User login
What are the reasons to use the Gail risk assessment model?
Text copyright DenseBreast-info.org.
Answer
B. The Gail risk model1-3 is used to predict 5-year and lifetime risks of developing invasive breast cancer, and to identify women who may benefit from risk-reducing medications such as tamoxifen. The Gail model should not be used to determine risk for purposes of screening magnetic resonance imaging (MRI)4 (or genetic testing).
Breast cancer risk models are used to stratify patients into risk categories to facilitate personalized screening and surveillance plans for clinical management. Several breast cancer risk assessment tools have been developed that include different combinations of known risk factors and are used for the following purposes:
1. To identify women who may benefit from risk-reducing medications. The Gail model is used to determine risk for purposes of advising on use of risk-reducing medications. Any woman with a 5-year risk ≥1.67% by the Gail model may be considered for treatment with tamoxifen (pre or postmenopausal), raloxifene (postmenopausal), or aromatase inhibitors (postmenopausal).5
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 study,6 women at increased risk for breast cancer were defined as follows:
- age 35 to 59 years with at least a 1.66% 5-year risk for developing breast cancer by the Gail model
- personal history of lobular carcinoma in situ (LCIS)
- age over 60 years.
More than 13,000 such women were randomly assigned to receive tamoxifen or placebo daily for 5 years. Tamoxifen reduced the risk of invasive breast cancer by 49% and reduced the risk of noninvasive cancer by 50% compared with placebo. The reduced risk of breast cancer was only seen for estrogen-receptor–expressing tumors. There was a 2.5-fold increase in risk of endometrial cancer in women taking tamoxifen and a decrease in hip and spine fracture risk. Blood clots causing stroke and deep vein thrombosis are increased in women taking tamoxifen.7,8
2. To identify women who may carry a pathogenic mutation in BRCA1 or BRCA2. Some models (eg, Tyrer-Cuzick [IBIS],9 Penn II,10 BOADICEA,11 and BRCAPRO12) estimate the probability of a BRCA1/2 mutation; however, most testing guidelines are now criterion based (eg, National Comprehensive Cancer Network [NCCN]) as opposed to probability based. In practical terms, clinical decision making around genetic testing is rarely based on a priori probabilities.
3. To identify women who meet criteria for high-risk screening MRI. Current American Cancer Society (ACS) guidelines4 recommend annual screening MRI, in addition to mammography, beginning by age 25 to 30 in women who have a lifetime risk of breast cancer ≥20%. Any of the models used to predict risk of a pathogenic mutation (Tyrer-Cuzick [IBIS], Penn II, BOADICEA, BRCAPRO),or the Claus model,13 but not the Gail model, can be used to estimate lifetime risk for purposes of screening MRI guidelines. The ACS and NCCN guidelines specifically recommend against using the Gail model to determine risk for purposes of MRI screening or risk of pathogenic mutation, as it does not include detailed family history such as age at diagnosis or second-degree relatives.
ACS and NCCN guidelines also recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic mutations in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are first-degree relatives of known mutation carriers but who are themselves untested.14
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome, Bannayan-Riley-Ruvalcaba syndrome, hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, Cowden syndrome, Neurofibromatosis type 1, or Fanconi anemia) are also recommended for annual screening MRI beginning between ages 20-35, depending on the mutation.14 Women with known pathogenic mutations in ATM, CHEK2, or NBN should consider annual MRI starting at age 40 or 5-10 years before the earliest known breast cancer in the family (whichever comes first).
Finally, women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,4,15,16 with risk similar in magnitude to pathogenic BRCA1 or BRCA2 carriers. These women are also recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
Currently the Tyrer-Cuzick Model (IBIS) version 817 and the Breast Cancer Surveillance Consortium (BCSC) models18 include breast density in risk calculations; the Gail, Penn II, and Claus models do not include breast density.
