User login
CASE: POSTMENSTRUAL BLEEDING, HISTORY OF CESAREAN DELIVERIES
A 36-year-old woman (G3P3) reports prolonged and postmenstrual bleeding. Her cycles are regular, every 28 to 30 days, and are associated with ovulatory symptoms. She bleeds for 8 to 10 days with each cycle, having heavy bleeding on cycle day 2 requiring use of super tampons every 3 hours. Beginning on day 5 of the cycle, the blood becomes much darker and scant requiring a small pad, which she changes twice daily. Often, she experiences dark bleeding with physical activity—specifically, running—usually several days after her cycle has ended. She is otherwise healthy and uses no medications. She uses condoms for contraception. She has had a prior vaginal delivery followed by two cesarean sections. Physical examination is normal.
What is causing this patient’s abnormal bleeding pattern?
From 1996 to 2009, the total US cesarean delivery rate increased steadily from 20.7% to 32.9% and has remained stable at 32.8% through 2012.1 With 3,952,841 registered births in 2012, the number of operative procedures performed annually approximates 1.3 million.2 This means, potentially, that one-third of pregnant American women will undergo cesarean delivery annually, translating into an increasing prevalence of long-term sequelae of this surgery.
An increasingly recognized etiology of AUB
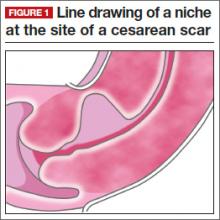
One long-term complication of cesarean delivery, not often discussed, is the presence of a defect within the uterine scar that is directly associated with a type of abnormal uterine bleeding (AUB) referred to as postmenstrual bleeding. Stewart first reported this post–cesarean delivery phenomenon in 1975.3 It is postulated that the cesarean scar defect (CSD)4 forms a pocket, which holds the menstrual effluent, allowing bleeding to occur after regular menstrual cycle bleeding has concluded. Often, remnant menstrual blood is extruded slowly over several days, and is generally dark brown, indicating old blood. Physical activity sometimes can initiate expulsion of the old blood even after the regular cycle has ceased (FIGURE 1).
As early as 1995, Morris reported the histopathologic changes within the cesarean scar in a series of 51 hysterectomy specimens with scar present for 2 to 15 years. His findings included distortion and widening of the lower uterine segment (75%), congested endometrium above the scar recess (61%), marked lymphocytic infiltration (65%), capillary dilation (65%), residual suture material with foreign body giant cell reaction (92%), fragmentation and breakdown of the endometrium of the scar (37%), and iatrogenic adenomyosis confined to the scar (28%). Morris concluded that in addition to AUB, these scar abnormalities could give rise to clinical symptoms such as pelvic pain, dyspareunia, and dysmenorrhea.5 It also has been suggested that otherwise unexplained infertility is associated with anatomic and physiologic changes seen with CSD.6 A recent review article published by Tower summarized additional clinical outcomes of CSD, such as ectopic pregnancy and increased surgical risks for such gynecologic procedures as uterine evacuation in the nonpregnant or postpartum state, hysterectomy, endometrial ablation, and intrauterine device placement.4
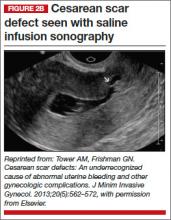
The CSD generally is described as a triangular or circular sonographically anechoic area in the myometrium of the anterior lower uterine segment or cervix at the site of a previous cesarean section. In nonpregnant patients, the defect is best evaluated with contrast infusion sonography (CIS), such as saline infusion or gel infusion, versus transvaginal ultrasound (TVUS) alone (FIGURE 2).4,7,8 However, the precise dimensions and definition of the scar defect vary among investigators.4,6,7,8,10
The reported prevalence of CSD has varied in the literature and appears to depend on the modality of diagnosis and the population studied. For instance, van der Voet and colleagues reported that in random populations of women who had undergone cesarean delivery, the defect was evident in 24% to 69% of women evaluated with transvaginal noncontrast ultrasound; the defect was evident in 56% to 78% of women evaluated with transvaginal contrast sonography.8
