User login
Beyond Borders: Tonsillar Squamous Cell Carcinoma with Intriguing Liver Metastasis
Background
Oropharyngeal squamous cell carcinoma (OPSCC) arises in the middle pharynx, including the tonsils, base of the tongue, and surrounding tissues. While OPSCC commonly metastasizes to regional lymph nodes, distant metastases to sites like the liver are rare, occurring in about 1-4% of cases with advanced disease.
Case Presentation
A 66-year-old male presented to the emergency department with recurrent right-sided facial swelling and a two-week history of sore throat. CT imaging revealed a large right tonsillar mass extending to the base of the tongue. Further evaluation with PET scan showed hypermetabolic activity in the right tonsil, multiple hypermetabolic lymph nodes in the right neck (stations 1B, 2, 3, 4, 5), right supraclavicular fossa, and small retropharyngeal nodes. Additionally, PET scan detected a hypermetabolic lesion in the liver and focal activity at T10 suggestive of bone metastasis. Fine needle aspiration (FNA) confirmed squamous cell carcinoma. Biopsy of the liver lesion revealed metastatic squamous cell carcinoma with basaloid differentiation, positive for p40 and p63 stains. Clinical staging was T2b cN2 cM1. The patient’s case was discussed in tumor boards, leading to a treatment plan of palliative radiotherapy with radiosensitizer (weekly carboplatin/paclitaxel) due to recent myocardial infarction, precluding cisplatin or 5FU use. Post-radiotherapy, Pembrolizumab was planned based on 60% PD-L1 expression. The patient opted to forego additional systemic chemotherapy and currently receives Keytruda every three weeks.
Discussion
Liver metastases from head and neck SCC are rare, highlighting the complexity of treatment decisions in such cases. Effective management requires a multidisciplinary approach to optimize therapeutic outcomes while considering patient-specific factors and comorbidities.
Conclusions
This case underscores the challenges and poor prognosis associated with tonsillar SCC with liver metastases. It underscores the need for personalized treatment strategies tailored to the unique characteristics of each patient’s disease.
Background
Oropharyngeal squamous cell carcinoma (OPSCC) arises in the middle pharynx, including the tonsils, base of the tongue, and surrounding tissues. While OPSCC commonly metastasizes to regional lymph nodes, distant metastases to sites like the liver are rare, occurring in about 1-4% of cases with advanced disease.
Case Presentation
A 66-year-old male presented to the emergency department with recurrent right-sided facial swelling and a two-week history of sore throat. CT imaging revealed a large right tonsillar mass extending to the base of the tongue. Further evaluation with PET scan showed hypermetabolic activity in the right tonsil, multiple hypermetabolic lymph nodes in the right neck (stations 1B, 2, 3, 4, 5), right supraclavicular fossa, and small retropharyngeal nodes. Additionally, PET scan detected a hypermetabolic lesion in the liver and focal activity at T10 suggestive of bone metastasis. Fine needle aspiration (FNA) confirmed squamous cell carcinoma. Biopsy of the liver lesion revealed metastatic squamous cell carcinoma with basaloid differentiation, positive for p40 and p63 stains. Clinical staging was T2b cN2 cM1. The patient’s case was discussed in tumor boards, leading to a treatment plan of palliative radiotherapy with radiosensitizer (weekly carboplatin/paclitaxel) due to recent myocardial infarction, precluding cisplatin or 5FU use. Post-radiotherapy, Pembrolizumab was planned based on 60% PD-L1 expression. The patient opted to forego additional systemic chemotherapy and currently receives Keytruda every three weeks.
Discussion
Liver metastases from head and neck SCC are rare, highlighting the complexity of treatment decisions in such cases. Effective management requires a multidisciplinary approach to optimize therapeutic outcomes while considering patient-specific factors and comorbidities.
Conclusions
This case underscores the challenges and poor prognosis associated with tonsillar SCC with liver metastases. It underscores the need for personalized treatment strategies tailored to the unique characteristics of each patient’s disease.
