Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Assistant Professor, Department of Pulmonary Critical Care Medicine
Baylor College of Medicine
Houston, TX
Dr. Narendra has disclosed no relevant financial relationships.

COPD is a common and preventable condition characterized by persistent respiratory symptoms and airflow obstruction. Its prevalence ranges from 7.4% to 12.6% among adults aged 40 years and older, with higher rates observed in non-Hispanic White individuals, women, and those aged 65 years and older.1,2 Despite declining mortality trends, COPD remains the third leading cause of death worldwide and sixth in the United States.2,3
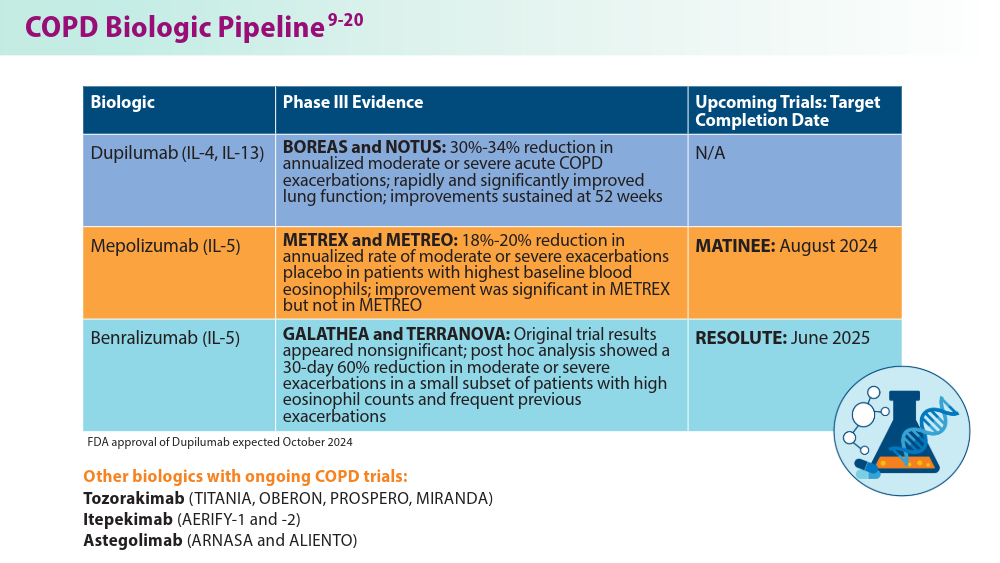
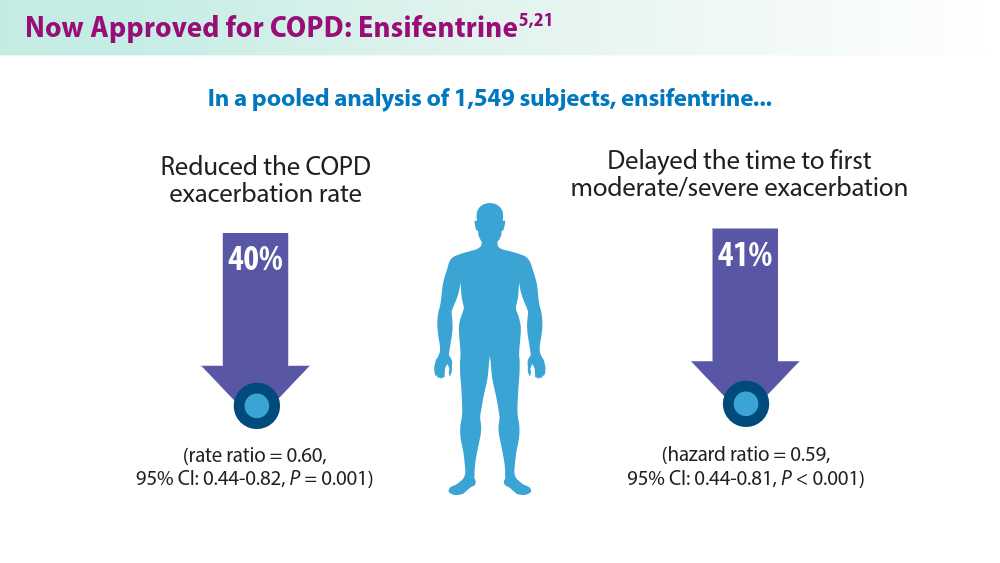
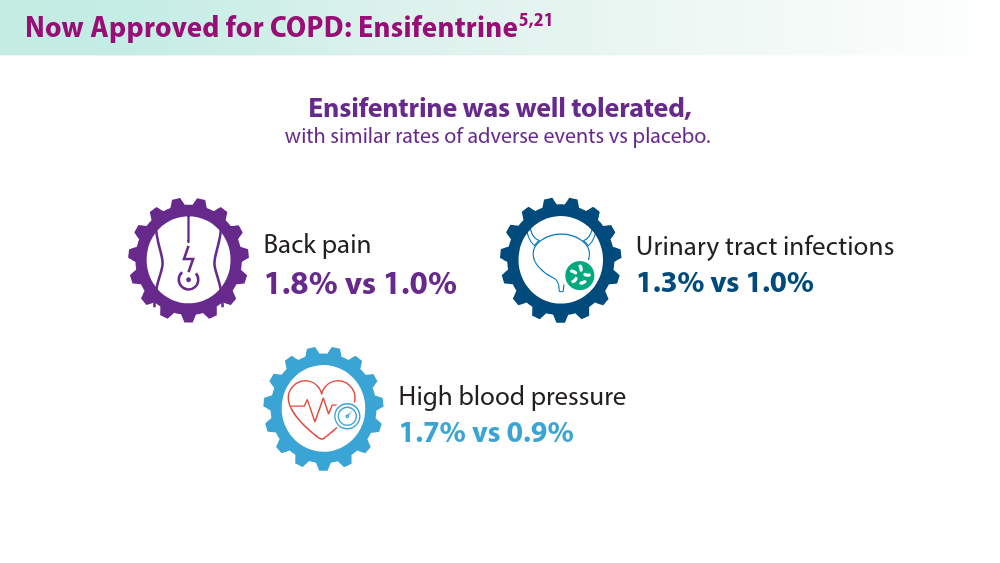