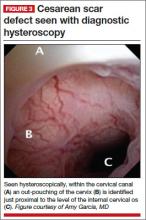
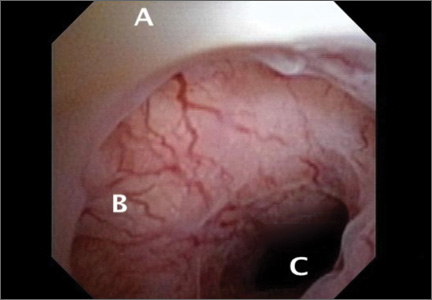
The scar defect also has been identified with magnetic resonance imaging (MRI) and found to be equal in sensitivity to TVUS.9,10 When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”6 Hysteroscopically, the defect also is visualized commonly within the cervical canal, indicating that cesarean incisions often are made through cervical tissue at the time of delivery (FIGURE 3, VIDEO 1, VIDEO 2 [see below]). Not all women with CSD report bleeding abnormalities, but it appears that the deeper and wider the defect, the more likely a woman is to present with postmenstrual AUB.7 According to the International Federation of Gynecology and Obstetrics (FIGO) Classification of AUB, CSD-associated postmenstrual bleeding falls into the “iatrogenic” category in the PALM-COIEN pneumonic.11
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.
MINIMALLY INVASIVE THERAPY FOR GYNECOLOGIC SYMPTOMS
van der Voet LF, Vervoort AJ, Veersema S, Bij de Vatte AJ, Brolmann HAM, Huirne JAF. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145-156.
CSD-related bleeding issues may not respond to hormonal management and are frequently underdiagnosed. This scenario often leads to hysterectomy. Because there are women who desire uterine preservation, van der Voet and colleagues sought to evaluate the results of nonhysterectomy treatments of CSD-related AUB. They limited this systematic review to include only published studies that were randomized controlled trials, cohort studies, case-control studies, and case series of at least five patients.
Additionally, they included only studies that reported on conservative therapies (hysteroscopic resection, laparoscopic repair, abdominal repair, vaginal repair, endometrial ablation, LNG-IUS, or medical management) as well as at least one of the following outcomes: AUB, pain relief, sexual function, quality of life, surgical outcome, anatomic reconstruction, fertility or pregnancy outcome. Of 1,629 publications that were screened, 12 ultimately met inclusion criteria for the review. The studies, 11 of which were peer reviewed and 1 abstract, were published between 1996 and 2013 and reported on a total of 455 women with postcesarean AUB.
Weaknesses of the study
The most poignant statements made by the investigators pertain to the methodologic quality of the included articles. No study met requisite quality criteria. A clear definition of outcomes, including standardized measurements, was lacking in most studies. Most of the studies reviewed did not report CSD measurements, and only one study provided an objective reproducible method of CSD measurement. Few studies reported AUB symptom evaluation methodology, and no study used validated questionnaires. In the majority of studies, methods of posttreatment outcome measurements either were not reported or differed from pretreatment evaluation methods, potentiating verification bias. Because their literature review yielded primarily small case series publications that reported positive effects of interventions, and because of a lack of large RCT and prospective cohort trials, little could be gleaned regarding the viability of treatment interventions for CSD-related AUB.
Only three studies provided sufficient data to be included in a meta-analysis. The number of days of bleeding was reduced with hysteroscopic defect resection by 2 to 4 days in two studies, and in one study, vaginal repair decreased days of bleeding by 4 to 7 days. Only one study with laparoscopic repair compared CSD characteristics before and after surgery. Residual myometrial thickness increased for laparoscopic repair to greater than 8.3 mm; however, it is not known if this will make a clinical difference in the risk of scar dehiscence or improved functionality of the lower uterine segment.
