligible practices contacted consented to participate in our trial.17 Practices were willing to evaluate their current preventive services, set goals, and develop improvement strategies. Practice members were receptive to the innovative conference and consultation formats and were especially positive about including all office staff. Many practices reported that the HEART conference was the first time they had ever met as an entire staff for any reason. Although there was initial resistance to the time involved in practicewide meetings, the vast majority of practices participated fully and were positive about their participation.
Our study also shows that practices can set goals, make changes in practice organization for prevention services, and increase risk factor screening and management documentation. Practice process change requires significant effort and is difficult to initiate without consensus and group support.10,14,20,21 Many physicians reported that before this study they had inadequate training or time for development and implementation of system changes and quality improvement. Physicians acknowledged that staff involvement is critical for office system change, and staff were enthusiastic about an expanded role in the prevention process. The greater number of practice meetings in the combined intervention group may have resulted in the larger preventive system changes. Group meetings facilitate communication and consensus development, which are important parts of organizational change.10,22,23
Medical records are appropriately viewed as tools to assist in optimal patient care. Practices were receptive to medical record changes that were designed to efficiently improve preventive care. Before this study, many practices were not routinely using problem lists, patient questionnaires, and flow sheets. Consultant faculty emphasized the benefits of these tools for organizing a prevention system, and as a result, combined intervention and prevention coordinator practices were more likely to use these tools for risk factor screening and management. Other studies support the need to make changes in practice systems to increase preventive services.11 In a randomized controlled trial in a staff model HMO, physician training and office systems changes increased physician counseling rates and resulted in significant decreases in patient cholesterol levels and body weight. The use of physician training alone did not produce an increase in counseling or change in patient outcomes, indicating that training is not adequate, and systems changes are needed to create a supportive office environment that will improve services.11
The study design allowed practices to set their own goals and timetable for change, as is recommended for quality improvement plans, and the resulting changes varied among the practices. The practices set screening goals more often than management goals, and smoking screening goals were set most often. This may have been due to physician and practice attitudes, exposure to previous practice guidelines, or because smoking screening requires fewer steps to accomplish than cholesterol screening. Screening goals may have been more common because screening is the first step in the prevention process. Screening, in general, is less complex than the provider and patient behavior change required for management services, and therefore, screening goals may be easier to achieve. Screening provides the foundation for management services, and our study results show that the combined intervention, consultation, and prevention coordinator practices achieved significant improvements not only in their documentation of screening but also in documenting management for patients with cardiovascular disease risk factors.
We noted differences in the effects of the consultation and prevention coordinator interventions. Consultation practices set more goals, but prevention coordinator practices achieved greater increases in the use of medical record tools and in the documentation of screening and management. The communication and collaboration involved in the consultations seemed to lead to more meetings and goal setting, while the additional dedicated prevention coordinator time appeared to improve implementation of the goals. Regarding documentation of cardiovascular disease screening after the 12-month intervention period, our 18-month results show screening rates continued to increase in the consultation group, but screening rates decreased in the prevention coordinator group. These results may indicate that the time and possibly leadership of the prevention coordinator was necessary to maintain changes in screening routines. The consultations produced smaller changes in screening during the intervention, but those changes seemed to be more durable.
Our study’s strengths include the participation of a variety of private practices in several regions, high completion and participation rates, and multiple data sources. The study methodology, including continuous monitoring, persistent reminders, modest financial incentives, and treatment of participating practices as “valued customers” throughout the study, helped achieve the high participation and completion rates. In addition, we demonstrated that this intervention model may be generalizable, as consultant faculty in 4 different regions implemented the same intervention and achieved similar results. This model could be used in managed care practice networks to improve preventive services.
Limitations
Selection bias from the volunteer practices is a possible limitation, although more than 60% of eligible practices participated, and this participation rate would have been higher if all practices that wanted to participate could have been accommodated.17 Nevertheless, our study results pertain only to the practices that participated. Although practices were randomized to interventions, each practice is different, and these differences may have affected the outcomes. For the practice variables we examined Table 2, no significant differences were noted. The intervention protocol encouraged practices to choose their own goals and allowed them to set multiple goals and change several practice systems simultaneously, providing the autonomy recommended in quality improvement projects. This flexibility may have diluted the effects of the inter- vention.,/p>
We designated the conference-only group as a control for secular changes, but the conference, the materials provided, the practices’ interest in prevention, and the knowledge that they were participating in a national trial may have influenced this group’s outcomes. On the basis of previous research, our initial hypothesis was that an educational intervention alone would have little effect.15 However, conference-only practices met with all staff and set goals at the 1-day conference, used system materials, and reported holding meetings and making system changes, which is more than expected with usual CME. There was no true control group in this study, and either secular change or the conference may explain the improvements in services noted in the conference-only group. It is also possible that because the conference-only practices set fewer goals, they were more likely to achieve them. Our results suggest that further research is needed on how a 1-day conference affects quality improvement, with protected time for group interaction and staff participation.
