User login
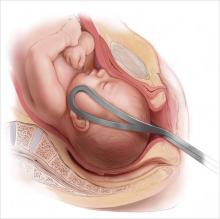
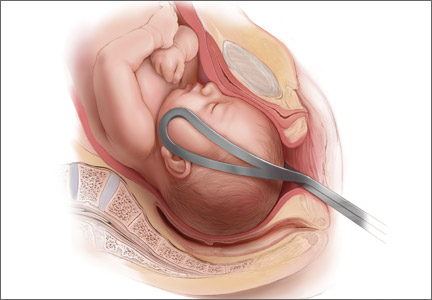
There’s a cyclical lament in obstetrics, and it goes something like this: Forceps are waning and are going to fade away completely if something isn’t done about it. This lament resounds every few decades, as a look at the literature confirms:
- 1963: “Midforceps delivery—a vanishing art?”1
- 1992: “Kielland’s forceps delivery: Is it a dying art?”2
- 2000: “Operative obstetrics: a lost art?”3
- 2015: “Forceps: towards obsolescence or revival?”4
In this, our latest cycle of lament, 4 or 5 papers have suggested that forceps in general and Kielland forceps in particular ought not be abandoned because outcomes are better than those suggested by the older literature. With the cesarean delivery rate hovering at about 31% in the United States, perhaps it is time to revisit the issue.
This Update is not intended to be a comprehensive review of the literature. Rather, it offers a snapshot of articles published within the past year—articles that highlight some new features of a very old debate:
- a nested observational study of 478 nulliparous women at term undergoing instrumental delivery, which found that instrument placement was “suboptimal” in a significant percentage of deliveries
- a retrospective study of major teaching hospitals, minor teaching facilities, and nonteaching institutions in 9 states, which found forceps delivery volumes so low they may make it difficult for clinicians to maintain their skills and prevent many trainees from acquiring proficiency
- a commentary calling for the discontinuation of forceps deliveries in light of an ultrasonographically identified injury to the pelvic floor—levator ani muscle avulsion—and a cadaveric study refuting this argument
- a systematic review and meta-analysis of maternal and neonatal morbidity following cesarean delivery in the first stage versus the second stage of labor.
Forceps and vacuum device placement is “suboptimal” in almost 30% of operative vaginal deliveries
Ramphul M, Kennelly MM, Burke G, Murphy DJ. Risk factors and morbidity associated with suboptimal instrument placement at instrumental delivery: observational study nested within the Instrumental Delivery & Ultrasound randomised controlled trial ISRCTN 72230496. BJOG. 2015;122(4):558–563.
Rouse DJ. Instrument placement is sub-optimal in three of ten attempted operative vaginal deliveries. BJOG. 2015;122(4):564.
Over the years, many clinicians have argued that we don’t do enough forceps deliveries to maintain our own competence with the procedure, let alone teach residents how to perform it. This observational study nested in a randomized clinical trial is intriguing because Ramphul and colleagues looked for objective evidence of clinicians’ skill at the vacuum and forceps. Specifically, they looked for evidence that the forceps or vacuum was malpositioned during attempts at operative vaginal delivery. In the process, they nicely documented the absolute rate of malpositioning of the forceps and vacuum, finding that it is much higher than expected, even in an institution that performs a lot of operative vaginal deliveries.
Details of the trial
A cohort of 478 nulliparous women at term (≥37 weeks) underwent instrumental delivery at 2 university-affiliated maternity hospitals in Ireland. Ramphul and colleagues documented fetal head position prior to application of the instrument and at delivery. The midwife or neonatologist attending each delivery examined the neonate after birth and recorded the markings of the instrument on the infant’s head to determine whether instrument placement had been optimal.
Instrument placement was considered optimal when the vacuum cup included the flexion point (3 cm anterior to the posterior fontanelle) and the posterior fontanelle, with central placement. For forceps, instrument placement was considered optimal when the blades were positioned bilaterally and symmetrically over the malar bones. Two main types of forceps were used in this study—direct-traction Neville Barnes forceps (n = 138) and rotational Kielland forceps (n = 13)—and the rates of optimal and suboptimal placement were similar between them.
Each case was labeled as “optimal” or “suboptimal” by 2 investigators, with a third observer arbitrating when the 2 investigators differed in opinion.
Instrument placement was clearly documented in 478 deliveries, 138 of which (28.8%) involved suboptimal placement. There was a lower rate of induction of labor among deliveries with suboptimal placement (42.8% vs 53.2%; odds ratio [OR], 0.66; 95% confidence interval [CI], 0.44–0.98; P = .038). There were no differences between the optimal and suboptimal groups in terms of duration of labor, use of oxytocin, and analgesia. In addition, the seniority of obstetricians performing operative vaginal delivery was similar between groups.
Fetal malposition was more common in the suboptimal group (58.7% vs 37.4%; OR, 2.44; 95% CI, 1.62–3.66; P<.0001). Midcavity station also was more common in the suboptimal group (82.6% vs 73.8%; OR, 1.68; 95% CI, 1.02–2.78; P = .042).
Maternal and neonatal outcomes
Postpartum hemorrhage was more common in the suboptimal placement group (24.6% vs 14.4%; OR, 1.94; 95% CI, 1.19–3.17; P = .008), as was prolonged hospitalization (26.8% vs 14.7%; OR, 2.13; 95% CI, 1.31–3.44; P = .02).
In addition, the incidence of neonatal trauma was higher in the group with suboptimal placement (15.9% vs 3.9%; OR, 4.64; 95% CI, 2.25–9.58; P<.0001) and included such effects as Erb’s palsy, fracture, retinal hemorrhage, cephalhematoma, and cerebral hemorrhage.