Background
Oropharyngeal squamous cell carcinoma (OPSCC) arises in the middle pharynx, including the tonsils, base of the tongue, and surrounding tissues. While OPSCC commonly metastasizes to regional lymph nodes, distant metastases to sites like the liver are rare, occurring in about 1-4% of cases with advanced disease.
Case Presentation
A 66-year-old male presented to the emergency department with recurrent right-sided facial swelling and a two-week history of sore throat. CT imaging revealed a large right tonsillar mass extending to the base of the tongue. Further evaluation with PET scan showed hypermetabolic activity in the right tonsil, multiple hypermetabolic lymph nodes in the right neck (stations 1B, 2, 3, 4, 5), right supraclavicular fossa, and small retropharyngeal nodes. Additionally, PET scan detected a hypermetabolic lesion in the liver and focal activity at T10 suggestive of bone metastasis. Fine needle aspiration (FNA) confirmed squamous cell carcinoma. Biopsy of the liver lesion revealed metastatic squamous cell carcinoma with basaloid differentiation, positive for p40 and p63 stains. Clinical staging was T2b cN2 cM1. The patient’s case was discussed in tumor boards, leading to a treatment plan of palliative radiotherapy with radiosensitizer (weekly carboplatin/paclitaxel) due to recent myocardial infarction, precluding cisplatin or 5FU use. Post-radiotherapy, Pembrolizumab was planned based on 60% PD-L1 expression. The patient opted to forego additional systemic chemotherapy and currently receives Keytruda every three weeks.
Discussion
Liver metastases from head and neck SCC are rare, highlighting the complexity of treatment decisions in such cases. Effective management requires a multidisciplinary approach to optimize therapeutic outcomes while considering patient-specific factors and comorbidities.
Conclusions
This case underscores the challenges and poor prognosis associated with tonsillar SCC with liver metastases. It underscores the need for personalized treatment strategies tailored to the unique characteristics of each patient’s disease.
Leiomyosarcoma of the Penis: A Case Report and Re-Appraisal
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
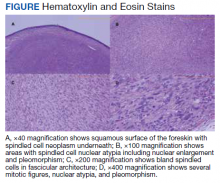
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
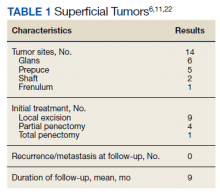
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
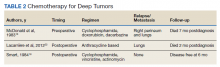
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
Factors Associated with Survival and Epidemiology of Gastrointestinal Neuroendocrine Tumors in the US Department of Veteran Affairs
Introduction
Rectal carcinoid tumors are rare but the second most common carcinoid in the gastrointestinal tract. They are usually found incidentally during endoscopic or rectal examination. They do not often produce carcinoid syndrome like manifestations although they may manifest as rectal bleeding. Rectal carcinoid patients also have a higher morbidity for other cancers such as stomach, small intestine, or secondary lung cancer.
Methods
We retrospectively explored factors associated with survival in Veterans with rectal carcinoid tumors over a ten-year period from 2007-2017 using the National Veterans Affairs Cancer Cube Registry using specific histological ICD-03 coding. We identified 1110 cases of rectal carcinoid. Chi-squared tests were used for statistical analysis.
Results
Regarding age distribution in our cohort, there were 2.61% of patients ages 40-50 group, 14.0% in the 50-60 age group, 41.5% in the 60-70 age group, and 40.7% above ages 70. There was a higher proportion of rectal cancer in stage 1 compared to other stages (86.3%). The majority of diagnoses occur after age 50 (89.8%). A higher proportion of rectal carcinoid was identified in the 60-70 years category compared to <60 and >70 years old. In the general VA population, there are 80.2% White and 12.8% Black patients. We found a higher proportion of rectal carcinoid in Black patients (47.8%) over White patients (42.8%, p=0.02), which differs significantly from the racial makeup of the VA population (12.8% Black vs 80.3% White). Looking at survival time based on diagnosis, it is notable that 82.7% of individuals survive longer than 5 years when the diagnosis is made in ages 50-60 when compared to 68.7% when the diagnosis is made between ages 60-70 (p<0.001).
