User login
Low-Carbohydrate and Ketogenic Dietary Patterns for Type 2 Diabetes Management
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
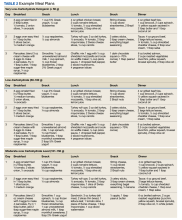
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent studyshowed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
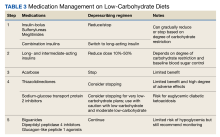
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent studyshowed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent studyshowed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
How best to diagnose iron-deficiency anemia in patients with inflammatory disease?
THE SERUM FERRITIN LEVEL is the most sensitive and specific initial laboratory test for iron-deficiency anemia (IDA) in patients with inflammation. Serum ferritin levels <45 ng/dL confirm IDA, and levels of ≥100 ng/dL essentially rule it out (strength of recommendation [SOR]: B, systematic review of prospective validating cohort studies with heterogeneity).
For patients with intermediate serum ferritin levels (45-99 ng/dL), the soluble transferrin receptor (sTfR) level and the sTfR-ferritin index (ratio of sTfR to log ferritin) are highly sensitive and specific. An sTfR-ferritin index of ≥1.5 is diagnostic of IDA, even in the presence of acute or chronic inflammation (SOR: B, systematic review of prospective validating cohort studies that lacked standardized reference values).
Evidence summary
A systematic review of 55 studies that included 45 validating prospective studies (total 2579 patients, of whom 919 had inflammatory, liver, or neoplastic disease) found that a low serum ferritin level was the most sensitive and specific initial test to diagnose IDA, even in the presence of inflammation.1 More than 80% of patients studied had confirmatory bone marrow aspiration.
Serum ferritin <45 ng/dL performed well for identifying IDA in all patients combined (sensitivity=85%; specificity=92%; positive likelihood ratio=11), although the authors didn’t calculate sensitivity and specificity for the subgroup of patients with an inflammatory disease process.
A serum ferritin level >100 ng/dL made IDA unlikely (sensitivity=5.5%; specificity=29%; negative likelihood ratio=0.08). Serum ferritin levels between 45 and 99 ng/dL weren’t useful in evaluating IDA. The subgroup analysis of the patients with inflammatory, liver, or neoplastic disease found that serum ferritin outperformed measurements of mean cell volume, transferrin saturation, red cell protoporphyrin, and red cell volume for diagnosing IDA.1
Follow up with the sTfR-ferritin index if necessary
The sTfR-ferritin index is sensitive and specific for diagnosing IDA in patients with concomitant inflammation, including those with intermediate serum ferritin levels of 45 to 99 ng/dL. A systematic review of 9 prospective validating cohort studies (total 818 patients, of whom 678 had inflammatory disease) compared sTfR levels and sTfR-ferritin index (sTfR-to-log ferritin ratio) values against bone marrow biopsy for identifying IDA.2
A mean sTfR level ≥2.5 mg/L diagnosed IDA with a sensitivity of 68% to 97% and a specificity of 47% to 100%. In patients with acute and chronic inflammation, a mean sTfR-ferritin index ≥1.5 mg/L had a higher sensitivity (88%-100%) and specificity (93%-100%). The studies were limited by heterogenous populations, small sample sizes, and nonstandardized techniques and reference ranges for sTfR levels.
Recommendations
No major professional organizations in the United States have issued recommendations or clinical guidelines for diagnosing iron deficiency in patients with chronic inflammation.
The British Society of Gastroenterology suggests that a serum ferritin level <50 ng/dL is consistent with iron deficiency but lists the use of sTfR as promising, but unproven, in the clinical setting.3
1. Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153.
2. Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352.
3. Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. 2005. Available at: www.bsg.org.uk/images/stories/docs/clinical/guidelines/sbn/iron_def.pdf. Accessed February 7, 2011.
THE SERUM FERRITIN LEVEL is the most sensitive and specific initial laboratory test for iron-deficiency anemia (IDA) in patients with inflammation. Serum ferritin levels <45 ng/dL confirm IDA, and levels of ≥100 ng/dL essentially rule it out (strength of recommendation [SOR]: B, systematic review of prospective validating cohort studies with heterogeneity).