After adjustment for potential confounding factors, including induction of labor, seniority of the obstetrician, fetal malposition, caput above +1, midcavity station, regional analgesia, and the instrument used, the association remained significant between suboptimal placement and prolonged hospitalization (adjusted OR, 2.28; 95% CI, 1.30–4.02) and neonatal trauma (adjusted OR, 4.25; 95% CI, 1.85–9.72).
Dwindling statistics for operative vaginal delivery
In an editorial accompanying the study by Ramphul and colleagues, Dwight J. Rouse, MD,points to the waning of instrumental vaginal delivery in many parts of the world, most notably the United States, where, in 2012, only 2.8% of live births involved use of a vacuum device and only 0.6% involved the forceps.5
“When the rate of cesarean delivery is 10 times the combined rate of vaginal vacuum and forceps delivery (as it is in the USA), it is fair to argue that operative vaginal delivery is underutilized,” Dr. Rouse writes. “So kudos to Ramphul et al for providing insight into how we might continue to perform operative vaginal delivery safely.”
What this EVIDENCE means for practice
The study by Ramphul and colleagues very clearly confirms that correct placement of the vacuum device or forceps is key to safety.
Should we continue forceps education using the apprenticeship model of training?
Kyser KL, Lu X, Santillan D, et al. Forceps delivery volumes in teaching and nonteaching hospitals: Are volumes sufficient for physicians to acquire and maintain competence? Acad Med. 2014;89(1):71–76.
Ericsson KA. Necessity is the mother of invention: video recording firsthand perspectives of critical medical procedures to make simulated training more effective. Acad Med. 2014;89(1):17–20.
Kyser and colleagues have provided the best current snapshot of the opportunity for teaching instrumental vaginal delivery in the United States. They conducted a retrospective cohort study using new state inpatient data from 9 states in diverse geographic locations to capture experience at large and small teaching hospitals, as well as nonteaching institutions. They demonstrated that the opportunity for hands-on experience with these difficult and technically demanding deliveries is extremely limited and probably insufficient for all practicing physicians to maintain their skills if we continue to rely on traditional ways of teaching.
Details of the study
Using State Inpatient Data from 9 states, Kyser and colleagues identified all women hospitalized for childbirth in 2008. Of 1,344,305 deliveries in 835 hospitals, the final cohort included 624,000 operative deliveries—424,224 cesarean deliveries, 174,036 vacuum extractions, 6,158 forceps deliveries, and 19,582 deliveries that required more than 1 method. Of the 835 hospitals in this study, 68 were major teaching hospitals, 130 were minor teaching facilities, and 637 were nonteaching institutions.
The mean annual volumes for cesarean delivery for major teaching, minor teaching, and nonteaching hospitals were 969.8, 757.8, and 406.9, respectively (P<.0001).
The mean annual volumes for vacuum delivery were 301.0, 304.2, and 190.4, respectively (P<.0001).
The mean annual volumes for forceps delivery were 25.2, 15.3, and 8.9, respectively (P<.0001).
Three hundred twenty hospitals (38.3% of all hospitals) failed to perform a single forceps delivery in 2008, including 11 major teaching hospitals (16.2% of major teaching hospitals), 30 minor teaching hospitals (23.1% of minor teaching hospitals), and 279 nonteaching hospitals (43.8% of nonteaching hospitals) (P<.0001).
We need to rethink the apprenticeship model
In a commentary accompanying the study by Kyser and colleagues, K. Anders Ericsson, PhD, revisits the “see one, do one, teach one” model that has long characterized medical education. “Both the limitations on learning opportunities available in the clinics and the restrictions on resident work hours have created a real problem for the traditional apprenticeship model for training doctors,” he writes.
Ericsson notes that other specialists, such as concert musicians, chess players, and professional athletes do not learn using an apprenticeship model. For example, chess players do not play game after game of chess to become expert. And when a game is concluded, usually after several hours have passed, they are unlikely to be aware of the specific moves that lost or won them the game (unless an observer points them out). That is why, when training, chess players focus on particular aspects of the game (often identified by a mentor) as being crucial to improve their overall performance.
In today’s chess-learning environment, Ericsson notes, the computer plays a key role and can provide accurate feedback on each move the player executes. Computer chess programs have evolved to the point that they “are far superior in skill to any human chess player. Most important, computers can provide more accurate feedback on each chess move and are available at any time for practice,” writes Ericsson.
The same is true in sports. A tennis player does not practice by playing an endless series of games—though an ability to win a game is the ultimate goal. Rather, the athlete focuses on aspects of the game—the serve, for example—that can make the difference between winning or losing. Ericsson also notes that most musicians, dancers, and athletes “spend most of their time training by themselves to get ready to exhibit their skills for the first time in front of a large audience.”
These approaches are a better model for improving performance than the apprenticeship model, Ericsson argues. In medicine, one alternative might be the video recording of medical procedures in the clinic from multiple points of view—so that later viewers get both the “big picture” and a close-up view from the point of technical performance. After the recording is digitized and stored on a server, it can serve as valuable teaching for an unlimited number of residents.
Simulator training offers another venue for education, as it makes possible the isolation of difficult aspects of a procedure, which can then be repeated by the trainee as many times as necessary. In the future, it should be possible to link video recordings directly to simulators “so trainees could focus on particular aspects of the procedures and be required to respond to prompts with recordable actions,” Ericsson writes.
What this EVIDENCE means for practice
Given the extremely limited opportunities for observing forceps deliveries in the United States, it is time for us to explore new avenues for teaching other than the traditional apprenticeship model.
Is ultrasound evidence of levator muscle “avulsion” a real anatomic entity?
