User login
A Pharmacist-Led Transitional Care Program to Reduce Hospital Readmissions in Older Adults
Medication reconciliation and patient education during admission and after discharge helped older patients remain independent at home.
There will be 53 million older adults in the US by 2020.1 Increasing age often brings medical comorbidities and prescriptions for multiple medications. An increasing number of prescribed medications combined with age-related changes in the ability to metabolize drugs makes older adults highly vulnerable to adverse drug events (ADEs).2 In addition, older adults often have difficulty self-managing their medications and adhering to prescribed regimens.3 As a result, ADEs can lead to poor health outcomes, including hospitalizations, in older adults.
Medication errors and ADEs are particularly common during transitions from hospital to home and can lead to unnecessary readmissions,a major cause of wasteful health care spending in the US.4,5 More than $25 billion are estimated to be spent annually on hospital readmissions, with Medicare picking up the bill for $17 billion of the total.6,7 Researchers have found that the majority of ADEs following hospital discharge are either entirely preventable or at least ameliorable (ie, the negative impact or harm resulting from the ADE could have been reduced).8
To address these issues, we undertook a clinical demonstration project that implemented a new transitional care program to improve the quality of care for older veterans transitioning from the Audie L. Murphy Veterans Memorial Hospital of the South Texas Veterans Health Care System (STVHCS) in San Antonio to home. The Geriatrics Medication Education at Discharge project (GMED) falls under the auspices of the San Antonio Geriatrics Research Education and Clinical Center (GRECC). Clinical demonstration projects are mandated for US Department of Veterans Affairs (VA) GRECCs to create and promote innovative models of care for older veterans. Dissemination of successful clinical demonstration projects to other VA sites is strongly encouraged. The GMED program was modeled after the Boston GRECC Pharmacological Intervention in Late Life (PILL) program.9 The PILL program, which focuses on serving older veterans with cognitive impairment, demonstrated that a postdischarge pharmacist telephone visit for medication reconciliation leads to a reduction in readmission within 60 days of discharge.9 The goals of the GMED program were to reduce polypharmacy, inappropriate prescribing and 30-day readmissions.
Methods
The project was conducted when a full-time clinical pharmacy specialist (CPS) was available (May-September 2013 and April 2014-March 2015). This project was approved as nonresearch/quality improvement by the University of Texas Health Science Center Institutional Review Board, which serves the STVHCS. Consent was not required.
Eligibility
Patients were identified via a daily hospital database query of all adults aged ≥ 65 years admitted to the hospital through Inpatient Medicine, Neurology, or Cardiology services within the prior 24 hours. Patients meeting any of the following criteria based on review of the Computerized Patient Record System (CPRS) by the team geriatrician and CPS were considered eligible: (1) aged ≥ 70 years prescribed ≥ 12 outpatient medications; (2) aged ≥ 65 years with a medical history of dementia; (3) aged ≥ 65 years prescribed outpatient medications meeting Beers criteria10; (4) age ≥ 65 years with ≥ 2 hospital admissions (including the current, index admission) within the past calendar year; or (5) aged ≥ 65 years with ≥ 3 emergency department visits within the past calendar year. For the first polypharmacy criterion, patients aged ≥ 70 years were selected instead of aged ≥ 65 years so as not to exceed the capacity of 1 CPS. Twelve or more medications were used as a cutoff for polypharmacy based on prior quality improvement information gathered from our VA geriatrics clinic examining the average number of medications taken by older veterans in the outpatient setting.
Related: Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Patients were excluded if they were expected to be discharged to any facility where the patient and/or the caregiver were not primarily responsible for medication administration after discharge. Patients who met eligibility criteria but were not seen by the transitional program pharmacist (due to staff capacity) were included in this analysis as a convenience comparison group of patients who received usual care. Patients were not randomized. All communication occurred in English, but this project did not exclude patients with limited English proficiency.
A program support assistant conducted the daily query of the hospital database. The pharmacist conducted the chart review to determine eligibility and delivered the intervention. Eligible patients were selected at random for the intervention with the intention of providing the intervention to as many veterans as possible.
The GMED Intervention
The GMED program included 2 phases, which were both conducted by a CPS with oversight from a senior CPS with geriatric pharmacology expertise and an internist/geriatrician.
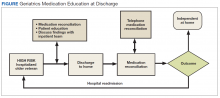
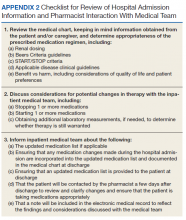
The first phase of the transitional care program included an individual, face-to-face meeting between the CPS and the patient during the hospitalization. If a veteran was not present in the room at the time of an attempted visit, the pharmacist made 2 additional attempts (3 total) to include the patient in the transitional care program during the hospitalization.
The second component of the transitional care program included a telephone visit within 2 to 3 days of discharge, conducted by the same CPS who performed the face-to-face visit. The purpose of the telephone visit was to perform medication reconciliation, identify and rectify medication errors, provide further patient education, and assist in facilitating appropriate follow-up by the patient’s primary care provider (PCP), if required. At a minimum, veterans were asked a series of questions pertaining to their concerns about medication regimens, receipt of newly prescribed medications at discharge, additional education regarding medications after the CPS encounter during hospitalization, and whether the veteran required assistance with the medication regimen in the home setting. Follow-up questions were asked as needed to clarify and identify potential medication problems. All information from this telephone encounter was communicated to the PCP through CPRS documentation and by telephone as needed.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Data Collection
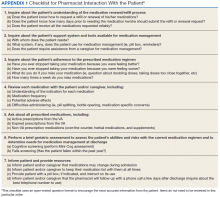
A standardized questionnaire was used prospectively for patients in the transitional care program group to assess patient education, primary residence, presence of a caregiver, fall history, medication adherence, and cognitive status (using Mini-Cog).13 Additional information (patient age, number of outpatient medications prior to and following the admission, presence of Beers criteria outpatient medications prior to and following the admission, new outpatient prescriptions, and changes to existing prescriptions as a result of the hospitalization) was gathered prospectively from patient interviews or from chart review.