Adding polygenic risk scores based on single nucleotide polymorphisms to traditional comprehensive risk models such as the Tyrer-Cuzick model has been shown to improve model performance.19 In addition, artificial intelligence is being used to identify textural and other findings beyond breast density on mammograms that predict increased risk. Such information, which is complementary to the Tyrer-Cuzick model (v.8),20 has more accurately identified high-risk patients than the Tyrer-Cuzick v8 risk model and prior deep learning models.21
In a study from the Karolinska Institute, a model that included computer-aided detection of microcalcifications and masses in addition to other traditional risk factors (including breast density) successfully identified women who would develop interval or advanced cancer in the 2 years after a normal mammogram and improved short-term (2-to-3-year) risk assessment over TyrerCuzick (v.7) or Gail models.22 This model proved more accurate than traditional risk models and can augment genetic/family history to help identify women who should and, importantly, who should not, have supplemental screening after 2D mammography. Risk models that include detailed family history should be used rather than the Gail model to identify women who meet high risk criteria for MRI screening. Research also supports the benefits of MRI in women with dense breasts who are not otherwise considered “high risk,” and while not widely available, lower cost, abbreviated MRI protocols have been validated for all women with dense breasts.23 For more details on risk models, including a risk models table with live links to commonly used breast cancer risk assessment tools, visit https://densebreast-info .org/for-providers/risk-model-tutorial/. ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- The Breast Cancer Risk Assessment Tool. https://bcrisktool .cancer.gov/calculator.html. Accessed March 15, 2022.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782-1792.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Network NCC. Breast Cancer Risk Reduction (Version 1.2022). https://www.nccn.org/professionals/physician_gls /pdf/breast_risk.pdf. Published 2022. Accessed February 8, 2022.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen
treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449. - Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Panchal SM, Ennis M, Canon S, et al. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med Genet. 2008;9:116.
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580-1590.
- Berry DA, Iversen ES, Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes.
J Clin Oncol. 2002;20:2701-2712. - Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643-651.
- Network NCC. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). https:// www.nccn.org/professionals/physician_gls/pdf/genetics _bop.pdf. Accessed February 9, 2022.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301: 404-414.
- Brentnall AR, Cuzick J, Buist DSM, et al. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
- Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337-347.
- Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer. 2020;146:2122-2129.
- Yala A, Lehman C, Schuster T, et al. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66.
- Yala A, Mikhael PG, Strand F, et al. Toward robust mammography-based models for breast cancer risk. Sci Transl Med. 2021;13.
- Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
Text copyright DenseBreast-info.org.
Answer
B. The Gail risk model1-3 is used to predict 5-year and lifetime risks of developing invasive breast cancer, and to identify women who may benefit from risk-reducing medications such as tamoxifen. The Gail model should not be used to determine risk for purposes of screening magnetic resonance imaging (MRI)4 (or genetic testing).
Breast cancer risk models are used to stratify patients into risk categories to facilitate personalized screening and surveillance plans for clinical management. Several breast cancer risk assessment tools have been developed that include different combinations of known risk factors and are used for the following purposes:
1. To identify women who may benefit from risk-reducing medications. The Gail model is used to determine risk for purposes of advising on use of risk-reducing medications. Any woman with a 5-year risk ≥1.67% by the Gail model may be considered for treatment with tamoxifen (pre or postmenopausal), raloxifene (postmenopausal), or aromatase inhibitors (postmenopausal).5
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 study,6 women at increased risk for breast cancer were defined as follows:
- age 35 to 59 years with at least a 1.66% 5-year risk for developing breast cancer by the Gail model
- personal history of lobular carcinoma in situ (LCIS)
- age over 60 years.
More than 13,000 such women were randomly assigned to receive tamoxifen or placebo daily for 5 years. Tamoxifen reduced the risk of invasive breast cancer by 49% and reduced the risk of noninvasive cancer by 50% compared with placebo. The reduced risk of breast cancer was only seen for estrogen-receptor–expressing tumors. There was a 2.5-fold increase in risk of endometrial cancer in women taking tamoxifen and a decrease in hip and spine fracture risk. Blood clots causing stroke and deep vein thrombosis are increased in women taking tamoxifen.7,8
2. To identify women who may carry a pathogenic mutation in BRCA1 or BRCA2. Some models (eg, Tyrer-Cuzick [IBIS],9 Penn II,10 BOADICEA,11 and BRCAPRO12) estimate the probability of a BRCA1/2 mutation; however, most testing guidelines are now criterion based (eg, National Comprehensive Cancer Network [NCCN]) as opposed to probability based. In practical terms, clinical decision making around genetic testing is rarely based on a priori probabilities.