Dietz HP. Forceps: toward obsolescence or revival? Acta Obstet Gynecol Scand. 2015;94(4):347–351.
Da Silva AS, Digesu GA, Dell’Utri C, Fritsch H, Piffarotti P, Khullar V. Do ultrasound findings of levator ani “avulsion” correlate with anatomical findings? A multicenter cadaveric study [published online ahead of print May 15, 2015]. Neurourol Urodyn. doi:10.1002 /nau.22781.
Dietz takes a new tack in the debate over cesarean versus forceps by pointing to a recently highlighted abnormality in women who deliver by forceps: levator ani muscle avulsion, or LMA—traumatic disconnection of the levator ani from the pelvic sidewall. It has long been known that forceps deliveries can increase the risk of obstetric anal sphincter injuries (OASIS). Dietz contends that OASIS occurs at a rate as high as 40% to 60% after forceps delivery. He also notes, with some consternation, that the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have advocated forceps as a way of reducing the high cesarean delivery rate.
When a parturient has been pushing for an extended period of time and there is a positional abnormality of the fetus, such as persistent occiput posterior position, cesarean delivery is often favored as a way of protecting the rectal sphincter. Dietz argues that cesarean delivery also protects against LMA, which “has only recently been recognized as a major etiological factor in pelvic floor dysfunction.” Dietz then presents a list of studies that have produced ultrasound findings of LMA in a high percentage of women undergoing forceps delivery—percentages on the order of 10% to 40%.
Enter Da Silva and colleagues, who argue that “the only true place to visualize the 3D structure of the human body, [and] thus validate imaging findings, [is] on cadaveric or live tissue dissections.” They undertook a cadaveric study to validate—or not—some of the findings of LMA summarized by Dietz.
Details of the study
The pubovisceral muscle (PVM) anatomy of 30 female cadavers was analyzed via 3D translabial ultrasonography to confirm LMA. The cadavers were then dissected to assess the finding anatomically. Da Silva and colleagues found LMA on imaging in 11 (36.7%) cadavers. LMA was unilateral in 10 (33.3%) cadavers and bilateral in 1 (3.3%). However, no LMA was found at dissection.
When an additional 39 cadavers were dissected, no LMA was identified.
On ultrasound, LMA is strongly associated with a narrower PVM insertion depth (mean of 4.79 mm vs 6.32 mm; P = .001). Da Silva and colleagues concluded that “there is a clear difference between anatomical and ultrasonographic findings. The imaged appearance of an ‘avulsion’ does not represent a true anatomical ‘avulsion’ as confirmed on dissection.”
What this EVIDENCE means for practice
Before we prematurely adopt ultrasound evidence of LMA as a significant morbidity, we need to learn more about its true etiology, pathophysiology, and epidemiology. We don’t yet know enough to say that it’s such a bad injury, when imaged via ultrasound, that it warrants cesarean delivery to avoid it.
When deciding between cesarean and forceps, keep the risks of second-stage cesarean in mind
Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
This expert systematic review and meta-analysis summarizes the morbidity of second-stage cesarean delivery. When an obstetrician has a patient who is arrested at persistent occiput posterior position, say, and is trying to decide on cesarean delivery versus Kielland’s rotation or other forceps delivery, it is necessary to balance the risks and benefits of the 2 options. And as all clinicians are aware, when cesarean delivery is performed late in labor and the patient has been pushing for a prolonged period of time in the second stage—cesarean can be a challenging procedure. Moreover, these late cesareans are associated with much greater risks than cesarean deliveries performed earlier in labor.
Details of the review
Pergialiotis and colleagues selected 10 studies comparing maternal and neonatal morbidity and mortality between cesarean delivery at full dilatation and cesarean delivery prior to full dilatation. These studies involved 23,104 women with a singleton fetus who underwent cesarean delivery in the first (n = 18,160) or second (n = 4,944) stage of labor.
They found that second-stage cesarean was associated with a higher rate of maternal death (OR, 7.96; 95% CI, 1.61–39.39), a higher rate of maternal admission to the intensive care unit (OR, 7.41; 95% CI, 2.47–22.5), and a higher maternal transfusion rate (OR, 2.60; 95% CI, 1.49–2.54).
The rate of neonatal death also was higher among second-stage cesareans (OR, 5.20; 95% CI, 2.49–10.85), as was admission to the neonatal intensive care unit (OR, 1.63; 95% CI, 0.91–2.91), and the 5-minute Apgar score was more likely to be less than 7 (OR, 2.77; 95% CI, 1.02–7.50).
According to the authors, this study is the “first systematic review and meta-analysis that investigates the impact of the stage of labor on maternal and neonatal outcomes among women delivering by cesarean section.” The findings demonstrate with authority that second-stage cesareans can be a risky undertaking.
What this EVIDENCE means for practice
Cesareans performed late in the second stage of labor are distinct from those performed in the first stage, carrying much higher risks, especially for the mother. When deciding whether to proceed with cesarean, vacuum, or forceps, the added risk of second-stage cesarean is an important aspect of both the consent conversation and clinical decision making.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Danforth DN, Ellis AH. Midforceps delivery—a vanishing art? Am J Obstet Gynecol. 1963;86:29–37.
2. Tan KH, Sim R, Yam KL. Kielland’s forceps delivery: Is it a dying art? Singapore Med J. 1992;33(4):380–382.
3. Bofill JA. Operative obstetrics: a lost art? Obstet Gynecol Surv. 2000;55(7):405–406.
4. Dietz HP. Forceps: towards obsolescence or revival? Acta Obstet Gynecol Scand. 2015;94(4):347–351.
5. Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Surtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):9.