For patients included in the comparison group, a retrospective administrative chart review was conducted to collect information such as age, sex, ethnic group, admission within 1 year prior to index admission, frailty, and Charlson Comorbidity Index (CCI) score, a method of categorizing comorbidities of patients based on the diagnosis codes found in administrative data.14 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient (0 indicates no comorbidities; higher scores predict greater risk of mortality or increased resource use).
We used the index developed from 17 disease categories. The range for CCI was 0 to 25. Frailty was defined as the presence of any of the following frailty-related diagnoses: anemia; fall, head injury, other injury; coagulopathy; electrolyte disturbance; or gait disorder. These diagnoses are either primary frailty characteristics within the frailty phenotype or have been shown in prior studies to be associated with the frailty phenotype.15-18 While more widely accepted frailty definitions exist,these other definitions require direct examination of the patient and could not be used in this project because we did not directly interact with the comparison group.16,19 The frailty definition used has been previously identified as a predictor of health care utilization and 30-day readmission in a veteran population.20 Whether or not the CPS detected a postdischarge medication error was recorded. All CPS recommendations were documented.
An index admission was defined as a hospital admission that occurred during the project period. Thirty-day readmission was defined as a hospital admission that occurred within 30 days of the discharge date of an index admission. Each index admission was considered individually for readmission (yes vs no) even if it occurred in the same patient over the project period. A 30-day readmission was not considered an index admission. An admission that occurred after a 30-day readmission was considered a subsequent index admission. Patients who died in the hospital were not included in this analysis, as they would not have participated in the entire intervention.
Statistical Analysis
We compared characteristics between patients who received GMED and patients who never received GMED (comparison group). Generalized estimating equations (GEE) were used to determine whether the rate of 30-day readmission (yes vs no) in the transitional care program group differed from that of the comparison group. In our GEE analysis, we assumed a binomial distribution and the logit link to model the log-odds of readmission as a linear function of transitional care program status (yes vs no) and other covariates, including age, frailty, hospital admission within 1 year prior to the index admission, and CCI score as covariates. Thirty-day readmission status associated with each index admission was coded as 1 for a readmission within 30 days of the discharge date of the index admission, or 0 for no readmission within 30 days.
Transitional care program status was determined whether or not the individual received the transitional care program for each index admission. This analysis allowed us to model repeated measures of index admissions as a function of the project period and whether the patient was seen by the GMED CPS during the index admission. The patient identifier was used as a cluster variable in the GEE analysis. Inverse propensity scores of receiving GMED at the index admission were adjusted as weights in the GEE analysis to minimize confounding and, hence, to strengthen the causal interpretation of the effect of the transitional care program. If there was ≥ 1 index admission, the GMED status (yes vs no) at the initial index admission was used as the dependent variable to calculate propensity scores. The propensity scores of transitional care program status were derived from the logistic regression analysis that modeled the log-odds of receiving the transitional care program at the index admission as a linear function of age, CCI, frailty, and prior hospitalization during the 1-year period prior to the index admission.
Related: Development and Implementation of a Geriatric Walking Clinic
Results
The GMED CPS saw 435 patients during the project period; 47 (10.8%) died prior to 30 days and were excluded, leaving 388 patients who received the transitional care program included in this evaluation.
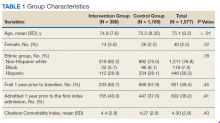
Data from the CPS-patient interviews and chart reviews were available for 378 of the 388 patients (Table 2). Patients were primarily male, non-Hispanic white, with a high school education. More than half (65%) the patients were admitted for a new diagnosis or clinical condition.
The 30-day readmission rate was 15.6% for the transitional care program group and 21.9% for the comparison group. Three hundred seventy-one patients received the transitional care program only once, 16 patients received the transitional care program twice (ie, they had 2 index admissions during the study period and received the intervention both times), and 1 patient received the transitional care program 3 times.
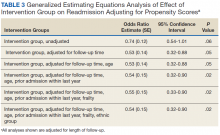
In an unadjusted GEE model, the odds ratio (OR) for readmission in the transitional care program group was 0.74 (95% CI, 0.54-1.0, P = .06) compared with the usual care group (Table 3).
Thirty-five percent of patients had ≥ 1 CPS-recommended change in their treatment at the time of the inpatient admission (Table 4).
Discussion
We developed a transitional care program for hospitalized older veterans to improve the transition from hospital to home. After adjusting for clinical factors, GMED was associated with 26% lower odds of readmission within 30 days of discharge compared with that of the control group. The GMED CPS made changes to the medical regimen both during the inpatient admission as well as after discharge to correct medication errors and educate patients.
In addition, GMED led to a reduction in the number of prescribed medications, which impacts inappropriate polypharmacy—a significant problem in older adults, which contributes to ADEs.21 Our intervention was patient centered, as all decisions and education regarding medication management were tailored to each patient, taking into account medical and psychosocial factors.
Studies of similar programs have shown that a pharmacist-based program can improve outcomes in patients transitioning from hospital to home. A meta-analysis of 19 studies that evaluated the effectiveness of pharmacy-led medication reconciliation interventions at the time of a care transition showed that compared with usual care a pharmacist intervention led to reduced medication discrepancies.22 In this meta-analysis, medication discrepancies of higher clinical impact were more easily identified through pharmacy-led interventions than with usual care, suggesting improved safety. Although not all studies have shown a clear reduction in readmission rates or other health care utilization, the addition of clinical pharmacist services in the care of inpatients has generally resulted in improved care with no evidence of harm.23
Based on these findings and collaboration with another GRECC, we designed our program to focus on older adults with polypharmacy, cognitive impairment, high-risk medication usage, and/or a history of high health care use.9 Our findings add to the growing body of evidence that a CPS-led transitional care program results in reduced polypharmacy and reduced unnecessary hospital readmissions. Further, our findings have demonstrated the effectiveness of this type of program in a practical, clinical setting with veteran patients.
