User login
Retrospective Evaluation of Drug-Drug Interactions With Erlotinib and Gefitinib Use in the Military Health System
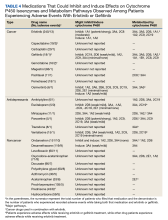
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib,or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
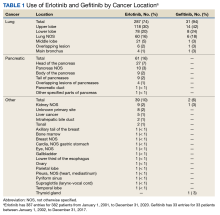
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
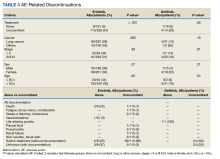
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
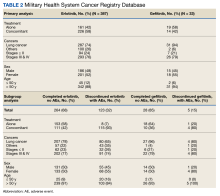
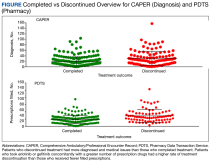
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib,or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib,or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480