There’s a cyclical lament in obstetrics, and it goes something like this: Forceps are waning and are going to fade away completely if something isn’t done about it. This lament resounds every few decades, as a look at the literature confirms:
- 1963: “Midforceps delivery—a vanishing art?”1
- 1992: “Kielland’s forceps delivery: Is it a dying art?”2
- 2000: “Operative obstetrics: a lost art?”3
- 2015: “Forceps: towards obsolescence or revival?”4
In this, our latest cycle of lament, 4 or 5 papers have suggested that forceps in general and Kielland forceps in particular ought not be abandoned because outcomes are better than those suggested by the older literature. With the cesarean delivery rate hovering at about 31% in the United States, perhaps it is time to revisit the issue.
This Update is not intended to be a comprehensive review of the literature. Rather, it offers a snapshot of articles published within the past year—articles that highlight some new features of a very old debate:
- a nested observational study of 478 nulliparous women at term undergoing instrumental delivery, which found that instrument placement was “suboptimal” in a significant percentage of deliveries
- a retrospective study of major teaching hospitals, minor teaching facilities, and nonteaching institutions in 9 states, which found forceps delivery volumes so low they may make it difficult for clinicians to maintain their skills and prevent many trainees from acquiring proficiency
- a commentary calling for the discontinuation of forceps deliveries in light of an ultrasonographically identified injury to the pelvic floor—levator ani muscle avulsion—and a cadaveric study refuting this argument
- a systematic review and meta-analysis of maternal and neonatal morbidity following cesarean delivery in the first stage versus the second stage of labor.
Forceps and vacuum device placement is “suboptimal” in almost 30% of operative vaginal deliveries
Ramphul M, Kennelly MM, Burke G, Murphy DJ. Risk factors and morbidity associated with suboptimal instrument placement at instrumental delivery: observational study nested within the Instrumental Delivery & Ultrasound randomised controlled trial ISRCTN 72230496. BJOG. 2015;122(4):558–563.
Rouse DJ. Instrument placement is sub-optimal in three of ten attempted operative vaginal deliveries. BJOG. 2015;122(4):564.
Over the years, many clinicians have argued that we don’t do enough forceps deliveries to maintain our own competence with the procedure, let alone teach residents how to perform it. This observational study nested in a randomized clinical trial is intriguing because Ramphul and colleagues looked for objective evidence of clinicians’ skill at the vacuum and forceps. Specifically, they looked for evidence that the forceps or vacuum was malpositioned during attempts at operative vaginal delivery. In the process, they nicely documented the absolute rate of malpositioning of the forceps and vacuum, finding that it is much higher than expected, even in an institution that performs a lot of operative vaginal deliveries.
Details of the trial
A cohort of 478 nulliparous women at term (≥37 weeks) underwent instrumental delivery at 2 university-affiliated maternity hospitals in Ireland. Ramphul and colleagues documented fetal head position prior to application of the instrument and at delivery. The midwife or neonatologist attending each delivery examined the neonate after birth and recorded the markings of the instrument on the infant’s head to determine whether instrument placement had been optimal.
Instrument placement was considered optimal when the vacuum cup included the flexion point (3 cm anterior to the posterior fontanelle) and the posterior fontanelle, with central placement. For forceps, instrument placement was considered optimal when the blades were positioned bilaterally and symmetrically over the malar bones. Two main types of forceps were used in this study—direct-traction Neville Barnes forceps (n = 138) and rotational Kielland forceps (n = 13)—and the rates of optimal and suboptimal placement were similar between them.
Each case was labeled as “optimal” or “suboptimal” by 2 investigators, with a third observer arbitrating when the 2 investigators differed in opinion.
Instrument placement was clearly documented in 478 deliveries, 138 of which (28.8%) involved suboptimal placement. There was a lower rate of induction of labor among deliveries with suboptimal placement (42.8% vs 53.2%; odds ratio [OR], 0.66; 95% confidence interval [CI], 0.44–0.98; P = .038). There were no differences between the optimal and suboptimal groups in terms of duration of labor, use of oxytocin, and analgesia. In addition, the seniority of obstetricians performing operative vaginal delivery was similar between groups.
Fetal malposition was more common in the suboptimal group (58.7% vs 37.4%; OR, 2.44; 95% CI, 1.62–3.66; P<.0001). Midcavity station also was more common in the suboptimal group (82.6% vs 73.8%; OR, 1.68; 95% CI, 1.02–2.78; P = .042).
Maternal and neonatal outcomes
Postpartum hemorrhage was more common in the suboptimal placement group (24.6% vs 14.4%; OR, 1.94; 95% CI, 1.19–3.17; P = .008), as was prolonged hospitalization (26.8% vs 14.7%; OR, 2.13; 95% CI, 1.31–3.44; P = .02).
In addition, the incidence of neonatal trauma was higher in the group with suboptimal placement (15.9% vs 3.9%; OR, 4.64; 95% CI, 2.25–9.58; P<.0001) and included such effects as Erb’s palsy, fracture, retinal hemorrhage, cephalhematoma, and cerebral hemorrhage.
After adjustment for potential confounding factors, including induction of labor, seniority of the obstetrician, fetal malposition, caput above +1, midcavity station, regional analgesia, and the instrument used, the association remained significant between suboptimal placement and prolonged hospitalization (adjusted OR, 2.28; 95% CI, 1.30–4.02) and neonatal trauma (adjusted OR, 4.25; 95% CI, 1.85–9.72).