At the time of project inception, we believed that the majority of our interventions would occur postdischarge. We were somewhat surprised that a major component of GMED was suggested interventions by our pharmacist at the time of admission. We believe that because the CPS made suggestions during admission, we prevented postdischarge ADEs. A frequent intervention corrected the medication reconciliation on file at admission. This finding also was seen in another study by Gleason and colleagues, which examined medication errors at admission for 651 adult medicine inpatients.24 This study found that more than one-third of patients had medication reconciliation errors. Further, older age (≥ 65 years) was associated with increased odds of medication errors in this study.
Of note, a survey of hospital-based pharmacists indicated medication reconciliation is the most important role of the pharmacist in improving care transitions.25 The pharmacists stated that detection of errors at the time of admission is very important. The pharmacists further reported that additional education and counseling for patients with poor understanding of their medications was also important. Our findings support these findings and the use of a pharmacist as part of the medical team to improve medication reconciliation and education.
Limitations
A limitation of GMED is that we monitored only admissions to our hospital; therefore, we did not account for any hospitalizations that may have occurred outside the STVHCS. Another limitation is that this was not a randomized controlled trial, and we used a convenience sample of patients who met our criteria for eligibility but were not seen due to time constraints. This introduces potential bias such that patients admitted and discharged on nights or weekends when the CPS was not available were not included in the transitional care program group, and these patients may fundamentally differ from those admitted and discharged Monday through Friday.
However, Khanna and colleagues found that night or weekend admission was not associated with 30-day readmission or other worse outcomes (such as length of stay, 30-day emergency department visit, or intensive care unit transfer) in 857 general medicine admissions at a tertiary care hospital.26 Every effort was made to include as many eligible patients as possible in the transitional program group, and we were able to demonstrate that the patients in the 2 groups were similar. Frailty and prior hospital admission were more prevalent, although not significantly so, in the transitional program group, suggesting that any selection bias would have actually attenuated—not enhanced—the observed effect of the transitional program. Although the transitional program group patients were slightly younger by 0.3 years, they were similar in frailty status and CCI score.
Conclusion
The GMED program was associated with reduced 30-day hospital readmission, discontinuation of unnecessary medications, and corrected medication errors and discrepancies. We propose that a CPS-based transitional care program can improve the quality of care for older patients being discharged to home.
Acknowledgments
Supported by funding from the Veterans Health Administration T21 Non-Institutional Long-Term Care Initiative and VA Office of Rural Health and the San Antonio Geriatrics Research, Education, and Clinical Center. The sponsor did not have any role in the design, methods, data collection, or analysis, and preparation.
Author Contributions
R. Rottman-Sagebiel developed the transitional program concept and design and executed the program implementation, interpretation of data, and preparation of the manuscript. S. Pastewait, N. Cupples, A. Conde, M. Moris, and E. Gonzalez assisted with program design and implementation. S. Cope assisted with interpretation of data and preparation of the manuscript. H. Braden assisted with interpretation of data. D. MacCarthy assisted with data management and statistical analysis. C. Wang and S. Espinoza developed the program concept and design, performed statistical analysis and interpretation of data, and helped prepare the manuscript.
Advances in Geriatrics
Advances in Geriatrics features articles focused on quality improvement/quality assurance initiatives, pilot studies, best practices, research, patient education, and patient-centered care written by health care providers associated with Veteran Health Administration Geriatric Research Education and Clinical Centers. Interested authors can submit articles at editorialmanager.com/fedprac or send a brief 2 to 3 sentence abstract to fedprac@mdedge.com for feedback and publication recommendations.
1. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010.
2. Merle L, Laroche ML, Dantoine T, Charmes JP. Predicting and preventing adverse drug reactions in the very old. Drugs Aging. 2005;22(5):375-392.
3. Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64(2):183-199.
4. Coleman EA, Min Sj, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465.
5. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516.
6. Price Waterhouse Coopers Health Research Institute. The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhouse Coopers Health Research Institute; 2008.
7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167.
9. Paquin AM, Salow M, Rudolph JL. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571-577.
10. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
11. Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477-485.
12. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027.
14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
15. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729-735.
16. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
17. Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Int Med. 2002;162(20):2333-2341.
18. Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty? J Am Geriatr Soc. 2004;52(8):1313-1320.
19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727.
20. Pugh JA, Wang CP, Espinoza SE, et al. Influence of frailty‐related diagnoses, high‐risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. J Am Geriatr Soc. 2014;62(2):291-298.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
22. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy‐led medication reconciliation programmes at hospital transitions: a systematic review and meta‐analysis. J Clin Pharm Ther. 2016;41(2):128-144.
23. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Int Med. 2006;166(9):955-964.
24. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
25. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother. 2012;46(9):1152-1159.
26. Khanna R, Wachsberg K, Marouni A, Feinglass J, Williams MV, Wayne DB. The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10-14.
Medication reconciliation and patient education during admission and after discharge helped older patients remain independent at home.
Medication reconciliation and patient education during admission and after discharge helped older patients remain independent at home.
There will be 53 million older adults in the US by 2020.1 Increasing age often brings medical comorbidities and prescriptions for multiple medications. An increasing number of prescribed medications combined with age-related changes in the ability to metabolize drugs makes older adults highly vulnerable to adverse drug events (ADEs).2 In addition, older adults often have difficulty self-managing their medications and adhering to prescribed regimens.3 As a result, ADEs can lead to poor health outcomes, including hospitalizations, in older adults.
Medication errors and ADEs are particularly common during transitions from hospital to home and can lead to unnecessary readmissions,a major cause of wasteful health care spending in the US.4,5 More than $25 billion are estimated to be spent annually on hospital readmissions, with Medicare picking up the bill for $17 billion of the total.6,7 Researchers have found that the majority of ADEs following hospital discharge are either entirely preventable or at least ameliorable (ie, the negative impact or harm resulting from the ADE could have been reduced).8
To address these issues, we undertook a clinical demonstration project that implemented a new transitional care program to improve the quality of care for older veterans transitioning from the Audie L. Murphy Veterans Memorial Hospital of the South Texas Veterans Health Care System (STVHCS) in San Antonio to home. The Geriatrics Medication Education at Discharge project (GMED) falls under the auspices of the San Antonio Geriatrics Research Education and Clinical Center (GRECC). Clinical demonstration projects are mandated for US Department of Veterans Affairs (VA) GRECCs to create and promote innovative models of care for older veterans. Dissemination of successful clinical demonstration projects to other VA sites is strongly encouraged. The GMED program was modeled after the Boston GRECC Pharmacological Intervention in Late Life (PILL) program.9 The PILL program, which focuses on serving older veterans with cognitive impairment, demonstrated that a postdischarge pharmacist telephone visit for medication reconciliation leads to a reduction in readmission within 60 days of discharge.9 The goals of the GMED program were to reduce polypharmacy, inappropriate prescribing and 30-day readmissions.