Two studies reported on the laparoscopic repair of scar defects in asymptomatic patients, which is not recommended by these investigators. It is not known what ramifications hysteroscopic resection of the scar will have for the risk of uterine rupture, malplacentation or cervical incompetence for women who conceive after hysteroscopic repair.
Meaningful conclusions are lacking
Despite the high success rates reported by investigators of various surgical intervention case series involving hysteroscopic resection, vaginal repair, or laparoscopic repair, van der Voet and colleagues ultimately state that the methodologies of these studies do not allow meaningful conclusions to be drawn regarding the effectiveness of any of these interventions. Consequently, the authors recommend that the outcomes of their meta-analysis be scrutinized. They also point out that the LNG-IUS has proven benefit for AUB and yet has not been studied in the treatment of AUB associated with a CSD.
They finally propose that women who are symptomatic be treated with oral contraceptives unless immediate fertility is desired, or by expectant management without intervention. While their primary focus was to assess AUB, given the stated shortcomings of the included studies and lack of long-term follow-up, the authors also warn against hysteroscopic, laparoscopic, or vaginal repair for fertility, as the risk to pregnancy or delivery after these therapies is unknown.
CASE RESOLVED
Suspecting a cesarean scar defect, you perform a saline infusion sonography and diagnose a 14 mm x 19 mm anechoic region within the scar, with no other intracavitary abnormalities found. You first reassure the patient that this is a benign finding and inform her why she likely is experiencing this type of bleeding pattern. After an informed discussion with you regarding the risks and benefits of possible surgical or nonsurgical options for management, she chooses to use oral contraceptive pills in a continuous fashion.
CONCLUSION
Consider a history of cesarean section in the evaluation of AUB, and be cognizant of the prevalence of CSD with cesarean delivery and the association of postmenstrual bleeding with CSD.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• A critical systematic review of available data suggests that there is not enough clinical evidence to support surgical intervention for the treatment of CSD for women symptomatic with AUB.
• Recommended nonhysterectomy treatments for AUB associated with CSD include oral contraceptives or expectant management.
• Surgical treatment should be limited to the research environment in the form of RCT to assess the long-term outcomes of intervention.
• An RCT of the LNG-IUS for the treatment of AUB associated with CSD is needed.
Acknowledgments
The author would like to thank Andrew Brill, MD, Lee Sloan-Garcia, MD, and William Parker, MD, for their thoughtful review of this manuscript.
We want to hear from you!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Osterman MJK, Martin JA. Primary cesarean delivery rates, by state: Results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63(1):1–11.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9). Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. Accessed March 19, 2014.
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975;82(8):682–686.
- Tower AM, Frishman GN. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
- Morris H. Surgical pathology of the lower uterine segment cesarean section scar: Is the scar a source of clinical symptoms? Intl J Gynecol Pathol. 1995;14(1):16–20.
- Gubbini G, Centini G, Nascetti D, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J Minim Invasive Gynecol. 2011;18(2):234–237.
- van der Voet LF, Bijde Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
- van der Voet LF, Vervoort AJ, Veersema S, Bijde Vatte AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145–156.
- Maldjian C, Adam R, Maldjian J, Smith R. MRI appearance of the pelvis in the post cesarean-section patient. Magn Reson Imaging. 1999;17(2):223–227.
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-Cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013;20(3):386–391.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COIEN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
CASE: POSTMENSTRUAL BLEEDING, HISTORY OF CESAREAN DELIVERIES
A 36-year-old woman (G3P3) reports prolonged and postmenstrual bleeding. Her cycles are regular, every 28 to 30 days, and are associated with ovulatory symptoms. She bleeds for 8 to 10 days with each cycle, having heavy bleeding on cycle day 2 requiring use of super tampons every 3 hours. Beginning on day 5 of the cycle, the blood becomes much darker and scant requiring a small pad, which she changes twice daily. Often, she experiences dark bleeding with physical activity—specifically, running—usually several days after her cycle has ended. She is otherwise healthy and uses no medications. She uses condoms for contraception. She has had a prior vaginal delivery followed by two cesarean sections. Physical examination is normal.