Dwindling statistics for operative vaginal delivery
In an editorial accompanying the study by Ramphul and colleagues, Dwight J. Rouse, MD,points to the waning of instrumental vaginal delivery in many parts of the world, most notably the United States, where, in 2012, only 2.8% of live births involved use of a vacuum device and only 0.6% involved the forceps.5
“When the rate of cesarean delivery is 10 times the combined rate of vaginal vacuum and forceps delivery (as it is in the USA), it is fair to argue that operative vaginal delivery is underutilized,” Dr. Rouse writes. “So kudos to Ramphul et al for providing insight into how we might continue to perform operative vaginal delivery safely.”
What this EVIDENCE means for practice
The study by Ramphul and colleagues very clearly confirms that correct placement of the vacuum device or forceps is key to safety.
Should we continue forceps education using the apprenticeship model of training?
Kyser KL, Lu X, Santillan D, et al. Forceps delivery volumes in teaching and nonteaching hospitals: Are volumes sufficient for physicians to acquire and maintain competence? Acad Med. 2014;89(1):71–76.
Ericsson KA. Necessity is the mother of invention: video recording firsthand perspectives of critical medical procedures to make simulated training more effective. Acad Med. 2014;89(1):17–20.
Kyser and colleagues have provided the best current snapshot of the opportunity for teaching instrumental vaginal delivery in the United States. They conducted a retrospective cohort study using new state inpatient data from 9 states in diverse geographic locations to capture experience at large and small teaching hospitals, as well as nonteaching institutions. They demonstrated that the opportunity for hands-on experience with these difficult and technically demanding deliveries is extremely limited and probably insufficient for all practicing physicians to maintain their skills if we continue to rely on traditional ways of teaching.
Details of the study
Using State Inpatient Data from 9 states, Kyser and colleagues identified all women hospitalized for childbirth in 2008. Of 1,344,305 deliveries in 835 hospitals, the final cohort included 624,000 operative deliveries—424,224 cesarean deliveries, 174,036 vacuum extractions, 6,158 forceps deliveries, and 19,582 deliveries that required more than 1 method. Of the 835 hospitals in this study, 68 were major teaching hospitals, 130 were minor teaching facilities, and 637 were nonteaching institutions.
The mean annual volumes for cesarean delivery for major teaching, minor teaching, and nonteaching hospitals were 969.8, 757.8, and 406.9, respectively (P<.0001).
The mean annual volumes for vacuum delivery were 301.0, 304.2, and 190.4, respectively (P<.0001).
The mean annual volumes for forceps delivery were 25.2, 15.3, and 8.9, respectively (P<.0001).
Three hundred twenty hospitals (38.3% of all hospitals) failed to perform a single forceps delivery in 2008, including 11 major teaching hospitals (16.2% of major teaching hospitals), 30 minor teaching hospitals (23.1% of minor teaching hospitals), and 279 nonteaching hospitals (43.8% of nonteaching hospitals) (P<.0001).
We need to rethink the apprenticeship model
In a commentary accompanying the study by Kyser and colleagues, K. Anders Ericsson, PhD, revisits the “see one, do one, teach one” model that has long characterized medical education. “Both the limitations on learning opportunities available in the clinics and the restrictions on resident work hours have created a real problem for the traditional apprenticeship model for training doctors,” he writes.
Ericsson notes that other specialists, such as concert musicians, chess players, and professional athletes do not learn using an apprenticeship model. For example, chess players do not play game after game of chess to become expert. And when a game is concluded, usually after several hours have passed, they are unlikely to be aware of the specific moves that lost or won them the game (unless an observer points them out). That is why, when training, chess players focus on particular aspects of the game (often identified by a mentor) as being crucial to improve their overall performance.
In today’s chess-learning environment, Ericsson notes, the computer plays a key role and can provide accurate feedback on each move the player executes. Computer chess programs have evolved to the point that they “are far superior in skill to any human chess player. Most important, computers can provide more accurate feedback on each chess move and are available at any time for practice,” writes Ericsson.
The same is true in sports. A tennis player does not practice by playing an endless series of games—though an ability to win a game is the ultimate goal. Rather, the athlete focuses on aspects of the game—the serve, for example—that can make the difference between winning or losing. Ericsson also notes that most musicians, dancers, and athletes “spend most of their time training by themselves to get ready to exhibit their skills for the first time in front of a large audience.”
These approaches are a better model for improving performance than the apprenticeship model, Ericsson argues. In medicine, one alternative might be the video recording of medical procedures in the clinic from multiple points of view—so that later viewers get both the “big picture” and a close-up view from the point of technical performance. After the recording is digitized and stored on a server, it can serve as valuable teaching for an unlimited number of residents.
Simulator training offers another venue for education, as it makes possible the isolation of difficult aspects of a procedure, which can then be repeated by the trainee as many times as necessary. In the future, it should be possible to link video recordings directly to simulators “so trainees could focus on particular aspects of the procedures and be required to respond to prompts with recordable actions,” Ericsson writes.
What this EVIDENCE means for practice
Given the extremely limited opportunities for observing forceps deliveries in the United States, it is time for us to explore new avenues for teaching other than the traditional apprenticeship model.
Is ultrasound evidence of levator muscle “avulsion” a real anatomic entity?
Dietz HP. Forceps: toward obsolescence or revival? Acta Obstet Gynecol Scand. 2015;94(4):347–351.
Da Silva AS, Digesu GA, Dell’Utri C, Fritsch H, Piffarotti P, Khullar V. Do ultrasound findings of levator ani “avulsion” correlate with anatomical findings? A multicenter cadaveric study [published online ahead of print May 15, 2015]. Neurourol Urodyn. doi:10.1002 /nau.22781.