Methods
The project was conducted when a full-time clinical pharmacy specialist (CPS) was available (May-September 2013 and April 2014-March 2015). This project was approved as nonresearch/quality improvement by the University of Texas Health Science Center Institutional Review Board, which serves the STVHCS. Consent was not required.
Eligibility
Patients were identified via a daily hospital database query of all adults aged ≥ 65 years admitted to the hospital through Inpatient Medicine, Neurology, or Cardiology services within the prior 24 hours. Patients meeting any of the following criteria based on review of the Computerized Patient Record System (CPRS) by the team geriatrician and CPS were considered eligible: (1) aged ≥ 70 years prescribed ≥ 12 outpatient medications; (2) aged ≥ 65 years with a medical history of dementia; (3) aged ≥ 65 years prescribed outpatient medications meeting Beers criteria10; (4) age ≥ 65 years with ≥ 2 hospital admissions (including the current, index admission) within the past calendar year; or (5) aged ≥ 65 years with ≥ 3 emergency department visits within the past calendar year. For the first polypharmacy criterion, patients aged ≥ 70 years were selected instead of aged ≥ 65 years so as not to exceed the capacity of 1 CPS. Twelve or more medications were used as a cutoff for polypharmacy based on prior quality improvement information gathered from our VA geriatrics clinic examining the average number of medications taken by older veterans in the outpatient setting.
Related: Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Patients were excluded if they were expected to be discharged to any facility where the patient and/or the caregiver were not primarily responsible for medication administration after discharge. Patients who met eligibility criteria but were not seen by the transitional program pharmacist (due to staff capacity) were included in this analysis as a convenience comparison group of patients who received usual care. Patients were not randomized. All communication occurred in English, but this project did not exclude patients with limited English proficiency.
A program support assistant conducted the daily query of the hospital database. The pharmacist conducted the chart review to determine eligibility and delivered the intervention. Eligible patients were selected at random for the intervention with the intention of providing the intervention to as many veterans as possible.
The GMED Intervention
The GMED program included 2 phases, which were both conducted by a CPS with oversight from a senior CPS with geriatric pharmacology expertise and an internist/geriatrician.
The first phase of the transitional care program included an individual, face-to-face meeting between the CPS and the patient during the hospitalization. If a veteran was not present in the room at the time of an attempted visit, the pharmacist made 2 additional attempts (3 total) to include the patient in the transitional care program during the hospitalization.
The second component of the transitional care program included a telephone visit within 2 to 3 days of discharge, conducted by the same CPS who performed the face-to-face visit. The purpose of the telephone visit was to perform medication reconciliation, identify and rectify medication errors, provide further patient education, and assist in facilitating appropriate follow-up by the patient’s primary care provider (PCP), if required. At a minimum, veterans were asked a series of questions pertaining to their concerns about medication regimens, receipt of newly prescribed medications at discharge, additional education regarding medications after the CPS encounter during hospitalization, and whether the veteran required assistance with the medication regimen in the home setting. Follow-up questions were asked as needed to clarify and identify potential medication problems. All information from this telephone encounter was communicated to the PCP through CPRS documentation and by telephone as needed.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Data Collection
A standardized questionnaire was used prospectively for patients in the transitional care program group to assess patient education, primary residence, presence of a caregiver, fall history, medication adherence, and cognitive status (using Mini-Cog).13 Additional information (patient age, number of outpatient medications prior to and following the admission, presence of Beers criteria outpatient medications prior to and following the admission, new outpatient prescriptions, and changes to existing prescriptions as a result of the hospitalization) was gathered prospectively from patient interviews or from chart review.
For patients included in the comparison group, a retrospective administrative chart review was conducted to collect information such as age, sex, ethnic group, admission within 1 year prior to index admission, frailty, and Charlson Comorbidity Index (CCI) score, a method of categorizing comorbidities of patients based on the diagnosis codes found in administrative data.14 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient (0 indicates no comorbidities; higher scores predict greater risk of mortality or increased resource use).
We used the index developed from 17 disease categories. The range for CCI was 0 to 25. Frailty was defined as the presence of any of the following frailty-related diagnoses: anemia; fall, head injury, other injury; coagulopathy; electrolyte disturbance; or gait disorder. These diagnoses are either primary frailty characteristics within the frailty phenotype or have been shown in prior studies to be associated with the frailty phenotype.15-18 While more widely accepted frailty definitions exist,these other definitions require direct examination of the patient and could not be used in this project because we did not directly interact with the comparison group.16,19 The frailty definition used has been previously identified as a predictor of health care utilization and 30-day readmission in a veteran population.20 Whether or not the CPS detected a postdischarge medication error was recorded. All CPS recommendations were documented.
An index admission was defined as a hospital admission that occurred during the project period. Thirty-day readmission was defined as a hospital admission that occurred within 30 days of the discharge date of an index admission. Each index admission was considered individually for readmission (yes vs no) even if it occurred in the same patient over the project period. A 30-day readmission was not considered an index admission. An admission that occurred after a 30-day readmission was considered a subsequent index admission. Patients who died in the hospital were not included in this analysis, as they would not have participated in the entire intervention.
Statistical Analysis
We compared characteristics between patients who received GMED and patients who never received GMED (comparison group). Generalized estimating equations (GEE) were used to determine whether the rate of 30-day readmission (yes vs no) in the transitional care program group differed from that of the comparison group. In our GEE analysis, we assumed a binomial distribution and the logit link to model the log-odds of readmission as a linear function of transitional care program status (yes vs no) and other covariates, including age, frailty, hospital admission within 1 year prior to the index admission, and CCI score as covariates. Thirty-day readmission status associated with each index admission was coded as 1 for a readmission within 30 days of the discharge date of the index admission, or 0 for no readmission within 30 days.