3. To identify women who meet criteria for high-risk screening MRI. Current American Cancer Society (ACS) guidelines4 recommend annual screening MRI, in addition to mammography, beginning by age 25 to 30 in women who have a lifetime risk of breast cancer ≥20%. Any of the models used to predict risk of a pathogenic mutation (Tyrer-Cuzick [IBIS], Penn II, BOADICEA, BRCAPRO),or the Claus model,13 but not the Gail model, can be used to estimate lifetime risk for purposes of screening MRI guidelines. The ACS and NCCN guidelines specifically recommend against using the Gail model to determine risk for purposes of MRI screening or risk of pathogenic mutation, as it does not include detailed family history such as age at diagnosis or second-degree relatives.
ACS and NCCN guidelines also recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic mutations in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are first-degree relatives of known mutation carriers but who are themselves untested.14
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome, Bannayan-Riley-Ruvalcaba syndrome, hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, Cowden syndrome, Neurofibromatosis type 1, or Fanconi anemia) are also recommended for annual screening MRI beginning between ages 20-35, depending on the mutation.14 Women with known pathogenic mutations in ATM, CHEK2, or NBN should consider annual MRI starting at age 40 or 5-10 years before the earliest known breast cancer in the family (whichever comes first).
Finally, women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,4,15,16 with risk similar in magnitude to pathogenic BRCA1 or BRCA2 carriers. These women are also recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
Currently the Tyrer-Cuzick Model (IBIS) version 817 and the Breast Cancer Surveillance Consortium (BCSC) models18 include breast density in risk calculations; the Gail, Penn II, and Claus models do not include breast density.
Adding polygenic risk scores based on single nucleotide polymorphisms to traditional comprehensive risk models such as the Tyrer-Cuzick model has been shown to improve model performance.19 In addition, artificial intelligence is being used to identify textural and other findings beyond breast density on mammograms that predict increased risk. Such information, which is complementary to the Tyrer-Cuzick model (v.8),20 has more accurately identified high-risk patients than the Tyrer-Cuzick v8 risk model and prior deep learning models.21
In a study from the Karolinska Institute, a model that included computer-aided detection of microcalcifications and masses in addition to other traditional risk factors (including breast density) successfully identified women who would develop interval or advanced cancer in the 2 years after a normal mammogram and improved short-term (2-to-3-year) risk assessment over TyrerCuzick (v.7) or Gail models.22 This model proved more accurate than traditional risk models and can augment genetic/family history to help identify women who should and, importantly, who should not, have supplemental screening after 2D mammography. Risk models that include detailed family history should be used rather than the Gail model to identify women who meet high risk criteria for MRI screening. Research also supports the benefits of MRI in women with dense breasts who are not otherwise considered “high risk,” and while not widely available, lower cost, abbreviated MRI protocols have been validated for all women with dense breasts.23 For more details on risk models, including a risk models table with live links to commonly used breast cancer risk assessment tools, visit https://densebreast-info .org/for-providers/risk-model-tutorial/. ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
B. The Gail risk model1-3 is used to predict 5-year and lifetime risks of developing invasive breast cancer, and to identify women who may benefit from risk-reducing medications such as tamoxifen. The Gail model should not be used to determine risk for purposes of screening magnetic resonance imaging (MRI)4 (or genetic testing).
Breast cancer risk models are used to stratify patients into risk categories to facilitate personalized screening and surveillance plans for clinical management. Several breast cancer risk assessment tools have been developed that include different combinations of known risk factors and are used for the following purposes:
1. To identify women who may benefit from risk-reducing medications. The Gail model is used to determine risk for purposes of advising on use of risk-reducing medications. Any woman with a 5-year risk ≥1.67% by the Gail model may be considered for treatment with tamoxifen (pre or postmenopausal), raloxifene (postmenopausal), or aromatase inhibitors (postmenopausal).5
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 study,6 women at increased risk for breast cancer were defined as follows:
- age 35 to 59 years with at least a 1.66% 5-year risk for developing breast cancer by the Gail model
- personal history of lobular carcinoma in situ (LCIS)
- age over 60 years.
More than 13,000 such women were randomly assigned to receive tamoxifen or placebo daily for 5 years. Tamoxifen reduced the risk of invasive breast cancer by 49% and reduced the risk of noninvasive cancer by 50% compared with placebo. The reduced risk of breast cancer was only seen for estrogen-receptor–expressing tumors. There was a 2.5-fold increase in risk of endometrial cancer in women taking tamoxifen and a decrease in hip and spine fracture risk. Blood clots causing stroke and deep vein thrombosis are increased in women taking tamoxifen.7,8
2. To identify women who may carry a pathogenic mutation in BRCA1 or BRCA2. Some models (eg, Tyrer-Cuzick [IBIS],9 Penn II,10 BOADICEA,11 and BRCAPRO12) estimate the probability of a BRCA1/2 mutation; however, most testing guidelines are now criterion based (eg, National Comprehensive Cancer Network [NCCN]) as opposed to probability based. In practical terms, clinical decision making around genetic testing is rarely based on a priori probabilities.