Dietz takes a new tack in the debate over cesarean versus forceps by pointing to a recently highlighted abnormality in women who deliver by forceps: levator ani muscle avulsion, or LMA—traumatic disconnection of the levator ani from the pelvic sidewall. It has long been known that forceps deliveries can increase the risk of obstetric anal sphincter injuries (OASIS). Dietz contends that OASIS occurs at a rate as high as 40% to 60% after forceps delivery. He also notes, with some consternation, that the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have advocated forceps as a way of reducing the high cesarean delivery rate.
When a parturient has been pushing for an extended period of time and there is a positional abnormality of the fetus, such as persistent occiput posterior position, cesarean delivery is often favored as a way of protecting the rectal sphincter. Dietz argues that cesarean delivery also protects against LMA, which “has only recently been recognized as a major etiological factor in pelvic floor dysfunction.” Dietz then presents a list of studies that have produced ultrasound findings of LMA in a high percentage of women undergoing forceps delivery—percentages on the order of 10% to 40%.
Enter Da Silva and colleagues, who argue that “the only true place to visualize the 3D structure of the human body, [and] thus validate imaging findings, [is] on cadaveric or live tissue dissections.” They undertook a cadaveric study to validate—or not—some of the findings of LMA summarized by Dietz.
Details of the study
The pubovisceral muscle (PVM) anatomy of 30 female cadavers was analyzed via 3D translabial ultrasonography to confirm LMA. The cadavers were then dissected to assess the finding anatomically. Da Silva and colleagues found LMA on imaging in 11 (36.7%) cadavers. LMA was unilateral in 10 (33.3%) cadavers and bilateral in 1 (3.3%). However, no LMA was found at dissection.
When an additional 39 cadavers were dissected, no LMA was identified.
On ultrasound, LMA is strongly associated with a narrower PVM insertion depth (mean of 4.79 mm vs 6.32 mm; P = .001). Da Silva and colleagues concluded that “there is a clear difference between anatomical and ultrasonographic findings. The imaged appearance of an ‘avulsion’ does not represent a true anatomical ‘avulsion’ as confirmed on dissection.”
What this EVIDENCE means for practice
Before we prematurely adopt ultrasound evidence of LMA as a significant morbidity, we need to learn more about its true etiology, pathophysiology, and epidemiology. We don’t yet know enough to say that it’s such a bad injury, when imaged via ultrasound, that it warrants cesarean delivery to avoid it.
When deciding between cesarean and forceps, keep the risks of second-stage cesarean in mind
Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
This expert systematic review and meta-analysis summarizes the morbidity of second-stage cesarean delivery. When an obstetrician has a patient who is arrested at persistent occiput posterior position, say, and is trying to decide on cesarean delivery versus Kielland’s rotation or other forceps delivery, it is necessary to balance the risks and benefits of the 2 options. And as all clinicians are aware, when cesarean delivery is performed late in labor and the patient has been pushing for a prolonged period of time in the second stage—cesarean can be a challenging procedure. Moreover, these late cesareans are associated with much greater risks than cesarean deliveries performed earlier in labor.
Details of the review
Pergialiotis and colleagues selected 10 studies comparing maternal and neonatal morbidity and mortality between cesarean delivery at full dilatation and cesarean delivery prior to full dilatation. These studies involved 23,104 women with a singleton fetus who underwent cesarean delivery in the first (n = 18,160) or second (n = 4,944) stage of labor.
They found that second-stage cesarean was associated with a higher rate of maternal death (OR, 7.96; 95% CI, 1.61–39.39), a higher rate of maternal admission to the intensive care unit (OR, 7.41; 95% CI, 2.47–22.5), and a higher maternal transfusion rate (OR, 2.60; 95% CI, 1.49–2.54).
The rate of neonatal death also was higher among second-stage cesareans (OR, 5.20; 95% CI, 2.49–10.85), as was admission to the neonatal intensive care unit (OR, 1.63; 95% CI, 0.91–2.91), and the 5-minute Apgar score was more likely to be less than 7 (OR, 2.77; 95% CI, 1.02–7.50).
According to the authors, this study is the “first systematic review and meta-analysis that investigates the impact of the stage of labor on maternal and neonatal outcomes among women delivering by cesarean section.” The findings demonstrate with authority that second-stage cesareans can be a risky undertaking.
What this EVIDENCE means for practice
Cesareans performed late in the second stage of labor are distinct from those performed in the first stage, carrying much higher risks, especially for the mother. When deciding whether to proceed with cesarean, vacuum, or forceps, the added risk of second-stage cesarean is an important aspect of both the consent conversation and clinical decision making.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
There’s a cyclical lament in obstetrics, and it goes something like this: Forceps are waning and are going to fade away completely if something isn’t done about it. This lament resounds every few decades, as a look at the literature confirms:
- 1963: “Midforceps delivery—a vanishing art?”1
- 1992: “Kielland’s forceps delivery: Is it a dying art?”2
- 2000: “Operative obstetrics: a lost art?”3
- 2015: “Forceps: towards obsolescence or revival?”4
In this, our latest cycle of lament, 4 or 5 papers have suggested that forceps in general and Kielland forceps in particular ought not be abandoned because outcomes are better than those suggested by the older literature. With the cesarean delivery rate hovering at about 31% in the United States, perhaps it is time to revisit the issue.
This Update is not intended to be a comprehensive review of the literature. Rather, it offers a snapshot of articles published within the past year—articles that highlight some new features of a very old debate:
- a nested observational study of 478 nulliparous women at term undergoing instrumental delivery, which found that instrument placement was “suboptimal” in a significant percentage of deliveries
- a retrospective study of major teaching hospitals, minor teaching facilities, and nonteaching institutions in 9 states, which found forceps delivery volumes so low they may make it difficult for clinicians to maintain their skills and prevent many trainees from acquiring proficiency
- a commentary calling for the discontinuation of forceps deliveries in light of an ultrasonographically identified injury to the pelvic floor—levator ani muscle avulsion—and a cadaveric study refuting this argument
- a systematic review and meta-analysis of maternal and neonatal morbidity following cesarean delivery in the first stage versus the second stage of labor.