What is causing this patient’s abnormal bleeding pattern?
From 1996 to 2009, the total US cesarean delivery rate increased steadily from 20.7% to 32.9% and has remained stable at 32.8% through 2012.1 With 3,952,841 registered births in 2012, the number of operative procedures performed annually approximates 1.3 million.2 This means, potentially, that one-third of pregnant American women will undergo cesarean delivery annually, translating into an increasing prevalence of long-term sequelae of this surgery.
An increasingly recognized etiology of AUB
One long-term complication of cesarean delivery, not often discussed, is the presence of a defect within the uterine scar that is directly associated with a type of abnormal uterine bleeding (AUB) referred to as postmenstrual bleeding. Stewart first reported this post–cesarean delivery phenomenon in 1975.3 It is postulated that the cesarean scar defect (CSD)4 forms a pocket, which holds the menstrual effluent, allowing bleeding to occur after regular menstrual cycle bleeding has concluded. Often, remnant menstrual blood is extruded slowly over several days, and is generally dark brown, indicating old blood. Physical activity sometimes can initiate expulsion of the old blood even after the regular cycle has ceased (FIGURE 1).
As early as 1995, Morris reported the histopathologic changes within the cesarean scar in a series of 51 hysterectomy specimens with scar present for 2 to 15 years. His findings included distortion and widening of the lower uterine segment (75%), congested endometrium above the scar recess (61%), marked lymphocytic infiltration (65%), capillary dilation (65%), residual suture material with foreign body giant cell reaction (92%), fragmentation and breakdown of the endometrium of the scar (37%), and iatrogenic adenomyosis confined to the scar (28%). Morris concluded that in addition to AUB, these scar abnormalities could give rise to clinical symptoms such as pelvic pain, dyspareunia, and dysmenorrhea.5 It also has been suggested that otherwise unexplained infertility is associated with anatomic and physiologic changes seen with CSD.6 A recent review article published by Tower summarized additional clinical outcomes of CSD, such as ectopic pregnancy and increased surgical risks for such gynecologic procedures as uterine evacuation in the nonpregnant or postpartum state, hysterectomy, endometrial ablation, and intrauterine device placement.4
The CSD generally is described as a triangular or circular sonographically anechoic area in the myometrium of the anterior lower uterine segment or cervix at the site of a previous cesarean section. In nonpregnant patients, the defect is best evaluated with contrast infusion sonography (CIS), such as saline infusion or gel infusion, versus transvaginal ultrasound (TVUS) alone (FIGURE 2).4,7,8 However, the precise dimensions and definition of the scar defect vary among investigators.4,6,7,8,10
The reported prevalence of CSD has varied in the literature and appears to depend on the modality of diagnosis and the population studied. For instance, van der Voet and colleagues reported that in random populations of women who had undergone cesarean delivery, the defect was evident in 24% to 69% of women evaluated with transvaginal noncontrast ultrasound; the defect was evident in 56% to 78% of women evaluated with transvaginal contrast sonography.8
The scar defect also has been identified with magnetic resonance imaging (MRI) and found to be equal in sensitivity to TVUS.9,10 When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”6 Hysteroscopically, the defect also is visualized commonly within the cervical canal, indicating that cesarean incisions often are made through cervical tissue at the time of delivery (FIGURE 3, VIDEO 1, VIDEO 2 [see below]). Not all women with CSD report bleeding abnormalities, but it appears that the deeper and wider the defect, the more likely a woman is to present with postmenstrual AUB.7 According to the International Federation of Gynecology and Obstetrics (FIGO) Classification of AUB, CSD-associated postmenstrual bleeding falls into the “iatrogenic” category in the PALM-COIEN pneumonic.11
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.
MINIMALLY INVASIVE THERAPY FOR GYNECOLOGIC SYMPTOMS
van der Voet LF, Vervoort AJ, Veersema S, Bij de Vatte AJ, Brolmann HAM, Huirne JAF. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145-156.
