User login
The Surviving Sepsis Campaign: Where have we been and where are we going?
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
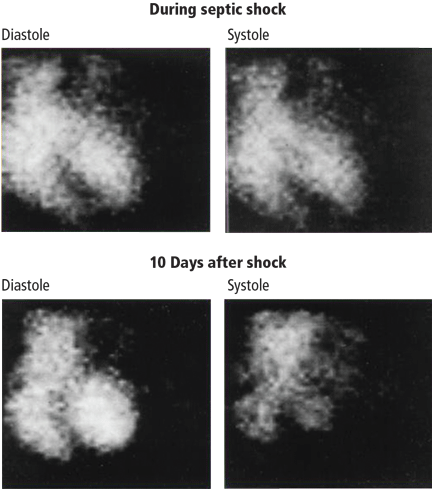
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).
NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
KEY POINTS
- Ideally, intravenous antibiotic therapy should start within the first hour after sepsis is recognized; performance improvement protocols set a target of within 3 hours.
- A specific source of infection that requires source control measures should be sought, diagnosed or excluded, and if located, treated as rapidly as possible.
- Crystalloids should be used for initial fluid resuscitation. Adding an albumin-based solution is suggested for patients who require substantial amounts of crystalloids.
- Vasopressors are indicated for those who remain hypotensive despite fluid resuscitation. Norepinephrine should be used initially, and if the target mean arterial pressure cannot be achieved, then epinephrine or low-dose vasopressin is added.
- Corticosteroids should be considered only for patients who remain unstable despite adequate fluid resuscitation and vasopressor therapy.
