User login
A continuous cardiac murmur
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.
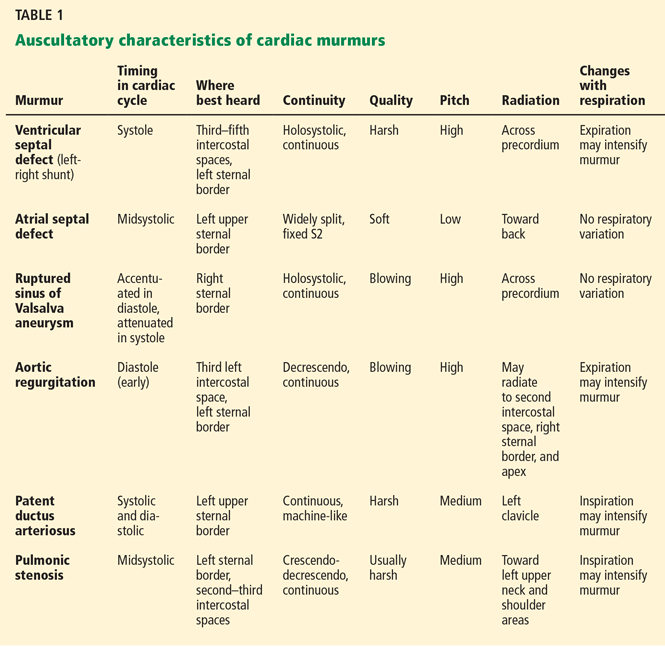
Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
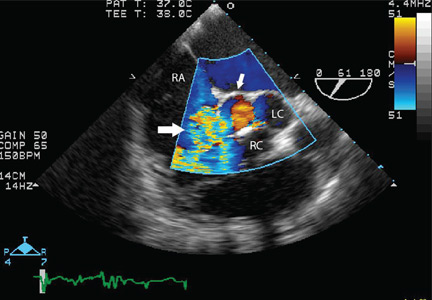
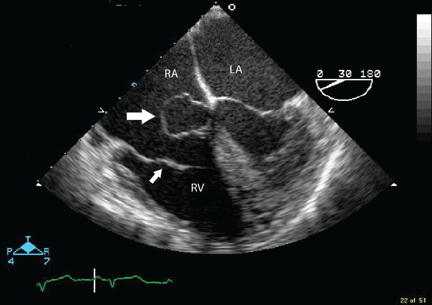
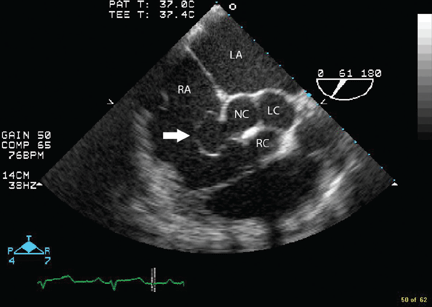
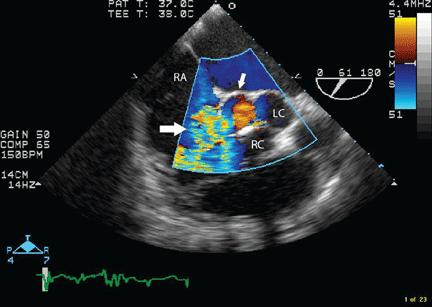
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.
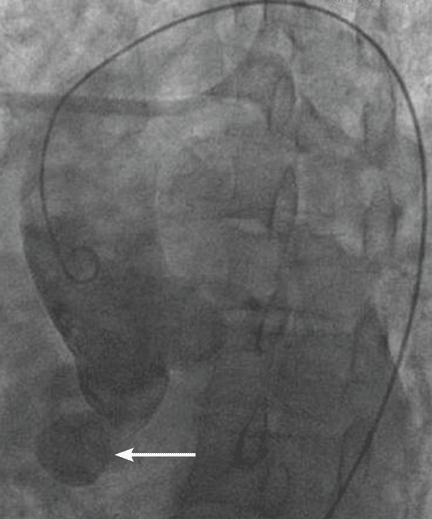
The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.
This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.

Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.



The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.

This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.

Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.



The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.

This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.