User login
Incorporating medication abortion into your ObGyn practice: Why and how
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
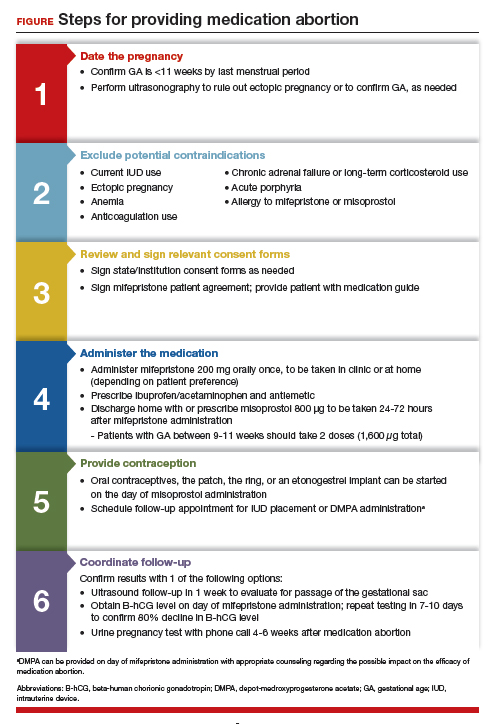
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.
Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.