For patients with intermediate serum ferritin levels (45-99 ng/dL), the soluble transferrin receptor (sTfR) level and the sTfR-ferritin index (ratio of sTfR to log ferritin) are highly sensitive and specific. An sTfR-ferritin index of ≥1.5 is diagnostic of IDA, even in the presence of acute or chronic inflammation (SOR: B, systematic review of prospective validating cohort studies that lacked standardized reference values).
Evidence summary
A systematic review of 55 studies that included 45 validating prospective studies (total 2579 patients, of whom 919 had inflammatory, liver, or neoplastic disease) found that a low serum ferritin level was the most sensitive and specific initial test to diagnose IDA, even in the presence of inflammation.1 More than 80% of patients studied had confirmatory bone marrow aspiration.
Serum ferritin <45 ng/dL performed well for identifying IDA in all patients combined (sensitivity=85%; specificity=92%; positive likelihood ratio=11), although the authors didn’t calculate sensitivity and specificity for the subgroup of patients with an inflammatory disease process.
A serum ferritin level >100 ng/dL made IDA unlikely (sensitivity=5.5%; specificity=29%; negative likelihood ratio=0.08). Serum ferritin levels between 45 and 99 ng/dL weren’t useful in evaluating IDA. The subgroup analysis of the patients with inflammatory, liver, or neoplastic disease found that serum ferritin outperformed measurements of mean cell volume, transferrin saturation, red cell protoporphyrin, and red cell volume for diagnosing IDA.1
Follow up with the sTfR-ferritin index if necessary
The sTfR-ferritin index is sensitive and specific for diagnosing IDA in patients with concomitant inflammation, including those with intermediate serum ferritin levels of 45 to 99 ng/dL. A systematic review of 9 prospective validating cohort studies (total 818 patients, of whom 678 had inflammatory disease) compared sTfR levels and sTfR-ferritin index (sTfR-to-log ferritin ratio) values against bone marrow biopsy for identifying IDA.2
A mean sTfR level ≥2.5 mg/L diagnosed IDA with a sensitivity of 68% to 97% and a specificity of 47% to 100%. In patients with acute and chronic inflammation, a mean sTfR-ferritin index ≥1.5 mg/L had a higher sensitivity (88%-100%) and specificity (93%-100%). The studies were limited by heterogenous populations, small sample sizes, and nonstandardized techniques and reference ranges for sTfR levels.
Recommendations
No major professional organizations in the United States have issued recommendations or clinical guidelines for diagnosing iron deficiency in patients with chronic inflammation.
The British Society of Gastroenterology suggests that a serum ferritin level <50 ng/dL is consistent with iron deficiency but lists the use of sTfR as promising, but unproven, in the clinical setting.3
THE SERUM FERRITIN LEVEL is the most sensitive and specific initial laboratory test for iron-deficiency anemia (IDA) in patients with inflammation. Serum ferritin levels <45 ng/dL confirm IDA, and levels of ≥100 ng/dL essentially rule it out (strength of recommendation [SOR]: B, systematic review of prospective validating cohort studies with heterogeneity).
For patients with intermediate serum ferritin levels (45-99 ng/dL), the soluble transferrin receptor (sTfR) level and the sTfR-ferritin index (ratio of sTfR to log ferritin) are highly sensitive and specific. An sTfR-ferritin index of ≥1.5 is diagnostic of IDA, even in the presence of acute or chronic inflammation (SOR: B, systematic review of prospective validating cohort studies that lacked standardized reference values).
Evidence summary
A systematic review of 55 studies that included 45 validating prospective studies (total 2579 patients, of whom 919 had inflammatory, liver, or neoplastic disease) found that a low serum ferritin level was the most sensitive and specific initial test to diagnose IDA, even in the presence of inflammation.1 More than 80% of patients studied had confirmatory bone marrow aspiration.
Serum ferritin <45 ng/dL performed well for identifying IDA in all patients combined (sensitivity=85%; specificity=92%; positive likelihood ratio=11), although the authors didn’t calculate sensitivity and specificity for the subgroup of patients with an inflammatory disease process.