3. To identify women who meet criteria for high-risk screening MRI. Current American Cancer Society (ACS) guidelines4 recommend annual screening MRI, in addition to mammography, beginning by age 25 to 30 in women who have a lifetime risk of breast cancer ≥20%. Any of the models used to predict risk of a pathogenic mutation (Tyrer-Cuzick [IBIS], Penn II, BOADICEA, BRCAPRO),or the Claus model,13 but not the Gail model, can be used to estimate lifetime risk for purposes of screening MRI guidelines. The ACS and NCCN guidelines specifically recommend against using the Gail model to determine risk for purposes of MRI screening or risk of pathogenic mutation, as it does not include detailed family history such as age at diagnosis or second-degree relatives.
ACS and NCCN guidelines also recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic mutations in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are first-degree relatives of known mutation carriers but who are themselves untested.14
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome, Bannayan-Riley-Ruvalcaba syndrome, hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, Cowden syndrome, Neurofibromatosis type 1, or Fanconi anemia) are also recommended for annual screening MRI beginning between ages 20-35, depending on the mutation.14 Women with known pathogenic mutations in ATM, CHEK2, or NBN should consider annual MRI starting at age 40 or 5-10 years before the earliest known breast cancer in the family (whichever comes first).
Finally, women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,4,15,16 with risk similar in magnitude to pathogenic BRCA1 or BRCA2 carriers. These women are also recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
Currently the Tyrer-Cuzick Model (IBIS) version 817 and the Breast Cancer Surveillance Consortium (BCSC) models18 include breast density in risk calculations; the Gail, Penn II, and Claus models do not include breast density.
Adding polygenic risk scores based on single nucleotide polymorphisms to traditional comprehensive risk models such as the Tyrer-Cuzick model has been shown to improve model performance.19 In addition, artificial intelligence is being used to identify textural and other findings beyond breast density on mammograms that predict increased risk. Such information, which is complementary to the Tyrer-Cuzick model (v.8),20 has more accurately identified high-risk patients than the Tyrer-Cuzick v8 risk model and prior deep learning models.21
In a study from the Karolinska Institute, a model that included computer-aided detection of microcalcifications and masses in addition to other traditional risk factors (including breast density) successfully identified women who would develop interval or advanced cancer in the 2 years after a normal mammogram and improved short-term (2-to-3-year) risk assessment over TyrerCuzick (v.7) or Gail models.22 This model proved more accurate than traditional risk models and can augment genetic/family history to help identify women who should and, importantly, who should not, have supplemental screening after 2D mammography. Risk models that include detailed family history should be used rather than the Gail model to identify women who meet high risk criteria for MRI screening. Research also supports the benefits of MRI in women with dense breasts who are not otherwise considered “high risk,” and while not widely available, lower cost, abbreviated MRI protocols have been validated for all women with dense breasts.23 For more details on risk models, including a risk models table with live links to commonly used breast cancer risk assessment tools, visit https://densebreast-info .org/for-providers/risk-model-tutorial/. ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- The Breast Cancer Risk Assessment Tool. https://bcrisktool .cancer.gov/calculator.html. Accessed March 15, 2022.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782-1792.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Network NCC. Breast Cancer Risk Reduction (Version 1.2022). https://www.nccn.org/professionals/physician_gls /pdf/breast_risk.pdf. Published 2022. Accessed February 8, 2022.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen
treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449. - Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Panchal SM, Ennis M, Canon S, et al. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med Genet. 2008;9:116.
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580-1590.
- Berry DA, Iversen ES, Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes.
J Clin Oncol. 2002;20:2701-2712. - Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643-651.
- Network NCC. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). https:// www.nccn.org/professionals/physician_gls/pdf/genetics _bop.pdf. Accessed February 9, 2022.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301: 404-414.
- Brentnall AR, Cuzick J, Buist DSM, et al. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
- Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337-347.
- Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer. 2020;146:2122-2129.
- Yala A, Lehman C, Schuster T, et al. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66.
- Yala A, Mikhael PG, Strand F, et al. Toward robust mammography-based models for breast cancer risk. Sci Transl Med. 2021;13.
- Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
- The Breast Cancer Risk Assessment Tool. https://bcrisktool .cancer.gov/calculator.html. Accessed March 15, 2022.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782-1792.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Network NCC. Breast Cancer Risk Reduction (Version 1.2022). https://www.nccn.org/professionals/physician_gls /pdf/breast_risk.pdf. Published 2022. Accessed February 8, 2022.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen
treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449. - Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Panchal SM, Ennis M, Canon S, et al. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med Genet. 2008;9:116.
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580-1590.
- Berry DA, Iversen ES, Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes.
J Clin Oncol. 2002;20:2701-2712. - Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643-651.
- Network NCC. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). https:// www.nccn.org/professionals/physician_gls/pdf/genetics _bop.pdf. Accessed February 9, 2022.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301: 404-414.
- Brentnall AR, Cuzick J, Buist DSM, et al. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
- Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337-347.
- Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer. 2020;146:2122-2129.
- Yala A, Lehman C, Schuster T, et al. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66.
- Yala A, Mikhael PG, Strand F, et al. Toward robust mammography-based models for breast cancer risk. Sci Transl Med. 2021;13.
- Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
High-risk women–What breast screening is appropriate?
Text copyright DenseBreast-info.org.
Answer
B. For a 28-year-old woman, herself untested, with a known pathogenic BRCA1 or BRCA2 mutation in a first-degree relative (mother/ father, sister/brother, daughter/son), screening annual magnetic resonance imaging (MRI) alone is recommended until age 30, followed by screening annual MRI and mammography/tomosynthesis at age 30 and beyond. If MRI is not an option, ultrasonography or contrast-enhanced mammography should be considered.
Most medical societies in the United States recommend mammography screening beginning at age 40 if a woman is at average risk. If a woman is determined to be at high risk, breast cancer screening may be recommended to begin by age 30. Breast cancer risk assessment, with a risk model based largely on family history, should begin by age 30 for all women.
The American College of Radiology (ACR),1 National Comprehensive Cancer Network (NCCN),2,3 and American Society of Breast Surgeons recommend annual MRI screening for the following high-risk subgroups of women:
- Women with known disease-causing BRCA1 or BRCA2 mutations, or other disease-causing mutations, or their untested first-degree relatives. (Age to begin screening MRI and screening mammography/tomosynthesis varies by mutation).1,3 Women with known pathogenic BRCA1 or BRCA2 mutations, or their untested first-degree relatives, should begin annual screening with MRI only between ages 25-29, adding annual digital mammography/tomosynthesis at age 30 and beyond (unless the woman has had bilateral mastectomy). Note: There is emerging evidence that the benefit of mammography is relatively small in pathogenic BRCA1 carriers prior to the age of 40; therefore, the ACR suggests BRCA1 mutation carriers may consider delaying mammography until age 40 only if they receive contrast-enhanced MRI annually starting at age 25.1 Annual mammography is of benefit beginning at age 30 in those with BRCA2 or other disease-causing mutations.Age to begin annual MRI screening and mammography/tomosynthesis in women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome [TP53]; Bannayan-Riley-Ruvalcaba syndrome or Cowden syndrome [PTEN]; hereditary diffuse gastric cancer [CDH1]; Peutz-Jeghers syndrome [STK11]; neurofibromatosis type 1 [NF1]; PALB2; ATM; CHEK2; or BARD1) ranges from 20-40 years depending on the mutation and family history.3 (See https://densebreast-info.org/providers-faqs /what-is-the-screening-management-for -various-other-mutation-carriers/.)
- Women who received chest/mantle radiation therapy by age 30 (such as for Hodgkin disease) and at least 8 years prior. Women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,1,2,4,5 with risk similar in magnitude to BRCA1 or BRCA2 carriers, and are recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
- Women with a calculated lifetime risk of breast cancer of ≥20% are recommended to begin annual screening MRI by age 25-30.1,2,5 Any of the models that include detailed family history such as the Tyrer-Cuzick (IBIS, which now includes breast density as a risk factor); BRCAPRO; BOADICEA; Claus; or Penn II; but not the Gail or Breast Cancer Surveillance Consortium (BCSC) models, can be used to estimate lifetime risk for the purposes of screening MRI guidelines. (See https://densebreast -info.org/for-providers/risk-model-tutorial/ for a summary table with live links.)
