User login
2019 Update on female sexual dysfunction
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
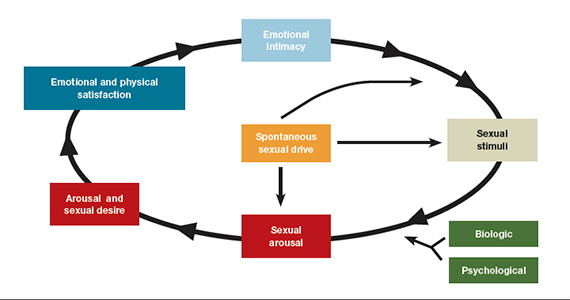
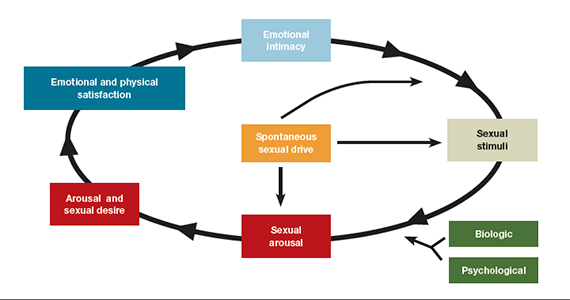
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4
Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4

Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4

Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.