User login
Pneumatic Tube-Induced Reverse Pseudohyperkalemia in a Patient With Chronic Lymphocytic Leukemia
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
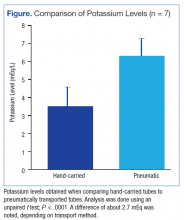
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.