A serum ferritin level >100 ng/dL made IDA unlikely (sensitivity=5.5%; specificity=29%; negative likelihood ratio=0.08). Serum ferritin levels between 45 and 99 ng/dL weren’t useful in evaluating IDA. The subgroup analysis of the patients with inflammatory, liver, or neoplastic disease found that serum ferritin outperformed measurements of mean cell volume, transferrin saturation, red cell protoporphyrin, and red cell volume for diagnosing IDA.1
Follow up with the sTfR-ferritin index if necessary
The sTfR-ferritin index is sensitive and specific for diagnosing IDA in patients with concomitant inflammation, including those with intermediate serum ferritin levels of 45 to 99 ng/dL. A systematic review of 9 prospective validating cohort studies (total 818 patients, of whom 678 had inflammatory disease) compared sTfR levels and sTfR-ferritin index (sTfR-to-log ferritin ratio) values against bone marrow biopsy for identifying IDA.2
A mean sTfR level ≥2.5 mg/L diagnosed IDA with a sensitivity of 68% to 97% and a specificity of 47% to 100%. In patients with acute and chronic inflammation, a mean sTfR-ferritin index ≥1.5 mg/L had a higher sensitivity (88%-100%) and specificity (93%-100%). The studies were limited by heterogenous populations, small sample sizes, and nonstandardized techniques and reference ranges for sTfR levels.
Recommendations
No major professional organizations in the United States have issued recommendations or clinical guidelines for diagnosing iron deficiency in patients with chronic inflammation.
The British Society of Gastroenterology suggests that a serum ferritin level <50 ng/dL is consistent with iron deficiency but lists the use of sTfR as promising, but unproven, in the clinical setting.3
1. Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153.
2. Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352.
3. Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. 2005. Available at: www.bsg.org.uk/images/stories/docs/clinical/guidelines/sbn/iron_def.pdf. Accessed February 7, 2011.
1. Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153.
2. Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352.
3. Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. 2005. Available at: www.bsg.org.uk/images/stories/docs/clinical/guidelines/sbn/iron_def.pdf. Accessed February 7, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
How do you evaluate macrocytosis without anemia?
Start with a detailed history, paying particular attention to medications and alcohol use (strength of recommendation [SOR]: B, prospective cohort studies). Blood testing can include a peripheral smear, evaluation for vitamin deficiencies (especially B12 deficiency), and liver function tests (SOR: B, inconsistent prospective cohort studies). Thyroid testing may be useful for older patients (SOR: B, prospective study). Reticulocyte count and bone marrow evaluation, although important to rule out hemolysis and myelodysplastic changes, may not be necessary for patients with isolated macrocytosis without anemia (SOR: B, prospective cohort studies). In unexplained macrocytosis, bone marrow evaluation may show early marrow changes, particularly in the elderly (SOR: B, prospective cohort study).
Evidence summary
Significant macrocytosis is usually defined as a mean corpuscular volume greater than 99 femtoliters (fL). The prevalence of macrocytosis (with or without anemia) ranges from 1.7% to 5.0%.1-4 As many as 60% to 80% of primary care patients may not have anemia.3,4
Because no study has looked specifically at evaluating macrocytosis without anemia, extrapolation from studies of all presentations of macrocytosis (with and without anemia) must help guide evaluation.1,3,5-7 The causes of macrocytosis vary depending on the population studied (TABLE). In primary care, alcohol use and vitamin deficiency are common causes. Even after evaluation, approximately 10% of cases remain unexplained.3
TABLE
Causes of macrocytosis: What prospective studies show
| CAUSE | PERCENT OF PATIENTS BY STUDY | ||||
|---|---|---|---|---|---|
| DAVIDSON 6 (N=200) | BREEDVELD 1 (N=70) | KEENAN 5 (N=80) | SAVAGE 7 (N=300) | MAHMOUD 10 (N=124) | |
| Alcohol | 18 | 27 | 36 | 26 | 14 |
| Vitamin deficiency | 13 | 39 (6% had both deficiencies) | 16 | 6 | 24 |
| B12 | 8 | 23 | 10 | 5 | 12 |
| Folate | 5 | 10 | 6 | 1 | 12 |
| Medications | 30 | 1 | —* | 37 | 2 |
| Liver disease | 16 | 3 | 9 | 6 | 2 |
| Hematologic disease | 15 | 19 | 14 | 14 | 20 |
| Malignancy/premalignancy | 15 | 13 | 11 | 6 | 20 |
| Reticulocytosis | 0 | 6 | 3 | 8 | —† |
| Hypothyroidism | —† | 3 | 6 | 1 | 12 |
| Unexplained | 23 | 9 | 28 | 7 | 19 |
| * Excluded patients on cytotoxic and chemotherapeutic medications | |||||
| † Not evaluated. | |||||
Clues in the history, physical exam, and lab results
A history focusing specifically on alcohol use and medications—especially chemotherapeutics, antiretroviral drugs, and antiseizure medications—can provide important clues to the cause of macrocytosis. During the physical examination, look for signs consistent with chronic liver disease.