CSD-related bleeding issues may not respond to hormonal management and are frequently underdiagnosed. This scenario often leads to hysterectomy. Because there are women who desire uterine preservation, van der Voet and colleagues sought to evaluate the results of nonhysterectomy treatments of CSD-related AUB. They limited this systematic review to include only published studies that were randomized controlled trials, cohort studies, case-control studies, and case series of at least five patients.
Additionally, they included only studies that reported on conservative therapies (hysteroscopic resection, laparoscopic repair, abdominal repair, vaginal repair, endometrial ablation, LNG-IUS, or medical management) as well as at least one of the following outcomes: AUB, pain relief, sexual function, quality of life, surgical outcome, anatomic reconstruction, fertility or pregnancy outcome. Of 1,629 publications that were screened, 12 ultimately met inclusion criteria for the review. The studies, 11 of which were peer reviewed and 1 abstract, were published between 1996 and 2013 and reported on a total of 455 women with postcesarean AUB.
Weaknesses of the study
The most poignant statements made by the investigators pertain to the methodologic quality of the included articles. No study met requisite quality criteria. A clear definition of outcomes, including standardized measurements, was lacking in most studies. Most of the studies reviewed did not report CSD measurements, and only one study provided an objective reproducible method of CSD measurement. Few studies reported AUB symptom evaluation methodology, and no study used validated questionnaires. In the majority of studies, methods of posttreatment outcome measurements either were not reported or differed from pretreatment evaluation methods, potentiating verification bias. Because their literature review yielded primarily small case series publications that reported positive effects of interventions, and because of a lack of large RCT and prospective cohort trials, little could be gleaned regarding the viability of treatment interventions for CSD-related AUB.
Only three studies provided sufficient data to be included in a meta-analysis. The number of days of bleeding was reduced with hysteroscopic defect resection by 2 to 4 days in two studies, and in one study, vaginal repair decreased days of bleeding by 4 to 7 days. Only one study with laparoscopic repair compared CSD characteristics before and after surgery. Residual myometrial thickness increased for laparoscopic repair to greater than 8.3 mm; however, it is not known if this will make a clinical difference in the risk of scar dehiscence or improved functionality of the lower uterine segment.
Two studies reported on the laparoscopic repair of scar defects in asymptomatic patients, which is not recommended by these investigators. It is not known what ramifications hysteroscopic resection of the scar will have for the risk of uterine rupture, malplacentation or cervical incompetence for women who conceive after hysteroscopic repair.
Meaningful conclusions are lacking
Despite the high success rates reported by investigators of various surgical intervention case series involving hysteroscopic resection, vaginal repair, or laparoscopic repair, van der Voet and colleagues ultimately state that the methodologies of these studies do not allow meaningful conclusions to be drawn regarding the effectiveness of any of these interventions. Consequently, the authors recommend that the outcomes of their meta-analysis be scrutinized. They also point out that the LNG-IUS has proven benefit for AUB and yet has not been studied in the treatment of AUB associated with a CSD.
They finally propose that women who are symptomatic be treated with oral contraceptives unless immediate fertility is desired, or by expectant management without intervention. While their primary focus was to assess AUB, given the stated shortcomings of the included studies and lack of long-term follow-up, the authors also warn against hysteroscopic, laparoscopic, or vaginal repair for fertility, as the risk to pregnancy or delivery after these therapies is unknown.
CASE RESOLVED
Suspecting a cesarean scar defect, you perform a saline infusion sonography and diagnose a 14 mm x 19 mm anechoic region within the scar, with no other intracavitary abnormalities found. You first reassure the patient that this is a benign finding and inform her why she likely is experiencing this type of bleeding pattern. After an informed discussion with you regarding the risks and benefits of possible surgical or nonsurgical options for management, she chooses to use oral contraceptive pills in a continuous fashion.
CONCLUSION
Consider a history of cesarean section in the evaluation of AUB, and be cognizant of the prevalence of CSD with cesarean delivery and the association of postmenstrual bleeding with CSD.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• A critical systematic review of available data suggests that there is not enough clinical evidence to support surgical intervention for the treatment of CSD for women symptomatic with AUB.