Current pharmacological treatments include bronchodilators, inhaled corticosteroids, combination inhalers,azithromycin, and phosphodiesterase-4 (PDE4) inhibitors, the latter two for exacerbation prevention. Each treatment has limitations, such as side effects, disease progression, and pneumonia risks.4 Ensifentrine,a breakthrough COPD treatment, was recently approved by the FDA and targets both PDE3 and PDE4 enzymes, offering significant benefits in managing moderate to severe COPD.5 Biologics are also emerging as promising therapies due to their targeted approach against specific inflammatory pathways.6
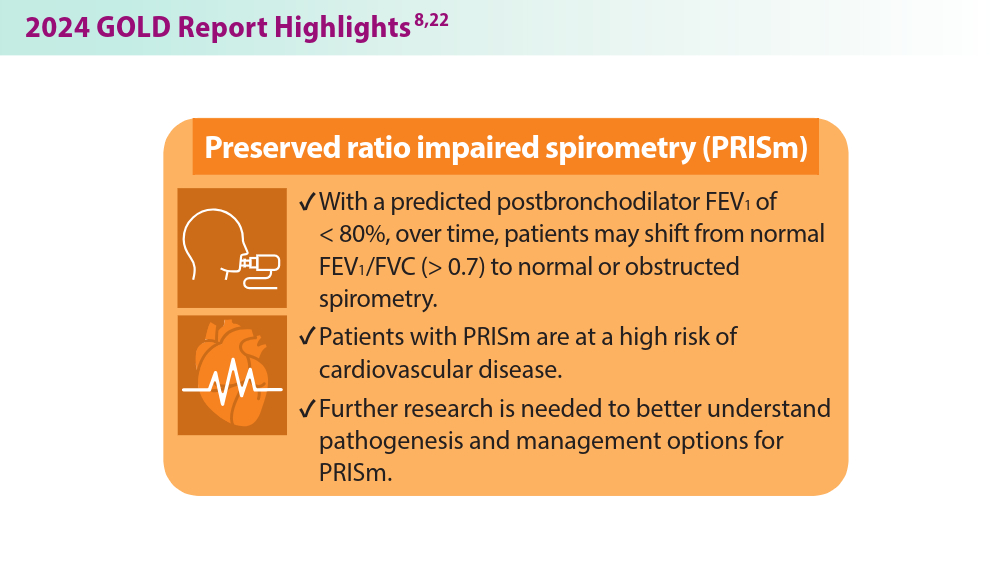
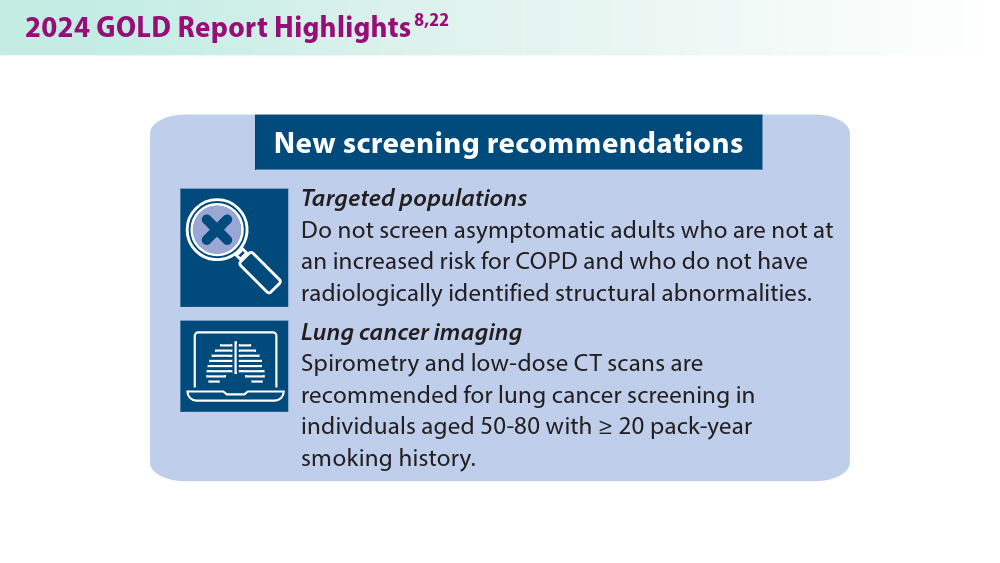
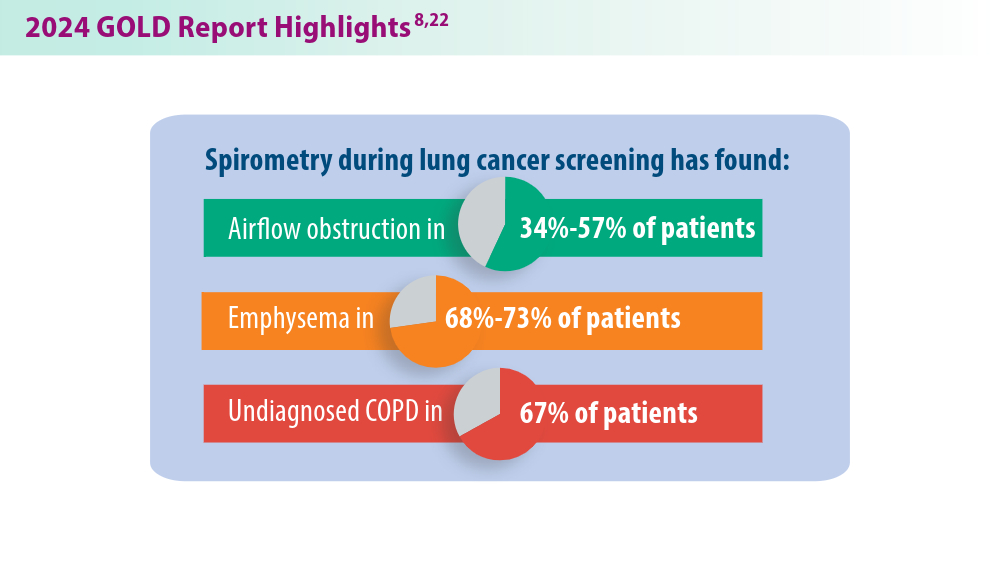
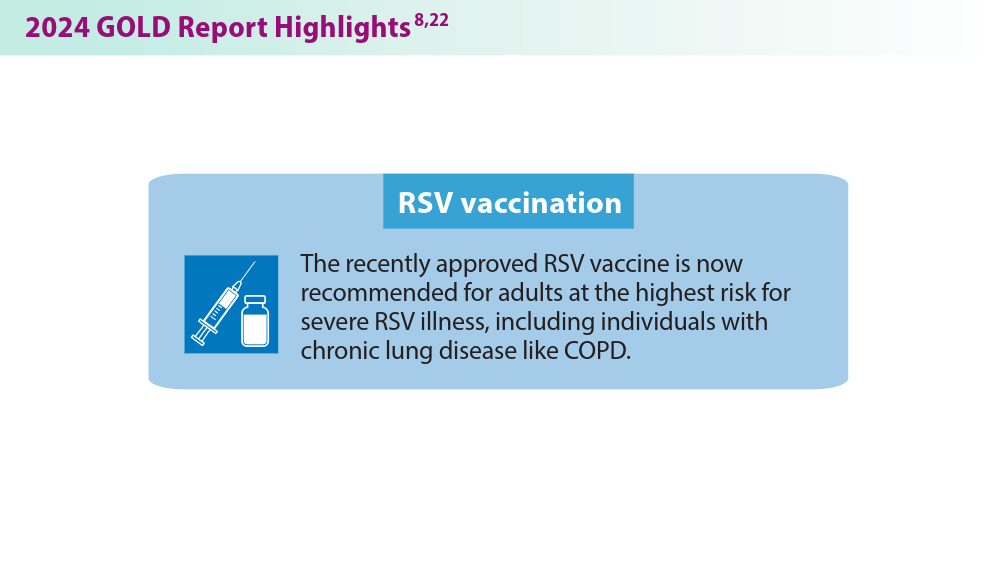
More nonpharmacological approaches are discussed in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, which is updated annually to align with our current understanding of COPD and the available literature. In 2023, GOLD significantly revised its COPD assessment tool, from ABCD to ABE, to simplify classification and focus on effectively treating patients with frequent exacerbations. This new tool helps clinicians identify patients who experience exacerbations and tailor treatments specifically for their needs.7 The 2024 GOLD report includes updated screening, vaccination, and spirometry guidelines, among many other changes that will be discussed below.8 These evolving recommendations, combined with the potential introduction of more targeted therapies, offer hope for improved COPD prevention and management in the future.
1