Transitional care program status was determined whether or not the individual received the transitional care program for each index admission. This analysis allowed us to model repeated measures of index admissions as a function of the project period and whether the patient was seen by the GMED CPS during the index admission. The patient identifier was used as a cluster variable in the GEE analysis. Inverse propensity scores of receiving GMED at the index admission were adjusted as weights in the GEE analysis to minimize confounding and, hence, to strengthen the causal interpretation of the effect of the transitional care program. If there was ≥ 1 index admission, the GMED status (yes vs no) at the initial index admission was used as the dependent variable to calculate propensity scores. The propensity scores of transitional care program status were derived from the logistic regression analysis that modeled the log-odds of receiving the transitional care program at the index admission as a linear function of age, CCI, frailty, and prior hospitalization during the 1-year period prior to the index admission.
Related: Development and Implementation of a Geriatric Walking Clinic
Results
The GMED CPS saw 435 patients during the project period; 47 (10.8%) died prior to 30 days and were excluded, leaving 388 patients who received the transitional care program included in this evaluation.
Data from the CPS-patient interviews and chart reviews were available for 378 of the 388 patients (Table 2). Patients were primarily male, non-Hispanic white, with a high school education. More than half (65%) the patients were admitted for a new diagnosis or clinical condition.
The 30-day readmission rate was 15.6% for the transitional care program group and 21.9% for the comparison group. Three hundred seventy-one patients received the transitional care program only once, 16 patients received the transitional care program twice (ie, they had 2 index admissions during the study period and received the intervention both times), and 1 patient received the transitional care program 3 times.
In an unadjusted GEE model, the odds ratio (OR) for readmission in the transitional care program group was 0.74 (95% CI, 0.54-1.0, P = .06) compared with the usual care group (Table 3).
Thirty-five percent of patients had ≥ 1 CPS-recommended change in their treatment at the time of the inpatient admission (Table 4).
Discussion
We developed a transitional care program for hospitalized older veterans to improve the transition from hospital to home. After adjusting for clinical factors, GMED was associated with 26% lower odds of readmission within 30 days of discharge compared with that of the control group. The GMED CPS made changes to the medical regimen both during the inpatient admission as well as after discharge to correct medication errors and educate patients.
In addition, GMED led to a reduction in the number of prescribed medications, which impacts inappropriate polypharmacy—a significant problem in older adults, which contributes to ADEs.21 Our intervention was patient centered, as all decisions and education regarding medication management were tailored to each patient, taking into account medical and psychosocial factors.
Studies of similar programs have shown that a pharmacist-based program can improve outcomes in patients transitioning from hospital to home. A meta-analysis of 19 studies that evaluated the effectiveness of pharmacy-led medication reconciliation interventions at the time of a care transition showed that compared with usual care a pharmacist intervention led to reduced medication discrepancies.22 In this meta-analysis, medication discrepancies of higher clinical impact were more easily identified through pharmacy-led interventions than with usual care, suggesting improved safety. Although not all studies have shown a clear reduction in readmission rates or other health care utilization, the addition of clinical pharmacist services in the care of inpatients has generally resulted in improved care with no evidence of harm.23
Based on these findings and collaboration with another GRECC, we designed our program to focus on older adults with polypharmacy, cognitive impairment, high-risk medication usage, and/or a history of high health care use.9 Our findings add to the growing body of evidence that a CPS-led transitional care program results in reduced polypharmacy and reduced unnecessary hospital readmissions. Further, our findings have demonstrated the effectiveness of this type of program in a practical, clinical setting with veteran patients.
At the time of project inception, we believed that the majority of our interventions would occur postdischarge. We were somewhat surprised that a major component of GMED was suggested interventions by our pharmacist at the time of admission. We believe that because the CPS made suggestions during admission, we prevented postdischarge ADEs. A frequent intervention corrected the medication reconciliation on file at admission. This finding also was seen in another study by Gleason and colleagues, which examined medication errors at admission for 651 adult medicine inpatients.24 This study found that more than one-third of patients had medication reconciliation errors. Further, older age (≥ 65 years) was associated with increased odds of medication errors in this study.
Of note, a survey of hospital-based pharmacists indicated medication reconciliation is the most important role of the pharmacist in improving care transitions.25 The pharmacists stated that detection of errors at the time of admission is very important. The pharmacists further reported that additional education and counseling for patients with poor understanding of their medications was also important. Our findings support these findings and the use of a pharmacist as part of the medical team to improve medication reconciliation and education.
Limitations
A limitation of GMED is that we monitored only admissions to our hospital; therefore, we did not account for any hospitalizations that may have occurred outside the STVHCS. Another limitation is that this was not a randomized controlled trial, and we used a convenience sample of patients who met our criteria for eligibility but were not seen due to time constraints. This introduces potential bias such that patients admitted and discharged on nights or weekends when the CPS was not available were not included in the transitional care program group, and these patients may fundamentally differ from those admitted and discharged Monday through Friday.
However, Khanna and colleagues found that night or weekend admission was not associated with 30-day readmission or other worse outcomes (such as length of stay, 30-day emergency department visit, or intensive care unit transfer) in 857 general medicine admissions at a tertiary care hospital.26 Every effort was made to include as many eligible patients as possible in the transitional program group, and we were able to demonstrate that the patients in the 2 groups were similar. Frailty and prior hospital admission were more prevalent, although not significantly so, in the transitional program group, suggesting that any selection bias would have actually attenuated—not enhanced—the observed effect of the transitional program. Although the transitional program group patients were slightly younger by 0.3 years, they were similar in frailty status and CCI score.
Conclusion
The GMED program was associated with reduced 30-day hospital readmission, discontinuation of unnecessary medications, and corrected medication errors and discrepancies. We propose that a CPS-based transitional care program can improve the quality of care for older patients being discharged to home.
Acknowledgments
Supported by funding from the Veterans Health Administration T21 Non-Institutional Long-Term Care Initiative and VA Office of Rural Health and the San Antonio Geriatrics Research, Education, and Clinical Center. The sponsor did not have any role in the design, methods, data collection, or analysis, and preparation.