Forceps and vacuum device placement is “suboptimal” in almost 30% of operative vaginal deliveries
Ramphul M, Kennelly MM, Burke G, Murphy DJ. Risk factors and morbidity associated with suboptimal instrument placement at instrumental delivery: observational study nested within the Instrumental Delivery & Ultrasound randomised controlled trial ISRCTN 72230496. BJOG. 2015;122(4):558–563.
Rouse DJ. Instrument placement is sub-optimal in three of ten attempted operative vaginal deliveries. BJOG. 2015;122(4):564.
Over the years, many clinicians have argued that we don’t do enough forceps deliveries to maintain our own competence with the procedure, let alone teach residents how to perform it. This observational study nested in a randomized clinical trial is intriguing because Ramphul and colleagues looked for objective evidence of clinicians’ skill at the vacuum and forceps. Specifically, they looked for evidence that the forceps or vacuum was malpositioned during attempts at operative vaginal delivery. In the process, they nicely documented the absolute rate of malpositioning of the forceps and vacuum, finding that it is much higher than expected, even in an institution that performs a lot of operative vaginal deliveries.
Details of the trial
A cohort of 478 nulliparous women at term (≥37 weeks) underwent instrumental delivery at 2 university-affiliated maternity hospitals in Ireland. Ramphul and colleagues documented fetal head position prior to application of the instrument and at delivery. The midwife or neonatologist attending each delivery examined the neonate after birth and recorded the markings of the instrument on the infant’s head to determine whether instrument placement had been optimal.
Instrument placement was considered optimal when the vacuum cup included the flexion point (3 cm anterior to the posterior fontanelle) and the posterior fontanelle, with central placement. For forceps, instrument placement was considered optimal when the blades were positioned bilaterally and symmetrically over the malar bones. Two main types of forceps were used in this study—direct-traction Neville Barnes forceps (n = 138) and rotational Kielland forceps (n = 13)—and the rates of optimal and suboptimal placement were similar between them.
Each case was labeled as “optimal” or “suboptimal” by 2 investigators, with a third observer arbitrating when the 2 investigators differed in opinion.
Instrument placement was clearly documented in 478 deliveries, 138 of which (28.8%) involved suboptimal placement. There was a lower rate of induction of labor among deliveries with suboptimal placement (42.8% vs 53.2%; odds ratio [OR], 0.66; 95% confidence interval [CI], 0.44–0.98; P = .038). There were no differences between the optimal and suboptimal groups in terms of duration of labor, use of oxytocin, and analgesia. In addition, the seniority of obstetricians performing operative vaginal delivery was similar between groups.
Fetal malposition was more common in the suboptimal group (58.7% vs 37.4%; OR, 2.44; 95% CI, 1.62–3.66; P<.0001). Midcavity station also was more common in the suboptimal group (82.6% vs 73.8%; OR, 1.68; 95% CI, 1.02–2.78; P = .042).
Maternal and neonatal outcomes
Postpartum hemorrhage was more common in the suboptimal placement group (24.6% vs 14.4%; OR, 1.94; 95% CI, 1.19–3.17; P = .008), as was prolonged hospitalization (26.8% vs 14.7%; OR, 2.13; 95% CI, 1.31–3.44; P = .02).
In addition, the incidence of neonatal trauma was higher in the group with suboptimal placement (15.9% vs 3.9%; OR, 4.64; 95% CI, 2.25–9.58; P<.0001) and included such effects as Erb’s palsy, fracture, retinal hemorrhage, cephalhematoma, and cerebral hemorrhage.
After adjustment for potential confounding factors, including induction of labor, seniority of the obstetrician, fetal malposition, caput above +1, midcavity station, regional analgesia, and the instrument used, the association remained significant between suboptimal placement and prolonged hospitalization (adjusted OR, 2.28; 95% CI, 1.30–4.02) and neonatal trauma (adjusted OR, 4.25; 95% CI, 1.85–9.72).
Dwindling statistics for operative vaginal delivery
In an editorial accompanying the study by Ramphul and colleagues, Dwight J. Rouse, MD,points to the waning of instrumental vaginal delivery in many parts of the world, most notably the United States, where, in 2012, only 2.8% of live births involved use of a vacuum device and only 0.6% involved the forceps.5
“When the rate of cesarean delivery is 10 times the combined rate of vaginal vacuum and forceps delivery (as it is in the USA), it is fair to argue that operative vaginal delivery is underutilized,” Dr. Rouse writes. “So kudos to Ramphul et al for providing insight into how we might continue to perform operative vaginal delivery safely.”
What this EVIDENCE means for practice
The study by Ramphul and colleagues very clearly confirms that correct placement of the vacuum device or forceps is key to safety.
Should we continue forceps education using the apprenticeship model of training?
Kyser KL, Lu X, Santillan D, et al. Forceps delivery volumes in teaching and nonteaching hospitals: Are volumes sufficient for physicians to acquire and maintain competence? Acad Med. 2014;89(1):71–76.
Ericsson KA. Necessity is the mother of invention: video recording firsthand perspectives of critical medical procedures to make simulated training more effective. Acad Med. 2014;89(1):17–20.