• Recommended nonhysterectomy treatments for AUB associated with CSD include oral contraceptives or expectant management.
• Surgical treatment should be limited to the research environment in the form of RCT to assess the long-term outcomes of intervention.
• An RCT of the LNG-IUS for the treatment of AUB associated with CSD is needed.
Acknowledgments
The author would like to thank Andrew Brill, MD, Lee Sloan-Garcia, MD, and William Parker, MD, for their thoughtful review of this manuscript.
We want to hear from you!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
CASE: POSTMENSTRUAL BLEEDING, HISTORY OF CESAREAN DELIVERIES
A 36-year-old woman (G3P3) reports prolonged and postmenstrual bleeding. Her cycles are regular, every 28 to 30 days, and are associated with ovulatory symptoms. She bleeds for 8 to 10 days with each cycle, having heavy bleeding on cycle day 2 requiring use of super tampons every 3 hours. Beginning on day 5 of the cycle, the blood becomes much darker and scant requiring a small pad, which she changes twice daily. Often, she experiences dark bleeding with physical activity—specifically, running—usually several days after her cycle has ended. She is otherwise healthy and uses no medications. She uses condoms for contraception. She has had a prior vaginal delivery followed by two cesarean sections. Physical examination is normal.
What is causing this patient’s abnormal bleeding pattern?
From 1996 to 2009, the total US cesarean delivery rate increased steadily from 20.7% to 32.9% and has remained stable at 32.8% through 2012.1 With 3,952,841 registered births in 2012, the number of operative procedures performed annually approximates 1.3 million.2 This means, potentially, that one-third of pregnant American women will undergo cesarean delivery annually, translating into an increasing prevalence of long-term sequelae of this surgery.
An increasingly recognized etiology of AUB
One long-term complication of cesarean delivery, not often discussed, is the presence of a defect within the uterine scar that is directly associated with a type of abnormal uterine bleeding (AUB) referred to as postmenstrual bleeding. Stewart first reported this post–cesarean delivery phenomenon in 1975.3 It is postulated that the cesarean scar defect (CSD)4 forms a pocket, which holds the menstrual effluent, allowing bleeding to occur after regular menstrual cycle bleeding has concluded. Often, remnant menstrual blood is extruded slowly over several days, and is generally dark brown, indicating old blood. Physical activity sometimes can initiate expulsion of the old blood even after the regular cycle has ceased (FIGURE 1).
As early as 1995, Morris reported the histopathologic changes within the cesarean scar in a series of 51 hysterectomy specimens with scar present for 2 to 15 years. His findings included distortion and widening of the lower uterine segment (75%), congested endometrium above the scar recess (61%), marked lymphocytic infiltration (65%), capillary dilation (65%), residual suture material with foreign body giant cell reaction (92%), fragmentation and breakdown of the endometrium of the scar (37%), and iatrogenic adenomyosis confined to the scar (28%). Morris concluded that in addition to AUB, these scar abnormalities could give rise to clinical symptoms such as pelvic pain, dyspareunia, and dysmenorrhea.5 It also has been suggested that otherwise unexplained infertility is associated with anatomic and physiologic changes seen with CSD.6 A recent review article published by Tower summarized additional clinical outcomes of CSD, such as ectopic pregnancy and increased surgical risks for such gynecologic procedures as uterine evacuation in the nonpregnant or postpartum state, hysterectomy, endometrial ablation, and intrauterine device placement.4
The CSD generally is described as a triangular or circular sonographically anechoic area in the myometrium of the anterior lower uterine segment or cervix at the site of a previous cesarean section. In nonpregnant patients, the defect is best evaluated with contrast infusion sonography (CIS), such as saline infusion or gel infusion, versus transvaginal ultrasound (TVUS) alone (FIGURE 2).4,7,8 However, the precise dimensions and definition of the scar defect vary among investigators.4,6,7,8,10
The reported prevalence of CSD has varied in the literature and appears to depend on the modality of diagnosis and the population studied. For instance, van der Voet and colleagues reported that in random populations of women who had undergone cesarean delivery, the defect was evident in 24% to 69% of women evaluated with transvaginal noncontrast ultrasound; the defect was evident in 56% to 78% of women evaluated with transvaginal contrast sonography.8
The scar defect also has been identified with magnetic resonance imaging (MRI) and found to be equal in sensitivity to TVUS.9,10 When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”6 Hysteroscopically, the defect also is visualized commonly within the cervical canal, indicating that cesarean incisions often are made through cervical tissue at the time of delivery (FIGURE 3, VIDEO 1, VIDEO 2 [see below]). Not all women with CSD report bleeding abnormalities, but it appears that the deeper and wider the defect, the more likely a woman is to present with postmenstrual AUB.7 According to the International Federation of Gynecology and Obstetrics (FIGO) Classification of AUB, CSD-associated postmenstrual bleeding falls into the “iatrogenic” category in the PALM-COIEN pneumonic.11
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.