Conclusions
Our data is consistent with the SEER data in that the incidence and prevalence of rectal carcinoid are higher in Black patients compared to White patients. Further analysis into reasons for this racial disparity may prove beneficial to our understanding of this malignancy in the Veteran population. Further research is needed to determine whether diagnosis at a younger age offers a survival advantage in rectal carcinoid.
Introduction
Rectal carcinoid tumors are rare but the second most common carcinoid in the gastrointestinal tract. They are usually found incidentally during endoscopic or rectal examination. They do not often produce carcinoid syndrome like manifestations although they may manifest as rectal bleeding. Rectal carcinoid patients also have a higher morbidity for other cancers such as stomach, small intestine, or secondary lung cancer.
Methods
We retrospectively explored factors associated with survival in Veterans with rectal carcinoid tumors over a ten-year period from 2007-2017 using the National Veterans Affairs Cancer Cube Registry using specific histological ICD-03 coding. We identified 1110 cases of rectal carcinoid. Chi-squared tests were used for statistical analysis.
Results
Regarding age distribution in our cohort, there were 2.61% of patients ages 40-50 group, 14.0% in the 50-60 age group, 41.5% in the 60-70 age group, and 40.7% above ages 70. There was a higher proportion of rectal cancer in stage 1 compared to other stages (86.3%). The majority of diagnoses occur after age 50 (89.8%). A higher proportion of rectal carcinoid was identified in the 60-70 years category compared to <60 and >70 years old. In the general VA population, there are 80.2% White and 12.8% Black patients. We found a higher proportion of rectal carcinoid in Black patients (47.8%) over White patients (42.8%, p=0.02), which differs significantly from the racial makeup of the VA population (12.8% Black vs 80.3% White). Looking at survival time based on diagnosis, it is notable that 82.7% of individuals survive longer than 5 years when the diagnosis is made in ages 50-60 when compared to 68.7% when the diagnosis is made between ages 60-70 (p<0.001).
Conclusions
Our data is consistent with the SEER data in that the incidence and prevalence of rectal carcinoid are higher in Black patients compared to White patients. Further analysis into reasons for this racial disparity may prove beneficial to our understanding of this malignancy in the Veteran population. Further research is needed to determine whether diagnosis at a younger age offers a survival advantage in rectal carcinoid.
Introduction
Rectal carcinoid tumors are rare but the second most common carcinoid in the gastrointestinal tract. They are usually found incidentally during endoscopic or rectal examination. They do not often produce carcinoid syndrome like manifestations although they may manifest as rectal bleeding. Rectal carcinoid patients also have a higher morbidity for other cancers such as stomach, small intestine, or secondary lung cancer.
Methods
We retrospectively explored factors associated with survival in Veterans with rectal carcinoid tumors over a ten-year period from 2007-2017 using the National Veterans Affairs Cancer Cube Registry using specific histological ICD-03 coding. We identified 1110 cases of rectal carcinoid. Chi-squared tests were used for statistical analysis.
Results
Regarding age distribution in our cohort, there were 2.61% of patients ages 40-50 group, 14.0% in the 50-60 age group, 41.5% in the 60-70 age group, and 40.7% above ages 70. There was a higher proportion of rectal cancer in stage 1 compared to other stages (86.3%). The majority of diagnoses occur after age 50 (89.8%). A higher proportion of rectal carcinoid was identified in the 60-70 years category compared to <60 and >70 years old. In the general VA population, there are 80.2% White and 12.8% Black patients. We found a higher proportion of rectal carcinoid in Black patients (47.8%) over White patients (42.8%, p=0.02), which differs significantly from the racial makeup of the VA population (12.8% Black vs 80.3% White). Looking at survival time based on diagnosis, it is notable that 82.7% of individuals survive longer than 5 years when the diagnosis is made in ages 50-60 when compared to 68.7% when the diagnosis is made between ages 60-70 (p<0.001).
Conclusions
Our data is consistent with the SEER data in that the incidence and prevalence of rectal carcinoid are higher in Black patients compared to White patients. Further analysis into reasons for this racial disparity may prove beneficial to our understanding of this malignancy in the Veteran population. Further research is needed to determine whether diagnosis at a younger age offers a survival advantage in rectal carcinoid.