- Women with a personal history of breast cancer and dense breasts or diagnosis by age 50, regardless of breast density. A personal history of breast cancer is not included in risk models, but all women diagnosed with breast cancer at or before age 50 and treated with breast-conserving therapy have a ≥20% lifetime risk for a new breast cancer.1,2 Annual MRI may be considered in addition to annual mammography or tomosynthesis in women with a personal history of breast cancer diagnosed after age 50 and without dense breasts, and/or a history of lobular carcinoma in situ (LCIS) or prior atypia (eg, atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), or atypical papilloma).1,2
Supplemental MRI screening should continue until age 75, after which management should be considered on an individual basis. If MRI screening is not an option, ultrasound or contrast-enhanced mammography (where available) should be considered as an alternative.6-8 ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15 (3 Pt A):408-414. doi: 10.1016/j.jacr.2017.11.034.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf. Accessed November 18, 2021.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf /genetics_bop.pdf. Accessed July 31, 2020.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301(4):404-414. DOI: 10.1001/jama.2008.1039.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89. doi: 10.3322/canjclin.57.2.75.
- Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol. 2018;211:W267-W274. doi: 10.2214/AJR.17.19355.
- Sung JS, Lebron L, Keating D, et al. Performance of dualenergy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology. 2019;293:81-88. doi: 10.1148/radiol.2019182660.
- Xiang W, Rao H, Zhou L. A meta-analysis of contrastenhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer. 2020;11:1423-1432. doi: 10.1111/1759-7714.13400.
Text copyright DenseBreast-info.org.
Answer
B. For a 28-year-old woman, herself untested, with a known pathogenic BRCA1 or BRCA2 mutation in a first-degree relative (mother/ father, sister/brother, daughter/son), screening annual magnetic resonance imaging (MRI) alone is recommended until age 30, followed by screening annual MRI and mammography/tomosynthesis at age 30 and beyond. If MRI is not an option, ultrasonography or contrast-enhanced mammography should be considered.
Most medical societies in the United States recommend mammography screening beginning at age 40 if a woman is at average risk. If a woman is determined to be at high risk, breast cancer screening may be recommended to begin by age 30. Breast cancer risk assessment, with a risk model based largely on family history, should begin by age 30 for all women.
The American College of Radiology (ACR),1 National Comprehensive Cancer Network (NCCN),2,3 and American Society of Breast Surgeons recommend annual MRI screening for the following high-risk subgroups of women:
- Women with known disease-causing BRCA1 or BRCA2 mutations, or other disease-causing mutations, or their untested first-degree relatives. (Age to begin screening MRI and screening mammography/tomosynthesis varies by mutation).1,3 Women with known pathogenic BRCA1 or BRCA2 mutations, or their untested first-degree relatives, should begin annual screening with MRI only between ages 25-29, adding annual digital mammography/tomosynthesis at age 30 and beyond (unless the woman has had bilateral mastectomy). Note: There is emerging evidence that the benefit of mammography is relatively small in pathogenic BRCA1 carriers prior to the age of 40; therefore, the ACR suggests BRCA1 mutation carriers may consider delaying mammography until age 40 only if they receive contrast-enhanced MRI annually starting at age 25.1 Annual mammography is of benefit beginning at age 30 in those with BRCA2 or other disease-causing mutations.Age to begin annual MRI screening and mammography/tomosynthesis in women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome [TP53]; Bannayan-Riley-Ruvalcaba syndrome or Cowden syndrome [PTEN]; hereditary diffuse gastric cancer [CDH1]; Peutz-Jeghers syndrome [STK11]; neurofibromatosis type 1 [NF1]; PALB2; ATM; CHEK2; or BARD1) ranges from 20-40 years depending on the mutation and family history.3 (See https://densebreast-info.org/providers-faqs /what-is-the-screening-management-for -various-other-mutation-carriers/.)
- Women who received chest/mantle radiation therapy by age 30 (such as for Hodgkin disease) and at least 8 years prior. Women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,1,2,4,5 with risk similar in magnitude to BRCA1 or BRCA2 carriers, and are recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
- Women with a calculated lifetime risk of breast cancer of ≥20% are recommended to begin annual screening MRI by age 25-30.1,2,5 Any of the models that include detailed family history such as the Tyrer-Cuzick (IBIS, which now includes breast density as a risk factor); BRCAPRO; BOADICEA; Claus; or Penn II; but not the Gail or Breast Cancer Surveillance Consortium (BCSC) models, can be used to estimate lifetime risk for the purposes of screening MRI guidelines. (See https://densebreast -info.org/for-providers/risk-model-tutorial/ for a summary table with live links.)