MINIMALLY INVASIVE THERAPY FOR GYNECOLOGIC SYMPTOMS
van der Voet LF, Vervoort AJ, Veersema S, Bij de Vatte AJ, Brolmann HAM, Huirne JAF. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145-156.
CSD-related bleeding issues may not respond to hormonal management and are frequently underdiagnosed. This scenario often leads to hysterectomy. Because there are women who desire uterine preservation, van der Voet and colleagues sought to evaluate the results of nonhysterectomy treatments of CSD-related AUB. They limited this systematic review to include only published studies that were randomized controlled trials, cohort studies, case-control studies, and case series of at least five patients.
Additionally, they included only studies that reported on conservative therapies (hysteroscopic resection, laparoscopic repair, abdominal repair, vaginal repair, endometrial ablation, LNG-IUS, or medical management) as well as at least one of the following outcomes: AUB, pain relief, sexual function, quality of life, surgical outcome, anatomic reconstruction, fertility or pregnancy outcome. Of 1,629 publications that were screened, 12 ultimately met inclusion criteria for the review. The studies, 11 of which were peer reviewed and 1 abstract, were published between 1996 and 2013 and reported on a total of 455 women with postcesarean AUB.
Weaknesses of the study
The most poignant statements made by the investigators pertain to the methodologic quality of the included articles. No study met requisite quality criteria. A clear definition of outcomes, including standardized measurements, was lacking in most studies. Most of the studies reviewed did not report CSD measurements, and only one study provided an objective reproducible method of CSD measurement. Few studies reported AUB symptom evaluation methodology, and no study used validated questionnaires. In the majority of studies, methods of posttreatment outcome measurements either were not reported or differed from pretreatment evaluation methods, potentiating verification bias. Because their literature review yielded primarily small case series publications that reported positive effects of interventions, and because of a lack of large RCT and prospective cohort trials, little could be gleaned regarding the viability of treatment interventions for CSD-related AUB.
Only three studies provided sufficient data to be included in a meta-analysis. The number of days of bleeding was reduced with hysteroscopic defect resection by 2 to 4 days in two studies, and in one study, vaginal repair decreased days of bleeding by 4 to 7 days. Only one study with laparoscopic repair compared CSD characteristics before and after surgery. Residual myometrial thickness increased for laparoscopic repair to greater than 8.3 mm; however, it is not known if this will make a clinical difference in the risk of scar dehiscence or improved functionality of the lower uterine segment.
Two studies reported on the laparoscopic repair of scar defects in asymptomatic patients, which is not recommended by these investigators. It is not known what ramifications hysteroscopic resection of the scar will have for the risk of uterine rupture, malplacentation or cervical incompetence for women who conceive after hysteroscopic repair.