Laboratory studies can help identify vitamin deficiencies, liver disease, and thyroid disease. A normal serum B12 level may not rule out a true B12 deficiency, but normal levels of the metabolites methylmalonic acid and homocysteine do essentially rule it out.8 In this era of folic acid fortification, the utility of the serum folate level is uncertain. Several studies suggest empiric treatment with folic acid instead of testing for a deficiency when B12 deficiency has been ruled out.7,9
Liver disease—which may be confounded by alcohol abuse, medications, or cancer—is a common cause of macrocytosis.5 Hypothyroidism is rarely a cause, but may be more prevalent in the elderly.10
What these 2 tests may, or may not, tell you
Although several authorities recommend a peripheral smear and reticulocyte count to help evaluate macrocytic anemia, no specific recommendations exist for these tests in the absence of anemia. A peripheral smear can detect megaloblastic changes typical of B12 and folate deficiency and other marrow disorders, especially myelodysplastic changes. Peripheral smear findings and reticulocytosis can also show evidence of hemolysis. However, megaloblastic changes and marrow-related changes on peripheral smear are typically seen with anemia.
In 2 prospective studies of primary care patients, 1 reported little diagnostic value for the peripheral smear,1 and the other found that reticulocytosis rarely caused macrocytosis.5 A prospective study of 300 hospitalized patients with macrocytosis found that 100% of marrow disorders and hemolysis that caused macrocytosis also caused an associated anemia.7 A retrospective chart review of 113 cases of macrocytosis in outpatients found that general practitioners often didn’t order a peripheral smear and reticulocyte count to complete their diagnostic workups.4
Bone marrow biopsy may reveal dysplastic changes, but not a Dx
A prospective study of the utility of bone marrow biopsy in 124 elderly patients with macrocytosis found that as many as 60% were diagnosed by blood tests alone. All the remaining patients with unexplained macrocytosis underwent bone marrow biopsy, which showed early dysplastic changes in 39%, but did not provide a diagnosis in nearly 50%. Twelve percent were found to have myelodysplastic syndrome, but they had a mean hemoglobin of 8.5 g/dL.10
Recommendations
We were unable to find published guidelines for the evaluation of macrocytosis without anemia by the American Society of Hematology, the British Committee for Standards in Haematology, or in an authoritative hematology text.11
Acknowledgments
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
1. Breedveld FC, Bieger R, van Wermeskerken RKA. The clinical significance of macrocytosis. Acta Med Scand. 1981;209:319-322.
2. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990;5:192-197.
3. Colon-Otero G, Menke D, Hook CC. A practical approach to the differential diagnosis and evaluation of the adult patient with macrocytic anemia. Med Clin North Am. 1992;76:581-597.
4. Seppa K, Heinila K, Sillanaukee P, et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996;57:97-100.
5. Keenan WF. Macrocytosis as an indicator of human disease. J Am Board Fam Pract. 1989;2:25-26.
6. Davidson RJL, Hamilton PJ. High mean red cell volume: its incidence and significance in routine haematology. J Clin Pathol. 1978;31:493-498.
7. Savage DG, Ogundipe A, Allen RH, et al. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319:343-352.
8. Cravens DD, Nashelsky J, Oh RC. How do we evaluate a marginally low B12 level? J Fam Pract. 2007;56:62-63.
9. Robinson AR, Mladenovic J. Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110:88-90.
10. Mahmoud MY, Lugon M, Anderson CC. Unexplained macrocytosis in elderly patients. Age Ageing. 1996;25:310-312.
11. Hoffman R, Benz EJ, Shattil SJ. Hematology: Basic Principles and Practice. 4th ed. Philadelphia: Churchill Livingstone; 2005.