- Women with a personal history of breast cancer and dense breasts or diagnosis by age 50, regardless of breast density. A personal history of breast cancer is not included in risk models, but all women diagnosed with breast cancer at or before age 50 and treated with breast-conserving therapy have a ≥20% lifetime risk for a new breast cancer.1,2 Annual MRI may be considered in addition to annual mammography or tomosynthesis in women with a personal history of breast cancer diagnosed after age 50 and without dense breasts, and/or a history of lobular carcinoma in situ (LCIS) or prior atypia (eg, atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), or atypical papilloma).1,2
Supplemental MRI screening should continue until age 75, after which management should be considered on an individual basis. If MRI screening is not an option, ultrasound or contrast-enhanced mammography (where available) should be considered as an alternative.6-8 ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
B. For a 28-year-old woman, herself untested, with a known pathogenic BRCA1 or BRCA2 mutation in a first-degree relative (mother/ father, sister/brother, daughter/son), screening annual magnetic resonance imaging (MRI) alone is recommended until age 30, followed by screening annual MRI and mammography/tomosynthesis at age 30 and beyond. If MRI is not an option, ultrasonography or contrast-enhanced mammography should be considered.
Most medical societies in the United States recommend mammography screening beginning at age 40 if a woman is at average risk. If a woman is determined to be at high risk, breast cancer screening may be recommended to begin by age 30. Breast cancer risk assessment, with a risk model based largely on family history, should begin by age 30 for all women.
The American College of Radiology (ACR),1 National Comprehensive Cancer Network (NCCN),2,3 and American Society of Breast Surgeons recommend annual MRI screening for the following high-risk subgroups of women:
- Women with known disease-causing BRCA1 or BRCA2 mutations, or other disease-causing mutations, or their untested first-degree relatives. (Age to begin screening MRI and screening mammography/tomosynthesis varies by mutation).1,3 Women with known pathogenic BRCA1 or BRCA2 mutations, or their untested first-degree relatives, should begin annual screening with MRI only between ages 25-29, adding annual digital mammography/tomosynthesis at age 30 and beyond (unless the woman has had bilateral mastectomy). Note: There is emerging evidence that the benefit of mammography is relatively small in pathogenic BRCA1 carriers prior to the age of 40; therefore, the ACR suggests BRCA1 mutation carriers may consider delaying mammography until age 40 only if they receive contrast-enhanced MRI annually starting at age 25.1 Annual mammography is of benefit beginning at age 30 in those with BRCA2 or other disease-causing mutations.Age to begin annual MRI screening and mammography/tomosynthesis in women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome [TP53]; Bannayan-Riley-Ruvalcaba syndrome or Cowden syndrome [PTEN]; hereditary diffuse gastric cancer [CDH1]; Peutz-Jeghers syndrome [STK11]; neurofibromatosis type 1 [NF1]; PALB2; ATM; CHEK2; or BARD1) ranges from 20-40 years depending on the mutation and family history.3 (See https://densebreast-info.org/providers-faqs /what-is-the-screening-management-for -various-other-mutation-carriers/.)
- Women who received chest/mantle radiation therapy by age 30 (such as for Hodgkin disease) and at least 8 years prior. Women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,1,2,4,5 with risk similar in magnitude to BRCA1 or BRCA2 carriers, and are recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
- Women with a calculated lifetime risk of breast cancer of ≥20% are recommended to begin annual screening MRI by age 25-30.1,2,5 Any of the models that include detailed family history such as the Tyrer-Cuzick (IBIS, which now includes breast density as a risk factor); BRCAPRO; BOADICEA; Claus; or Penn II; but not the Gail or Breast Cancer Surveillance Consortium (BCSC) models, can be used to estimate lifetime risk for the purposes of screening MRI guidelines. (See https://densebreast -info.org/for-providers/risk-model-tutorial/ for a summary table with live links.)