Conclusions
This trial demonstrates that private primary care practices will work to improve the quality of their prevention service systems. The interventions used were well received and were internalized by the practices to a significant degree. The practice system changes increased the provision and documentation of cardiovascular disease screening and management. Our study suggests that higher rates of screening are clearly related to more documentation of risk factor management, which is consistent with other studies.24 Further analysis is underway to assess practice characteristics associated with improvements, to further explain intervention effects, and to evaluate patient care outcomes. Although the practice efforts and improvements in this trial were positive, further research is needed to develop more effective methods and incentives to improve preventive services in community practices.
Acknowledgments
This research is supported by a Public Health Services Grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (RO1 HL-47554). We thank the Wisconsin Research Network (Madison) and HealthPartners (Minneapolis, Minn) for their collaboration. We also express our gratitude to the HEART practice physicians, staff, and patients for their willingness to participate. Wisconsin: Blue Diamond Family Practice Center, Bloomer; Community Health Center, Union Grove; Family Health Associates, Chippewa Falls; Family Health Specialists (PrimeCare Centers, SC), Wausau; Family Practice Associates, Dodgeville; Franciscan Skemp Healthcare/Mayo Health System/Onalaska Clinic, Onalaska; Franciscan Skemp Healthcare/Mayo Health System/Sparta Campus, Sparta; Grant Community Clinic, Lancaster; Grantsburg Clinic, Grantsburg; Group Health Cooperative of Eau Claire-Riverview, Eau Claire; Health Directions Delafield, Delafield; Kickapoo Valley Medical Clinic-VMH, Soldiers Grove; LacCourte Oreilles Community Health Center, Hayward; Lodi Medical Clinic, Lodi; Marshfield Clinic-Colby/Abbotsford Center, Colby; Medical Associates of Watertown, Watertown; Mercy Whitewater Medical Center, Whitewater; Milwaukee Physicians and Therapists, SC, Mequon; North Woods Community Health Center, Minong; Roche-A-Cri Clinic, SC, Friendship; Sinai Samaritan-Johnston Primary Care Clinic, Milwaukee; and United Internists of Milwaukee, SC, New Berlin. Illinois: Drs Dorsey, Rone, and Savic, Rockford; and Family Care Affiliates, Silvis. Minnesota: Camden Physicians Ltd–Camden, Minneapolis; Camden Physicians Ltd–Grove Square, Maple Grove; Consultants-Internal Medicine, Edina; Eden Prairie Clinic, Ltd, Eden Prairie; Glencoe Medical Clinic, Glencoe; Health Partners Inver Grove Heights Clinic, Inver Grove Heights; Health Partners Ridgedale Clinic, Minnetonka; Health Partners West Medical and Dental Clinic, St. Louis Park; and Health Partners White Bear Lake Clinic, White Bear Lake. Iowa: Center for Family Medicine, McFarland Clinic, PC, Marshalltown; Dyersville Family Practice, Dyersville; Elkader Medical Associates, Elkader; Family Medical Center, PC, Marion; Family Medical Center, PC, Oskaloosa; Family Medicine of Mt. Pleasant, PC, Mt. Pleasant; Manchester Family Medical Associates, PC, Manchester; Maquoketa Family Clinic, Maquoketa; Marshalltown Family Medical Services, McFarland Clinic, PC, Marshalltown; and Monticello Medical Center, Monticello.
1. PE, Plane MB, Underbakke G, Brown RL, Solberg LI. Smoking screening and management in primary care practices. Arch Fam Med 1997;6:165-72.
2. McBride PE, Schrott HG, Plane MB, Underbakke G, Brown RL. Primary care practice adherence to National Cholesterol Education Program guidelines for patients with coronary heart disease. Arch Intern Med 1998;158:1238-44.
3. Pearson TA, McBride PE, Houston-Miller N, Smith S. Organization of preventive cardiology service (27th Bethesda Conference-Task Force 8). J Am Coll Cardiol 1996;27:1039-47.
4. Eaton CB, Monroe A, McQuade W, Eimer MJ. Cholesterol testing and management: a national comparison of family physicians, general internists, and cardiologists. J Am Board Fam Pract 1998;11:180-6.
5. Stafford RS, Blumenthal D. Specialty differences in cardiovascular disease prevention practices. J Am Coll Cardiol 1998;32:1238-43.
6. Stafford RS, Blumenthal D, Pasternak RC. Variations in cholesterol management practices of U.S. physicians. J Am Coll Cardiol 1997;29:139-46.
7. Ockene JK, McBride PE, Sallis JF, Bonollo DP, Ockene IS. Synthesis of lessons learned from cardiopulmonary preventive interventions in healthcare practice settings. Ann Epidemiol 1997;S7:S32-45.
8. Kottke TE, Solberg LI, Brekke ML, Cabrera A, Marquez M. Delivery rates for preventive services in 44 midwestern primary care clinics. Mayo Clinic Proc 1997;72:515-23.
9. Nutting PA. Health promotion in primary medical care: problems and potential. Prev Med 1986;15:537-48.
10. LI, Kottke TE, Brekke ML. Will primary care clinics organize themselves to improve the delivery of preventive service? A randomized controlled trial. Prev Med 1998;27:623-31.