Meaningful conclusions are lacking
Despite the high success rates reported by investigators of various surgical intervention case series involving hysteroscopic resection, vaginal repair, or laparoscopic repair, van der Voet and colleagues ultimately state that the methodologies of these studies do not allow meaningful conclusions to be drawn regarding the effectiveness of any of these interventions. Consequently, the authors recommend that the outcomes of their meta-analysis be scrutinized. They also point out that the LNG-IUS has proven benefit for AUB and yet has not been studied in the treatment of AUB associated with a CSD.
They finally propose that women who are symptomatic be treated with oral contraceptives unless immediate fertility is desired, or by expectant management without intervention. While their primary focus was to assess AUB, given the stated shortcomings of the included studies and lack of long-term follow-up, the authors also warn against hysteroscopic, laparoscopic, or vaginal repair for fertility, as the risk to pregnancy or delivery after these therapies is unknown.
CASE RESOLVED
Suspecting a cesarean scar defect, you perform a saline infusion sonography and diagnose a 14 mm x 19 mm anechoic region within the scar, with no other intracavitary abnormalities found. You first reassure the patient that this is a benign finding and inform her why she likely is experiencing this type of bleeding pattern. After an informed discussion with you regarding the risks and benefits of possible surgical or nonsurgical options for management, she chooses to use oral contraceptive pills in a continuous fashion.
CONCLUSION
Consider a history of cesarean section in the evaluation of AUB, and be cognizant of the prevalence of CSD with cesarean delivery and the association of postmenstrual bleeding with CSD.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• A critical systematic review of available data suggests that there is not enough clinical evidence to support surgical intervention for the treatment of CSD for women symptomatic with AUB.
• Recommended nonhysterectomy treatments for AUB associated with CSD include oral contraceptives or expectant management.
• Surgical treatment should be limited to the research environment in the form of RCT to assess the long-term outcomes of intervention.
• An RCT of the LNG-IUS for the treatment of AUB associated with CSD is needed.
Acknowledgments
The author would like to thank Andrew Brill, MD, Lee Sloan-Garcia, MD, and William Parker, MD, for their thoughtful review of this manuscript.
We want to hear from you!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Osterman MJK, Martin JA. Primary cesarean delivery rates, by state: Results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63(1):1–11.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9). Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. Accessed March 19, 2014.
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975;82(8):682–686.
- Tower AM, Frishman GN. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
- Morris H. Surgical pathology of the lower uterine segment cesarean section scar: Is the scar a source of clinical symptoms? Intl J Gynecol Pathol. 1995;14(1):16–20.
- Gubbini G, Centini G, Nascetti D, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J Minim Invasive Gynecol. 2011;18(2):234–237.
- van der Voet LF, Bijde Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
- van der Voet LF, Vervoort AJ, Veersema S, Bijde Vatte AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145–156.
- Maldjian C, Adam R, Maldjian J, Smith R. MRI appearance of the pelvis in the post cesarean-section patient. Magn Reson Imaging. 1999;17(2):223–227.
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-Cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013;20(3):386–391.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COIEN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
- Osterman MJK, Martin JA. Primary cesarean delivery rates, by state: Results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63(1):1–11.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9). Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. Accessed March 19, 2014.
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975;82(8):682–686.
- Tower AM, Frishman GN. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
- Morris H. Surgical pathology of the lower uterine segment cesarean section scar: Is the scar a source of clinical symptoms? Intl J Gynecol Pathol. 1995;14(1):16–20.
- Gubbini G, Centini G, Nascetti D, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J Minim Invasive Gynecol. 2011;18(2):234–237.
- van der Voet LF, Bijde Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
- van der Voet LF, Vervoort AJ, Veersema S, Bijde Vatte AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145–156.
- Maldjian C, Adam R, Maldjian J, Smith R. MRI appearance of the pelvis in the post cesarean-section patient. Magn Reson Imaging. 1999;17(2):223–227.
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-Cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013;20(3):386–391.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COIEN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
CESAREAN SCAR DEFECT DIAGNOSED WITH HYSTEROSCOPY
![]()
![]()
Videos courtesy of Amy Garcia, MD