- Women with a personal history of breast cancer and dense breasts or diagnosis by age 50, regardless of breast density. A personal history of breast cancer is not included in risk models, but all women diagnosed with breast cancer at or before age 50 and treated with breast-conserving therapy have a ≥20% lifetime risk for a new breast cancer.1,2 Annual MRI may be considered in addition to annual mammography or tomosynthesis in women with a personal history of breast cancer diagnosed after age 50 and without dense breasts, and/or a history of lobular carcinoma in situ (LCIS) or prior atypia (eg, atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), or atypical papilloma).1,2
Supplemental MRI screening should continue until age 75, after which management should be considered on an individual basis. If MRI screening is not an option, ultrasound or contrast-enhanced mammography (where available) should be considered as an alternative.6-8 ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15 (3 Pt A):408-414. doi: 10.1016/j.jacr.2017.11.034.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf. Accessed November 18, 2021.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf /genetics_bop.pdf. Accessed July 31, 2020.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301(4):404-414. DOI: 10.1001/jama.2008.1039.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89. doi: 10.3322/canjclin.57.2.75.
- Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol. 2018;211:W267-W274. doi: 10.2214/AJR.17.19355.
- Sung JS, Lebron L, Keating D, et al. Performance of dualenergy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology. 2019;293:81-88. doi: 10.1148/radiol.2019182660.
- Xiang W, Rao H, Zhou L. A meta-analysis of contrastenhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer. 2020;11:1423-1432. doi: 10.1111/1759-7714.13400.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15 (3 Pt A):408-414. doi: 10.1016/j.jacr.2017.11.034.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf. Accessed November 18, 2021.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf /genetics_bop.pdf. Accessed July 31, 2020.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301(4):404-414. DOI: 10.1001/jama.2008.1039.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89. doi: 10.3322/canjclin.57.2.75.
- Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol. 2018;211:W267-W274. doi: 10.2214/AJR.17.19355.
- Sung JS, Lebron L, Keating D, et al. Performance of dualenergy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology. 2019;293:81-88. doi: 10.1148/radiol.2019182660.
- Xiang W, Rao H, Zhou L. A meta-analysis of contrastenhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer. 2020;11:1423-1432. doi: 10.1111/1759-7714.13400.
Average-risk women with dense breasts—What breast screening is appropriate?
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
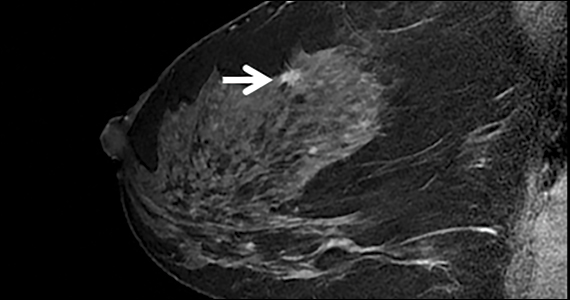
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Quiz developed in collaboration with 
3D vs 2D mammography for detecting cancer in dense breasts
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.
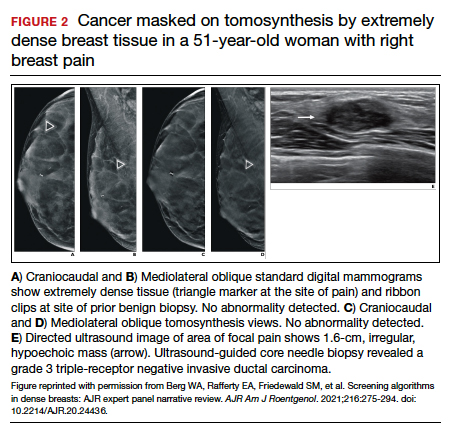
In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Quiz developed in collaboration with 
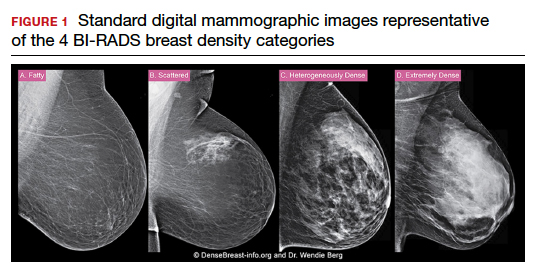
DenseBreast-info.org: What this resource can offer you, and your patients
Read the article Get smart about dense breasts by Wendie Berg, MD, PhD, JoAnn Pushkin, and Cindy Henke-Sarmento (October 2015)
Read the article Get smart about dense breasts by Wendie Berg, MD, PhD, JoAnn Pushkin, and Cindy Henke-Sarmento (October 2015)
Read the article Get smart about dense breasts by Wendie Berg, MD, PhD, JoAnn Pushkin, and Cindy Henke-Sarmento (October 2015)
Access DenseBreast-info.org