11. IS, Herbert JR, Ockene JK, Merriam PA, Hurley TG, Saperia GM. Effect of training and a structured office practice on physician-delivered nutrition counseling: the Worcester-Area Trial for Counseling in Hyperlipoproteinemia. Am J Prev Med 1996;12:252-8.
12. AJ, O’Connor GT, Keller A, Carney PA, Levy D, Whaley FS. Cancer: improving early detection and prevention. A community practice randomized trial. BMJ 1992;304:687-91.
13. DM. From measuring to managing the improvement of prevention. Am J Prev Med 1995;11:385-7.
14. AJ, Woodruff CB, Carney PA. Changing office routines to enhance preventive care. The preventive GAPS approach. Arch Fam Med 1994;3:176-83.
15. D, Thomason MA, Oxman AD, Haynes B. Changing physician performance: a systematic review of the effect of continuing medical education strategies. JAMA 1995;274:700-5.
16. BC, Lyone TF, Neuhsus E, Kolton M, Dwarshius L. Method of evaluating and improving ambulatory medical care. Health Serv Res 1984;19:218-45.
17. PE, Massoth KM, Underbakke G, Solberg LI, Beasley JW, Plane MB. Recruitment of practices for primary care research: experiences in a preventive services clinical trial. J Fam Pract 1996;43:389-95.
18. D, Schulte AC. A social learning model of consultation. Prof Psych Res Pract 1987;18:283-7.
19. E, Fowler G, Gray M. Promoting prevention in primary care: controlled trial of low technology, low cost approach. BMJ 1987;294:1080-2.
1. PE, Plane MB, Underbakke G, Brown RL, Solberg LI. Smoking screening and management in primary care practices. Arch Fam Med 1997;6:165-72.
2. McBride PE, Schrott HG, Plane MB, Underbakke G, Brown RL. Primary care practice adherence to National Cholesterol Education Program guidelines for patients with coronary heart disease. Arch Intern Med 1998;158:1238-44.
3. Pearson TA, McBride PE, Houston-Miller N, Smith S. Organization of preventive cardiology service (27th Bethesda Conference-Task Force 8). J Am Coll Cardiol 1996;27:1039-47.
4. Eaton CB, Monroe A, McQuade W, Eimer MJ. Cholesterol testing and management: a national comparison of family physicians, general internists, and cardiologists. J Am Board Fam Pract 1998;11:180-6.
5. Stafford RS, Blumenthal D. Specialty differences in cardiovascular disease prevention practices. J Am Coll Cardiol 1998;32:1238-43.
6. Stafford RS, Blumenthal D, Pasternak RC. Variations in cholesterol management practices of U.S. physicians. J Am Coll Cardiol 1997;29:139-46.
7. Ockene JK, McBride PE, Sallis JF, Bonollo DP, Ockene IS. Synthesis of lessons learned from cardiopulmonary preventive interventions in healthcare practice settings. Ann Epidemiol 1997;S7:S32-45.
8. Kottke TE, Solberg LI, Brekke ML, Cabrera A, Marquez M. Delivery rates for preventive services in 44 midwestern primary care clinics. Mayo Clinic Proc 1997;72:515-23.
9. Nutting PA. Health promotion in primary medical care: problems and potential. Prev Med 1986;15:537-48.
10. LI, Kottke TE, Brekke ML. Will primary care clinics organize themselves to improve the delivery of preventive service? A randomized controlled trial. Prev Med 1998;27:623-31.
11. IS, Herbert JR, Ockene JK, Merriam PA, Hurley TG, Saperia GM. Effect of training and a structured office practice on physician-delivered nutrition counseling: the Worcester-Area Trial for Counseling in Hyperlipoproteinemia. Am J Prev Med 1996;12:252-8.
12. AJ, O’Connor GT, Keller A, Carney PA, Levy D, Whaley FS. Cancer: improving early detection and prevention. A community practice randomized trial. BMJ 1992;304:687-91.
13. DM. From measuring to managing the improvement of prevention. Am J Prev Med 1995;11:385-7.
14. AJ, Woodruff CB, Carney PA. Changing office routines to enhance preventive care. The preventive GAPS approach. Arch Fam Med 1994;3:176-83.
15. D, Thomason MA, Oxman AD, Haynes B. Changing physician performance: a systematic review of the effect of continuing medical education strategies. JAMA 1995;274:700-5.
16. BC, Lyone TF, Neuhsus E, Kolton M, Dwarshius L. Method of evaluating and improving ambulatory medical care. Health Serv Res 1984;19:218-45.
17. PE, Massoth KM, Underbakke G, Solberg LI, Beasley JW, Plane MB. Recruitment of practices for primary care research: experiences in a preventive services clinical trial. J Fam Pract 1996;43:389-95.
18. D, Schulte AC. A social learning model of consultation. Prof Psych Res Pract 1987;18:283-7.
19. E, Fowler G, Gray M. Promoting prevention in primary care: controlled trial of low technology, low cost approach. BMJ 1987;294:1080-2.