Author Contributions
R. Rottman-Sagebiel developed the transitional program concept and design and executed the program implementation, interpretation of data, and preparation of the manuscript. S. Pastewait, N. Cupples, A. Conde, M. Moris, and E. Gonzalez assisted with program design and implementation. S. Cope assisted with interpretation of data and preparation of the manuscript. H. Braden assisted with interpretation of data. D. MacCarthy assisted with data management and statistical analysis. C. Wang and S. Espinoza developed the program concept and design, performed statistical analysis and interpretation of data, and helped prepare the manuscript.
Advances in Geriatrics
Advances in Geriatrics features articles focused on quality improvement/quality assurance initiatives, pilot studies, best practices, research, patient education, and patient-centered care written by health care providers associated with Veteran Health Administration Geriatric Research Education and Clinical Centers. Interested authors can submit articles at editorialmanager.com/fedprac or send a brief 2 to 3 sentence abstract to fedprac@mdedge.com for feedback and publication recommendations.
There will be 53 million older adults in the US by 2020.1 Increasing age often brings medical comorbidities and prescriptions for multiple medications. An increasing number of prescribed medications combined with age-related changes in the ability to metabolize drugs makes older adults highly vulnerable to adverse drug events (ADEs).2 In addition, older adults often have difficulty self-managing their medications and adhering to prescribed regimens.3 As a result, ADEs can lead to poor health outcomes, including hospitalizations, in older adults.
Medication errors and ADEs are particularly common during transitions from hospital to home and can lead to unnecessary readmissions,a major cause of wasteful health care spending in the US.4,5 More than $25 billion are estimated to be spent annually on hospital readmissions, with Medicare picking up the bill for $17 billion of the total.6,7 Researchers have found that the majority of ADEs following hospital discharge are either entirely preventable or at least ameliorable (ie, the negative impact or harm resulting from the ADE could have been reduced).8
To address these issues, we undertook a clinical demonstration project that implemented a new transitional care program to improve the quality of care for older veterans transitioning from the Audie L. Murphy Veterans Memorial Hospital of the South Texas Veterans Health Care System (STVHCS) in San Antonio to home. The Geriatrics Medication Education at Discharge project (GMED) falls under the auspices of the San Antonio Geriatrics Research Education and Clinical Center (GRECC). Clinical demonstration projects are mandated for US Department of Veterans Affairs (VA) GRECCs to create and promote innovative models of care for older veterans. Dissemination of successful clinical demonstration projects to other VA sites is strongly encouraged. The GMED program was modeled after the Boston GRECC Pharmacological Intervention in Late Life (PILL) program.9 The PILL program, which focuses on serving older veterans with cognitive impairment, demonstrated that a postdischarge pharmacist telephone visit for medication reconciliation leads to a reduction in readmission within 60 days of discharge.9 The goals of the GMED program were to reduce polypharmacy, inappropriate prescribing and 30-day readmissions.
Methods
The project was conducted when a full-time clinical pharmacy specialist (CPS) was available (May-September 2013 and April 2014-March 2015). This project was approved as nonresearch/quality improvement by the University of Texas Health Science Center Institutional Review Board, which serves the STVHCS. Consent was not required.
Eligibility
Patients were identified via a daily hospital database query of all adults aged ≥ 65 years admitted to the hospital through Inpatient Medicine, Neurology, or Cardiology services within the prior 24 hours. Patients meeting any of the following criteria based on review of the Computerized Patient Record System (CPRS) by the team geriatrician and CPS were considered eligible: (1) aged ≥ 70 years prescribed ≥ 12 outpatient medications; (2) aged ≥ 65 years with a medical history of dementia; (3) aged ≥ 65 years prescribed outpatient medications meeting Beers criteria10; (4) age ≥ 65 years with ≥ 2 hospital admissions (including the current, index admission) within the past calendar year; or (5) aged ≥ 65 years with ≥ 3 emergency department visits within the past calendar year. For the first polypharmacy criterion, patients aged ≥ 70 years were selected instead of aged ≥ 65 years so as not to exceed the capacity of 1 CPS. Twelve or more medications were used as a cutoff for polypharmacy based on prior quality improvement information gathered from our VA geriatrics clinic examining the average number of medications taken by older veterans in the outpatient setting.
Related: Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Patients were excluded if they were expected to be discharged to any facility where the patient and/or the caregiver were not primarily responsible for medication administration after discharge. Patients who met eligibility criteria but were not seen by the transitional program pharmacist (due to staff capacity) were included in this analysis as a convenience comparison group of patients who received usual care. Patients were not randomized. All communication occurred in English, but this project did not exclude patients with limited English proficiency.
A program support assistant conducted the daily query of the hospital database. The pharmacist conducted the chart review to determine eligibility and delivered the intervention. Eligible patients were selected at random for the intervention with the intention of providing the intervention to as many veterans as possible.
The GMED Intervention
The GMED program included 2 phases, which were both conducted by a CPS with oversight from a senior CPS with geriatric pharmacology expertise and an internist/geriatrician.
The first phase of the transitional care program included an individual, face-to-face meeting between the CPS and the patient during the hospitalization. If a veteran was not present in the room at the time of an attempted visit, the pharmacist made 2 additional attempts (3 total) to include the patient in the transitional care program during the hospitalization.
The second component of the transitional care program included a telephone visit within 2 to 3 days of discharge, conducted by the same CPS who performed the face-to-face visit. The purpose of the telephone visit was to perform medication reconciliation, identify and rectify medication errors, provide further patient education, and assist in facilitating appropriate follow-up by the patient’s primary care provider (PCP), if required. At a minimum, veterans were asked a series of questions pertaining to their concerns about medication regimens, receipt of newly prescribed medications at discharge, additional education regarding medications after the CPS encounter during hospitalization, and whether the veteran required assistance with the medication regimen in the home setting. Follow-up questions were asked as needed to clarify and identify potential medication problems. All information from this telephone encounter was communicated to the PCP through CPRS documentation and by telephone as needed.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Data Collection
A standardized questionnaire was used prospectively for patients in the transitional care program group to assess patient education, primary residence, presence of a caregiver, fall history, medication adherence, and cognitive status (using Mini-Cog).13 Additional information (patient age, number of outpatient medications prior to and following the admission, presence of Beers criteria outpatient medications prior to and following the admission, new outpatient prescriptions, and changes to existing prescriptions as a result of the hospitalization) was gathered prospectively from patient interviews or from chart review.