Double Hit: Epstein-Barr Virus Causing Infectious Mononucleosis Followed by Hemolytic Uremic Syndrome
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Survival Analysis of Untreated Early-Stage Non-Small Cell Lung Cancer (NSCLC) in a Veteran Population
Introduction
Veterans with early-stage NSCLC who do not receive any form of treatment have been shown to have a worse overall survival compared to those who receive treatment. Factors that may influence the decision to administer treatment including age, performance status (PS), comorbidities, and racial disparity have not been assessed on a national level in recent years.
Methods
Data for 31,966 veterans diagnosed with early-stage (0, I) NSCLC between 2003-2017 was obtained from the Cancer cube registry (VACCR). IRB approval was obtained.
Results
Patients were divided into treatment (26,833/31,966, 83.16%) and no-treatment group (3096/31966, 9.68%). Of the no-treatment group, 3004 patients were stage I and 92 were stage 0 whereas in the treatment group, the distribution was 26,584 and 249 respectively. Gender, race, and histology distribution were comparable between the two. Patients with poor PS (defined as ECOG III and IV) received less treatment with any modality compared to those with good PS (ECOG I and II) (15.07% in no treatment group vs 4.03% in treatment group, p<0.05). The treatment group had a better 5-year overall survival (OS) as compared to no-treatment group (43.1% vs 14.7%, p<0.05). Regardless of treatment, patients above the age of 60 (41% vs 13.4%, p<0.05) and those with poor PS (19.6% vs 5.8%, p<0.05) had worse 5-year survival, with the effect being greater in the treatment group. Adenocarcinoma had a better 5-year survival compared to squamous cell carcinoma (SCC) in both groups (49.56% vs 39.1% p<0.05). There was no clinically significant OS difference in terms of race (Caucasian or African American) or tumor location (upper, middle, or lower lobe) in between the two groups. Our study was limited by lack of patient- level data including smoking status or reason why no treatment was given.
Conclusion
Patients with early-stage NSCLC who receive no treatment based on poor PS have a worse overall survival compared to the patients that receive treatment. Further investigation is required to assess what other criteria are used to decide treatment eligibility and whether these patients would be candidates for immunotherapy or targeted therapy in the future.
Introduction
Veterans with early-stage NSCLC who do not receive any form of treatment have been shown to have a worse overall survival compared to those who receive treatment. Factors that may influence the decision to administer treatment including age, performance status (PS), comorbidities, and racial disparity have not been assessed on a national level in recent years.
Methods
Data for 31,966 veterans diagnosed with early-stage (0, I) NSCLC between 2003-2017 was obtained from the Cancer cube registry (VACCR). IRB approval was obtained.
Results
Patients were divided into treatment (26,833/31,966, 83.16%) and no-treatment group (3096/31966, 9.68%). Of the no-treatment group, 3004 patients were stage I and 92 were stage 0 whereas in the treatment group, the distribution was 26,584 and 249 respectively. Gender, race, and histology distribution were comparable between the two. Patients with poor PS (defined as ECOG III and IV) received less treatment with any modality compared to those with good PS (ECOG I and II) (15.07% in no treatment group vs 4.03% in treatment group, p<0.05). The treatment group had a better 5-year overall survival (OS) as compared to no-treatment group (43.1% vs 14.7%, p<0.05). Regardless of treatment, patients above the age of 60 (41% vs 13.4%, p<0.05) and those with poor PS (19.6% vs 5.8%, p<0.05) had worse 5-year survival, with the effect being greater in the treatment group. Adenocarcinoma had a better 5-year survival compared to squamous cell carcinoma (SCC) in both groups (49.56% vs 39.1% p<0.05). There was no clinically significant OS difference in terms of race (Caucasian or African American) or tumor location (upper, middle, or lower lobe) in between the two groups. Our study was limited by lack of patient- level data including smoking status or reason why no treatment was given.
Conclusion
Patients with early-stage NSCLC who receive no treatment based on poor PS have a worse overall survival compared to the patients that receive treatment. Further investigation is required to assess what other criteria are used to decide treatment eligibility and whether these patients would be candidates for immunotherapy or targeted therapy in the future.