Start with a detailed history, paying particular attention to medications and alcohol use (strength of recommendation [SOR]: B, prospective cohort studies). Blood testing can include a peripheral smear, evaluation for vitamin deficiencies (especially B12 deficiency), and liver function tests (SOR: B, inconsistent prospective cohort studies). Thyroid testing may be useful for older patients (SOR: B, prospective study). Reticulocyte count and bone marrow evaluation, although important to rule out hemolysis and myelodysplastic changes, may not be necessary for patients with isolated macrocytosis without anemia (SOR: B, prospective cohort studies). In unexplained macrocytosis, bone marrow evaluation may show early marrow changes, particularly in the elderly (SOR: B, prospective cohort study).
Evidence summary
Significant macrocytosis is usually defined as a mean corpuscular volume greater than 99 femtoliters (fL). The prevalence of macrocytosis (with or without anemia) ranges from 1.7% to 5.0%.1-4 As many as 60% to 80% of primary care patients may not have anemia.3,4
Because no study has looked specifically at evaluating macrocytosis without anemia, extrapolation from studies of all presentations of macrocytosis (with and without anemia) must help guide evaluation.1,3,5-7 The causes of macrocytosis vary depending on the population studied (TABLE). In primary care, alcohol use and vitamin deficiency are common causes. Even after evaluation, approximately 10% of cases remain unexplained.3
TABLE
Causes of macrocytosis: What prospective studies show
| CAUSE | PERCENT OF PATIENTS BY STUDY | ||||
|---|---|---|---|---|---|
| DAVIDSON 6 (N=200) | BREEDVELD 1 (N=70) | KEENAN 5 (N=80) | SAVAGE 7 (N=300) | MAHMOUD 10 (N=124) | |
| Alcohol | 18 | 27 | 36 | 26 | 14 |
| Vitamin deficiency | 13 | 39 (6% had both deficiencies) | 16 | 6 | 24 |
| B12 | 8 | 23 | 10 | 5 | 12 |
| Folate | 5 | 10 | 6 | 1 | 12 |
| Medications | 30 | 1 | —* | 37 | 2 |
| Liver disease | 16 | 3 | 9 | 6 | 2 |
| Hematologic disease | 15 | 19 | 14 | 14 | 20 |
| Malignancy/premalignancy | 15 | 13 | 11 | 6 | 20 |
| Reticulocytosis | 0 | 6 | 3 | 8 | —† |
| Hypothyroidism | —† | 3 | 6 | 1 | 12 |
| Unexplained | 23 | 9 | 28 | 7 | 19 |
| * Excluded patients on cytotoxic and chemotherapeutic medications | |||||
| † Not evaluated. | |||||
Clues in the history, physical exam, and lab results
A history focusing specifically on alcohol use and medications—especially chemotherapeutics, antiretroviral drugs, and antiseizure medications—can provide important clues to the cause of macrocytosis. During the physical examination, look for signs consistent with chronic liver disease.
Laboratory studies can help identify vitamin deficiencies, liver disease, and thyroid disease. A normal serum B12 level may not rule out a true B12 deficiency, but normal levels of the metabolites methylmalonic acid and homocysteine do essentially rule it out.8 In this era of folic acid fortification, the utility of the serum folate level is uncertain. Several studies suggest empiric treatment with folic acid instead of testing for a deficiency when B12 deficiency has been ruled out.7,9
Liver disease—which may be confounded by alcohol abuse, medications, or cancer—is a common cause of macrocytosis.5 Hypothyroidism is rarely a cause, but may be more prevalent in the elderly.10
What these 2 tests may, or may not, tell you
Although several authorities recommend a peripheral smear and reticulocyte count to help evaluate macrocytic anemia, no specific recommendations exist for these tests in the absence of anemia. A peripheral smear can detect megaloblastic changes typical of B12 and folate deficiency and other marrow disorders, especially myelodysplastic changes. Peripheral smear findings and reticulocytosis can also show evidence of hemolysis. However, megaloblastic changes and marrow-related changes on peripheral smear are typically seen with anemia.