For patients included in the comparison group, a retrospective administrative chart review was conducted to collect information such as age, sex, ethnic group, admission within 1 year prior to index admission, frailty, and Charlson Comorbidity Index (CCI) score, a method of categorizing comorbidities of patients based on the diagnosis codes found in administrative data.14 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient (0 indicates no comorbidities; higher scores predict greater risk of mortality or increased resource use).
We used the index developed from 17 disease categories. The range for CCI was 0 to 25. Frailty was defined as the presence of any of the following frailty-related diagnoses: anemia; fall, head injury, other injury; coagulopathy; electrolyte disturbance; or gait disorder. These diagnoses are either primary frailty characteristics within the frailty phenotype or have been shown in prior studies to be associated with the frailty phenotype.15-18 While more widely accepted frailty definitions exist,these other definitions require direct examination of the patient and could not be used in this project because we did not directly interact with the comparison group.16,19 The frailty definition used has been previously identified as a predictor of health care utilization and 30-day readmission in a veteran population.20 Whether or not the CPS detected a postdischarge medication error was recorded. All CPS recommendations were documented.
An index admission was defined as a hospital admission that occurred during the project period. Thirty-day readmission was defined as a hospital admission that occurred within 30 days of the discharge date of an index admission. Each index admission was considered individually for readmission (yes vs no) even if it occurred in the same patient over the project period. A 30-day readmission was not considered an index admission. An admission that occurred after a 30-day readmission was considered a subsequent index admission. Patients who died in the hospital were not included in this analysis, as they would not have participated in the entire intervention.
Statistical Analysis
We compared characteristics between patients who received GMED and patients who never received GMED (comparison group). Generalized estimating equations (GEE) were used to determine whether the rate of 30-day readmission (yes vs no) in the transitional care program group differed from that of the comparison group. In our GEE analysis, we assumed a binomial distribution and the logit link to model the log-odds of readmission as a linear function of transitional care program status (yes vs no) and other covariates, including age, frailty, hospital admission within 1 year prior to the index admission, and CCI score as covariates. Thirty-day readmission status associated with each index admission was coded as 1 for a readmission within 30 days of the discharge date of the index admission, or 0 for no readmission within 30 days.
Transitional care program status was determined whether or not the individual received the transitional care program for each index admission. This analysis allowed us to model repeated measures of index admissions as a function of the project period and whether the patient was seen by the GMED CPS during the index admission. The patient identifier was used as a cluster variable in the GEE analysis. Inverse propensity scores of receiving GMED at the index admission were adjusted as weights in the GEE analysis to minimize confounding and, hence, to strengthen the causal interpretation of the effect of the transitional care program. If there was ≥ 1 index admission, the GMED status (yes vs no) at the initial index admission was used as the dependent variable to calculate propensity scores. The propensity scores of transitional care program status were derived from the logistic regression analysis that modeled the log-odds of receiving the transitional care program at the index admission as a linear function of age, CCI, frailty, and prior hospitalization during the 1-year period prior to the index admission.
Related: Development and Implementation of a Geriatric Walking Clinic
Results
The GMED CPS saw 435 patients during the project period; 47 (10.8%) died prior to 30 days and were excluded, leaving 388 patients who received the transitional care program included in this evaluation.
Data from the CPS-patient interviews and chart reviews were available for 378 of the 388 patients (Table 2). Patients were primarily male, non-Hispanic white, with a high school education. More than half (65%) the patients were admitted for a new diagnosis or clinical condition.
The 30-day readmission rate was 15.6% for the transitional care program group and 21.9% for the comparison group. Three hundred seventy-one patients received the transitional care program only once, 16 patients received the transitional care program twice (ie, they had 2 index admissions during the study period and received the intervention both times), and 1 patient received the transitional care program 3 times.
In an unadjusted GEE model, the odds ratio (OR) for readmission in the transitional care program group was 0.74 (95% CI, 0.54-1.0, P = .06) compared with the usual care group (Table 3).
Thirty-five percent of patients had ≥ 1 CPS-recommended change in their treatment at the time of the inpatient admission (Table 4).
Discussion
We developed a transitional care program for hospitalized older veterans to improve the transition from hospital to home. After adjusting for clinical factors, GMED was associated with 26% lower odds of readmission within 30 days of discharge compared with that of the control group. The GMED CPS made changes to the medical regimen both during the inpatient admission as well as after discharge to correct medication errors and educate patients.
In addition, GMED led to a reduction in the number of prescribed medications, which impacts inappropriate polypharmacy—a significant problem in older adults, which contributes to ADEs.21 Our intervention was patient centered, as all decisions and education regarding medication management were tailored to each patient, taking into account medical and psychosocial factors.
Studies of similar programs have shown that a pharmacist-based program can improve outcomes in patients transitioning from hospital to home. A meta-analysis of 19 studies that evaluated the effectiveness of pharmacy-led medication reconciliation interventions at the time of a care transition showed that compared with usual care a pharmacist intervention led to reduced medication discrepancies.22 In this meta-analysis, medication discrepancies of higher clinical impact were more easily identified through pharmacy-led interventions than with usual care, suggesting improved safety. Although not all studies have shown a clear reduction in readmission rates or other health care utilization, the addition of clinical pharmacist services in the care of inpatients has generally resulted in improved care with no evidence of harm.23
Based on these findings and collaboration with another GRECC, we designed our program to focus on older adults with polypharmacy, cognitive impairment, high-risk medication usage, and/or a history of high health care use.9 Our findings add to the growing body of evidence that a CPS-led transitional care program results in reduced polypharmacy and reduced unnecessary hospital readmissions. Further, our findings have demonstrated the effectiveness of this type of program in a practical, clinical setting with veteran patients.