Introduction
Veterans with early-stage NSCLC who do not receive any form of treatment have been shown to have a worse overall survival compared to those who receive treatment. Factors that may influence the decision to administer treatment including age, performance status (PS), comorbidities, and racial disparity have not been assessed on a national level in recent years.
Methods
Data for 31,966 veterans diagnosed with early-stage (0, I) NSCLC between 2003-2017 was obtained from the Cancer cube registry (VACCR). IRB approval was obtained.
Results
Patients were divided into treatment (26,833/31,966, 83.16%) and no-treatment group (3096/31966, 9.68%). Of the no-treatment group, 3004 patients were stage I and 92 were stage 0 whereas in the treatment group, the distribution was 26,584 and 249 respectively. Gender, race, and histology distribution were comparable between the two. Patients with poor PS (defined as ECOG III and IV) received less treatment with any modality compared to those with good PS (ECOG I and II) (15.07% in no treatment group vs 4.03% in treatment group, p<0.05). The treatment group had a better 5-year overall survival (OS) as compared to no-treatment group (43.1% vs 14.7%, p<0.05). Regardless of treatment, patients above the age of 60 (41% vs 13.4%, p<0.05) and those with poor PS (19.6% vs 5.8%, p<0.05) had worse 5-year survival, with the effect being greater in the treatment group. Adenocarcinoma had a better 5-year survival compared to squamous cell carcinoma (SCC) in both groups (49.56% vs 39.1% p<0.05). There was no clinically significant OS difference in terms of race (Caucasian or African American) or tumor location (upper, middle, or lower lobe) in between the two groups. Our study was limited by lack of patient- level data including smoking status or reason why no treatment was given.
Conclusion
Patients with early-stage NSCLC who receive no treatment based on poor PS have a worse overall survival compared to the patients that receive treatment. Further investigation is required to assess what other criteria are used to decide treatment eligibility and whether these patients would be candidates for immunotherapy or targeted therapy in the future.
Cerebral Venous Thrombosis, an Extremely Rare Complication of Iron Deficiency Anemia
INTRODUCTION: Cerebral venous thrombosis (CVT) is a rare type of stroke and can be challenging to diagnose. It is seen in most commonly young females and has been linked to thrombophilia, pregnancy, and contraceptive pills. Here we present a rare case of CVT in a young female with iron deficiency anemia.
CASE REPORT: A 19-year-old female patient presented with severe headache, CT scan of the head on admission showed acute superior sagittal sinus thrombosis which was confirmed with CT venogram and MRI of the brain. The patient had intact neurologic exam upon admission. She was started on heparin and admitted for monitoring. Later on she developed expressive aphasia and right sided weakness. She ultimately underwent catheter directed thrombolysis. Follow up CT and MRI scans showed significant decrease in clot burden, and the patient’s neurologic function started to improve.
Her initial labs were significant for thrombocytosis with platelet count 840,000/μL, and microcytic anemia with hemoglobin 9.6 g/dL and MCV 79 fL. She had low serum ferritin and iron levels with high total iron binding capacity consistent with iron deficiency anemia. An extensive hypercoagulable work up was done including antithrombin, protein C and S, factor V Leiden mutation, prothrombin gene mutation, hyperhomocysteinemia, antiphospholipid antibodies, anti-nuclear antibodies which all came back negative. Given her high platelet count, a myeloproliferative disorder was entertained however testing of mutations JAK2V617F, CALR, MPL, and BCR-ABL was negative. She also had a bone marrow biopsy that revealed normal bone marrow. The patient had no prior personal or family history of venous thrombosis, she was not taking any hormonal mediation and pregnancy test was negative. She did report menorrhagia for couple of months prior to admission.
CONCLUSION: After ruling out genetic prothrombotic states, autoimmune disease, and bone marrow disorders. We determined this was a case of cerebral venous thrombosis secondary to reactive thrombocytosis in setting of untreated iron deficiency and menorrhagia. The patient was started on iron supplements with improvement in her iron and hemoglobin levels, and subsequent decrease in her platelet count to normal values. She continued anticoagulation with rivaroxaban for 3-6 months period.