In 2 prospective studies of primary care patients, 1 reported little diagnostic value for the peripheral smear,1 and the other found that reticulocytosis rarely caused macrocytosis.5 A prospective study of 300 hospitalized patients with macrocytosis found that 100% of marrow disorders and hemolysis that caused macrocytosis also caused an associated anemia.7 A retrospective chart review of 113 cases of macrocytosis in outpatients found that general practitioners often didn’t order a peripheral smear and reticulocyte count to complete their diagnostic workups.4
Bone marrow biopsy may reveal dysplastic changes, but not a Dx
A prospective study of the utility of bone marrow biopsy in 124 elderly patients with macrocytosis found that as many as 60% were diagnosed by blood tests alone. All the remaining patients with unexplained macrocytosis underwent bone marrow biopsy, which showed early dysplastic changes in 39%, but did not provide a diagnosis in nearly 50%. Twelve percent were found to have myelodysplastic syndrome, but they had a mean hemoglobin of 8.5 g/dL.10
Recommendations
We were unable to find published guidelines for the evaluation of macrocytosis without anemia by the American Society of Hematology, the British Committee for Standards in Haematology, or in an authoritative hematology text.11
Acknowledgments
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Start with a detailed history, paying particular attention to medications and alcohol use (strength of recommendation [SOR]: B, prospective cohort studies). Blood testing can include a peripheral smear, evaluation for vitamin deficiencies (especially B12 deficiency), and liver function tests (SOR: B, inconsistent prospective cohort studies). Thyroid testing may be useful for older patients (SOR: B, prospective study). Reticulocyte count and bone marrow evaluation, although important to rule out hemolysis and myelodysplastic changes, may not be necessary for patients with isolated macrocytosis without anemia (SOR: B, prospective cohort studies). In unexplained macrocytosis, bone marrow evaluation may show early marrow changes, particularly in the elderly (SOR: B, prospective cohort study).
Evidence summary
Significant macrocytosis is usually defined as a mean corpuscular volume greater than 99 femtoliters (fL). The prevalence of macrocytosis (with or without anemia) ranges from 1.7% to 5.0%.1-4 As many as 60% to 80% of primary care patients may not have anemia.3,4
Because no study has looked specifically at evaluating macrocytosis without anemia, extrapolation from studies of all presentations of macrocytosis (with and without anemia) must help guide evaluation.1,3,5-7 The causes of macrocytosis vary depending on the population studied (TABLE). In primary care, alcohol use and vitamin deficiency are common causes. Even after evaluation, approximately 10% of cases remain unexplained.3
TABLE
Causes of macrocytosis: What prospective studies show
| CAUSE | PERCENT OF PATIENTS BY STUDY | ||||
|---|---|---|---|---|---|
| DAVIDSON 6 (N=200) | BREEDVELD 1 (N=70) | KEENAN 5 (N=80) | SAVAGE 7 (N=300) | MAHMOUD 10 (N=124) | |
| Alcohol | 18 | 27 | 36 | 26 | 14 |
| Vitamin deficiency | 13 | 39 (6% had both deficiencies) | 16 | 6 | 24 |
| B12 | 8 | 23 | 10 | 5 | 12 |
| Folate | 5 | 10 | 6 | 1 | 12 |
| Medications | 30 | 1 | —* | 37 | 2 |
| Liver disease | 16 | 3 | 9 | 6 | 2 |
| Hematologic disease | 15 | 19 | 14 | 14 | 20 |
| Malignancy/premalignancy | 15 | 13 | 11 | 6 | 20 |
| Reticulocytosis | 0 | 6 | 3 | 8 | —† |
| Hypothyroidism | —† | 3 | 6 | 1 | 12 |
| Unexplained | 23 | 9 | 28 | 7 | 19 |
| * Excluded patients on cytotoxic and chemotherapeutic medications | |||||
| † Not evaluated. | |||||
Clues in the history, physical exam, and lab results
A history focusing specifically on alcohol use and medications—especially chemotherapeutics, antiretroviral drugs, and antiseizure medications—can provide important clues to the cause of macrocytosis. During the physical examination, look for signs consistent with chronic liver disease.
Laboratory studies can help identify vitamin deficiencies, liver disease, and thyroid disease. A normal serum B12 level may not rule out a true B12 deficiency, but normal levels of the metabolites methylmalonic acid and homocysteine do essentially rule it out.8 In this era of folic acid fortification, the utility of the serum folate level is uncertain. Several studies suggest empiric treatment with folic acid instead of testing for a deficiency when B12 deficiency has been ruled out.7,9
Liver disease—which may be confounded by alcohol abuse, medications, or cancer—is a common cause of macrocytosis.5 Hypothyroidism is rarely a cause, but may be more prevalent in the elderly.10
What these 2 tests may, or may not, tell you
Although several authorities recommend a peripheral smear and reticulocyte count to help evaluate macrocytic anemia, no specific recommendations exist for these tests in the absence of anemia. A peripheral smear can detect megaloblastic changes typical of B12 and folate deficiency and other marrow disorders, especially myelodysplastic changes. Peripheral smear findings and reticulocytosis can also show evidence of hemolysis. However, megaloblastic changes and marrow-related changes on peripheral smear are typically seen with anemia.