At the time of project inception, we believed that the majority of our interventions would occur postdischarge. We were somewhat surprised that a major component of GMED was suggested interventions by our pharmacist at the time of admission. We believe that because the CPS made suggestions during admission, we prevented postdischarge ADEs. A frequent intervention corrected the medication reconciliation on file at admission. This finding also was seen in another study by Gleason and colleagues, which examined medication errors at admission for 651 adult medicine inpatients.24 This study found that more than one-third of patients had medication reconciliation errors. Further, older age (≥ 65 years) was associated with increased odds of medication errors in this study.
Of note, a survey of hospital-based pharmacists indicated medication reconciliation is the most important role of the pharmacist in improving care transitions.25 The pharmacists stated that detection of errors at the time of admission is very important. The pharmacists further reported that additional education and counseling for patients with poor understanding of their medications was also important. Our findings support these findings and the use of a pharmacist as part of the medical team to improve medication reconciliation and education.
Limitations
A limitation of GMED is that we monitored only admissions to our hospital; therefore, we did not account for any hospitalizations that may have occurred outside the STVHCS. Another limitation is that this was not a randomized controlled trial, and we used a convenience sample of patients who met our criteria for eligibility but were not seen due to time constraints. This introduces potential bias such that patients admitted and discharged on nights or weekends when the CPS was not available were not included in the transitional care program group, and these patients may fundamentally differ from those admitted and discharged Monday through Friday.
However, Khanna and colleagues found that night or weekend admission was not associated with 30-day readmission or other worse outcomes (such as length of stay, 30-day emergency department visit, or intensive care unit transfer) in 857 general medicine admissions at a tertiary care hospital.26 Every effort was made to include as many eligible patients as possible in the transitional program group, and we were able to demonstrate that the patients in the 2 groups were similar. Frailty and prior hospital admission were more prevalent, although not significantly so, in the transitional program group, suggesting that any selection bias would have actually attenuated—not enhanced—the observed effect of the transitional program. Although the transitional program group patients were slightly younger by 0.3 years, they were similar in frailty status and CCI score.
Conclusion
The GMED program was associated with reduced 30-day hospital readmission, discontinuation of unnecessary medications, and corrected medication errors and discrepancies. We propose that a CPS-based transitional care program can improve the quality of care for older patients being discharged to home.
Acknowledgments
Supported by funding from the Veterans Health Administration T21 Non-Institutional Long-Term Care Initiative and VA Office of Rural Health and the San Antonio Geriatrics Research, Education, and Clinical Center. The sponsor did not have any role in the design, methods, data collection, or analysis, and preparation.
Author Contributions
R. Rottman-Sagebiel developed the transitional program concept and design and executed the program implementation, interpretation of data, and preparation of the manuscript. S. Pastewait, N. Cupples, A. Conde, M. Moris, and E. Gonzalez assisted with program design and implementation. S. Cope assisted with interpretation of data and preparation of the manuscript. H. Braden assisted with interpretation of data. D. MacCarthy assisted with data management and statistical analysis. C. Wang and S. Espinoza developed the program concept and design, performed statistical analysis and interpretation of data, and helped prepare the manuscript.
Advances in Geriatrics
Advances in Geriatrics features articles focused on quality improvement/quality assurance initiatives, pilot studies, best practices, research, patient education, and patient-centered care written by health care providers associated with Veteran Health Administration Geriatric Research Education and Clinical Centers. Interested authors can submit articles at editorialmanager.com/fedprac or send a brief 2 to 3 sentence abstract to fedprac@mdedge.com for feedback and publication recommendations.
1. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010.
2. Merle L, Laroche ML, Dantoine T, Charmes JP. Predicting and preventing adverse drug reactions in the very old. Drugs Aging. 2005;22(5):375-392.
3. Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64(2):183-199.
4. Coleman EA, Min Sj, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465.
5. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516.
6. Price Waterhouse Coopers Health Research Institute. The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhouse Coopers Health Research Institute; 2008.
7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167.
9. Paquin AM, Salow M, Rudolph JL. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571-577.
10. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
11. Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477-485.
12. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027.
14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
15. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729-735.
16. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
17. Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Int Med. 2002;162(20):2333-2341.
18. Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty? J Am Geriatr Soc. 2004;52(8):1313-1320.
19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727.
20. Pugh JA, Wang CP, Espinoza SE, et al. Influence of frailty‐related diagnoses, high‐risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. J Am Geriatr Soc. 2014;62(2):291-298.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
22. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy‐led medication reconciliation programmes at hospital transitions: a systematic review and meta‐analysis. J Clin Pharm Ther. 2016;41(2):128-144.
23. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Int Med. 2006;166(9):955-964.
24. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
25. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother. 2012;46(9):1152-1159.
26. Khanna R, Wachsberg K, Marouni A, Feinglass J, Williams MV, Wayne DB. The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10-14.
1. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010.
2. Merle L, Laroche ML, Dantoine T, Charmes JP. Predicting and preventing adverse drug reactions in the very old. Drugs Aging. 2005;22(5):375-392.
3. Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64(2):183-199.
4. Coleman EA, Min Sj, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465.
5. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516.
6. Price Waterhouse Coopers Health Research Institute. The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhouse Coopers Health Research Institute; 2008.
7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167.
9. Paquin AM, Salow M, Rudolph JL. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571-577.
10. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
11. Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477-485.
12. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027.
14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
15. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729-735.
16. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
17. Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Int Med. 2002;162(20):2333-2341.
18. Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty? J Am Geriatr Soc. 2004;52(8):1313-1320.
19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727.
20. Pugh JA, Wang CP, Espinoza SE, et al. Influence of frailty‐related diagnoses, high‐risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. J Am Geriatr Soc. 2014;62(2):291-298.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
22. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy‐led medication reconciliation programmes at hospital transitions: a systematic review and meta‐analysis. J Clin Pharm Ther. 2016;41(2):128-144.
23. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Int Med. 2006;166(9):955-964.
24. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
25. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother. 2012;46(9):1152-1159.
26. Khanna R, Wachsberg K, Marouni A, Feinglass J, Williams MV, Wayne DB. The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10-14.