INTRODUCTION: Cerebral venous thrombosis (CVT) is a rare type of stroke and can be challenging to diagnose. It is seen in most commonly young females and has been linked to thrombophilia, pregnancy, and contraceptive pills. Here we present a rare case of CVT in a young female with iron deficiency anemia.
CASE REPORT: A 19-year-old female patient presented with severe headache, CT scan of the head on admission showed acute superior sagittal sinus thrombosis which was confirmed with CT venogram and MRI of the brain. The patient had intact neurologic exam upon admission. She was started on heparin and admitted for monitoring. Later on she developed expressive aphasia and right sided weakness. She ultimately underwent catheter directed thrombolysis. Follow up CT and MRI scans showed significant decrease in clot burden, and the patient’s neurologic function started to improve.
Her initial labs were significant for thrombocytosis with platelet count 840,000/μL, and microcytic anemia with hemoglobin 9.6 g/dL and MCV 79 fL. She had low serum ferritin and iron levels with high total iron binding capacity consistent with iron deficiency anemia. An extensive hypercoagulable work up was done including antithrombin, protein C and S, factor V Leiden mutation, prothrombin gene mutation, hyperhomocysteinemia, antiphospholipid antibodies, anti-nuclear antibodies which all came back negative. Given her high platelet count, a myeloproliferative disorder was entertained however testing of mutations JAK2V617F, CALR, MPL, and BCR-ABL was negative. She also had a bone marrow biopsy that revealed normal bone marrow. The patient had no prior personal or family history of venous thrombosis, she was not taking any hormonal mediation and pregnancy test was negative. She did report menorrhagia for couple of months prior to admission.
CONCLUSION: After ruling out genetic prothrombotic states, autoimmune disease, and bone marrow disorders. We determined this was a case of cerebral venous thrombosis secondary to reactive thrombocytosis in setting of untreated iron deficiency and menorrhagia. The patient was started on iron supplements with improvement in her iron and hemoglobin levels, and subsequent decrease in her platelet count to normal values. She continued anticoagulation with rivaroxaban for 3-6 months period.
INTRODUCTION: Cerebral venous thrombosis (CVT) is a rare type of stroke and can be challenging to diagnose. It is seen in most commonly young females and has been linked to thrombophilia, pregnancy, and contraceptive pills. Here we present a rare case of CVT in a young female with iron deficiency anemia.
CASE REPORT: A 19-year-old female patient presented with severe headache, CT scan of the head on admission showed acute superior sagittal sinus thrombosis which was confirmed with CT venogram and MRI of the brain. The patient had intact neurologic exam upon admission. She was started on heparin and admitted for monitoring. Later on she developed expressive aphasia and right sided weakness. She ultimately underwent catheter directed thrombolysis. Follow up CT and MRI scans showed significant decrease in clot burden, and the patient’s neurologic function started to improve.
Her initial labs were significant for thrombocytosis with platelet count 840,000/μL, and microcytic anemia with hemoglobin 9.6 g/dL and MCV 79 fL. She had low serum ferritin and iron levels with high total iron binding capacity consistent with iron deficiency anemia. An extensive hypercoagulable work up was done including antithrombin, protein C and S, factor V Leiden mutation, prothrombin gene mutation, hyperhomocysteinemia, antiphospholipid antibodies, anti-nuclear antibodies which all came back negative. Given her high platelet count, a myeloproliferative disorder was entertained however testing of mutations JAK2V617F, CALR, MPL, and BCR-ABL was negative. She also had a bone marrow biopsy that revealed normal bone marrow. The patient had no prior personal or family history of venous thrombosis, she was not taking any hormonal mediation and pregnancy test was negative. She did report menorrhagia for couple of months prior to admission.
CONCLUSION: After ruling out genetic prothrombotic states, autoimmune disease, and bone marrow disorders. We determined this was a case of cerebral venous thrombosis secondary to reactive thrombocytosis in setting of untreated iron deficiency and menorrhagia. The patient was started on iron supplements with improvement in her iron and hemoglobin levels, and subsequent decrease in her platelet count to normal values. She continued anticoagulation with rivaroxaban for 3-6 months period.