In 2 prospective studies of primary care patients, 1 reported little diagnostic value for the peripheral smear,1 and the other found that reticulocytosis rarely caused macrocytosis.5 A prospective study of 300 hospitalized patients with macrocytosis found that 100% of marrow disorders and hemolysis that caused macrocytosis also caused an associated anemia.7 A retrospective chart review of 113 cases of macrocytosis in outpatients found that general practitioners often didn’t order a peripheral smear and reticulocyte count to complete their diagnostic workups.4
Bone marrow biopsy may reveal dysplastic changes, but not a Dx
A prospective study of the utility of bone marrow biopsy in 124 elderly patients with macrocytosis found that as many as 60% were diagnosed by blood tests alone. All the remaining patients with unexplained macrocytosis underwent bone marrow biopsy, which showed early dysplastic changes in 39%, but did not provide a diagnosis in nearly 50%. Twelve percent were found to have myelodysplastic syndrome, but they had a mean hemoglobin of 8.5 g/dL.10
Recommendations
We were unable to find published guidelines for the evaluation of macrocytosis without anemia by the American Society of Hematology, the British Committee for Standards in Haematology, or in an authoritative hematology text.11
Acknowledgments
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
1. Breedveld FC, Bieger R, van Wermeskerken RKA. The clinical significance of macrocytosis. Acta Med Scand. 1981;209:319-322.
2. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990;5:192-197.
3. Colon-Otero G, Menke D, Hook CC. A practical approach to the differential diagnosis and evaluation of the adult patient with macrocytic anemia. Med Clin North Am. 1992;76:581-597.
4. Seppa K, Heinila K, Sillanaukee P, et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996;57:97-100.
5. Keenan WF. Macrocytosis as an indicator of human disease. J Am Board Fam Pract. 1989;2:25-26.
6. Davidson RJL, Hamilton PJ. High mean red cell volume: its incidence and significance in routine haematology. J Clin Pathol. 1978;31:493-498.
7. Savage DG, Ogundipe A, Allen RH, et al. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319:343-352.
8. Cravens DD, Nashelsky J, Oh RC. How do we evaluate a marginally low B12 level? J Fam Pract. 2007;56:62-63.
9. Robinson AR, Mladenovic J. Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110:88-90.
10. Mahmoud MY, Lugon M, Anderson CC. Unexplained macrocytosis in elderly patients. Age Ageing. 1996;25:310-312.
11. Hoffman R, Benz EJ, Shattil SJ. Hematology: Basic Principles and Practice. 4th ed. Philadelphia: Churchill Livingstone; 2005.
1. Breedveld FC, Bieger R, van Wermeskerken RKA. The clinical significance of macrocytosis. Acta Med Scand. 1981;209:319-322.
2. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990;5:192-197.
3. Colon-Otero G, Menke D, Hook CC. A practical approach to the differential diagnosis and evaluation of the adult patient with macrocytic anemia. Med Clin North Am. 1992;76:581-597.
4. Seppa K, Heinila K, Sillanaukee P, et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996;57:97-100.
5. Keenan WF. Macrocytosis as an indicator of human disease. J Am Board Fam Pract. 1989;2:25-26.
6. Davidson RJL, Hamilton PJ. High mean red cell volume: its incidence and significance in routine haematology. J Clin Pathol. 1978;31:493-498.
7. Savage DG, Ogundipe A, Allen RH, et al. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319:343-352.
8. Cravens DD, Nashelsky J, Oh RC. How do we evaluate a marginally low B12 level? J Fam Pract. 2007;56:62-63.
9. Robinson AR, Mladenovic J. Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110:88-90.
10. Mahmoud MY, Lugon M, Anderson CC. Unexplained macrocytosis in elderly patients. Age Ageing. 1996;25:310-312.
11. Hoffman R, Benz EJ, Shattil SJ. Hematology: Basic Principles and Practice. 4th ed. Philadelphia: Churchill Livingstone; 2005.
Evidence-based answers from the Family Physicians Inquiries Network