Kyser and colleagues have provided the best current snapshot of the opportunity for teaching instrumental vaginal delivery in the United States. They conducted a retrospective cohort study using new state inpatient data from 9 states in diverse geographic locations to capture experience at large and small teaching hospitals, as well as nonteaching institutions. They demonstrated that the opportunity for hands-on experience with these difficult and technically demanding deliveries is extremely limited and probably insufficient for all practicing physicians to maintain their skills if we continue to rely on traditional ways of teaching.
Details of the study
Using State Inpatient Data from 9 states, Kyser and colleagues identified all women hospitalized for childbirth in 2008. Of 1,344,305 deliveries in 835 hospitals, the final cohort included 624,000 operative deliveries—424,224 cesarean deliveries, 174,036 vacuum extractions, 6,158 forceps deliveries, and 19,582 deliveries that required more than 1 method. Of the 835 hospitals in this study, 68 were major teaching hospitals, 130 were minor teaching facilities, and 637 were nonteaching institutions.
The mean annual volumes for cesarean delivery for major teaching, minor teaching, and nonteaching hospitals were 969.8, 757.8, and 406.9, respectively (P<.0001).
The mean annual volumes for vacuum delivery were 301.0, 304.2, and 190.4, respectively (P<.0001).
The mean annual volumes for forceps delivery were 25.2, 15.3, and 8.9, respectively (P<.0001).
Three hundred twenty hospitals (38.3% of all hospitals) failed to perform a single forceps delivery in 2008, including 11 major teaching hospitals (16.2% of major teaching hospitals), 30 minor teaching hospitals (23.1% of minor teaching hospitals), and 279 nonteaching hospitals (43.8% of nonteaching hospitals) (P<.0001).
We need to rethink the apprenticeship model
In a commentary accompanying the study by Kyser and colleagues, K. Anders Ericsson, PhD, revisits the “see one, do one, teach one” model that has long characterized medical education. “Both the limitations on learning opportunities available in the clinics and the restrictions on resident work hours have created a real problem for the traditional apprenticeship model for training doctors,” he writes.
Ericsson notes that other specialists, such as concert musicians, chess players, and professional athletes do not learn using an apprenticeship model. For example, chess players do not play game after game of chess to become expert. And when a game is concluded, usually after several hours have passed, they are unlikely to be aware of the specific moves that lost or won them the game (unless an observer points them out). That is why, when training, chess players focus on particular aspects of the game (often identified by a mentor) as being crucial to improve their overall performance.
In today’s chess-learning environment, Ericsson notes, the computer plays a key role and can provide accurate feedback on each move the player executes. Computer chess programs have evolved to the point that they “are far superior in skill to any human chess player. Most important, computers can provide more accurate feedback on each chess move and are available at any time for practice,” writes Ericsson.
The same is true in sports. A tennis player does not practice by playing an endless series of games—though an ability to win a game is the ultimate goal. Rather, the athlete focuses on aspects of the game—the serve, for example—that can make the difference between winning or losing. Ericsson also notes that most musicians, dancers, and athletes “spend most of their time training by themselves to get ready to exhibit their skills for the first time in front of a large audience.”
These approaches are a better model for improving performance than the apprenticeship model, Ericsson argues. In medicine, one alternative might be the video recording of medical procedures in the clinic from multiple points of view—so that later viewers get both the “big picture” and a close-up view from the point of technical performance. After the recording is digitized and stored on a server, it can serve as valuable teaching for an unlimited number of residents.
Simulator training offers another venue for education, as it makes possible the isolation of difficult aspects of a procedure, which can then be repeated by the trainee as many times as necessary. In the future, it should be possible to link video recordings directly to simulators “so trainees could focus on particular aspects of the procedures and be required to respond to prompts with recordable actions,” Ericsson writes.
What this EVIDENCE means for practice
Given the extremely limited opportunities for observing forceps deliveries in the United States, it is time for us to explore new avenues for teaching other than the traditional apprenticeship model.
Is ultrasound evidence of levator muscle “avulsion” a real anatomic entity?
Dietz HP. Forceps: toward obsolescence or revival? Acta Obstet Gynecol Scand. 2015;94(4):347–351.
Da Silva AS, Digesu GA, Dell’Utri C, Fritsch H, Piffarotti P, Khullar V. Do ultrasound findings of levator ani “avulsion” correlate with anatomical findings? A multicenter cadaveric study [published online ahead of print May 15, 2015]. Neurourol Urodyn. doi:10.1002 /nau.22781.
Dietz takes a new tack in the debate over cesarean versus forceps by pointing to a recently highlighted abnormality in women who deliver by forceps: levator ani muscle avulsion, or LMA—traumatic disconnection of the levator ani from the pelvic sidewall. It has long been known that forceps deliveries can increase the risk of obstetric anal sphincter injuries (OASIS). Dietz contends that OASIS occurs at a rate as high as 40% to 60% after forceps delivery. He also notes, with some consternation, that the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have advocated forceps as a way of reducing the high cesarean delivery rate.
When a parturient has been pushing for an extended period of time and there is a positional abnormality of the fetus, such as persistent occiput posterior position, cesarean delivery is often favored as a way of protecting the rectal sphincter. Dietz argues that cesarean delivery also protects against LMA, which “has only recently been recognized as a major etiological factor in pelvic floor dysfunction.” Dietz then presents a list of studies that have produced ultrasound findings of LMA in a high percentage of women undergoing forceps delivery—percentages on the order of 10% to 40%.
Enter Da Silva and colleagues, who argue that “the only true place to visualize the 3D structure of the human body, [and] thus validate imaging findings, [is] on cadaveric or live tissue dissections.” They undertook a cadaveric study to validate—or not—some of the findings of LMA summarized by Dietz.
Details of the study
The pubovisceral muscle (PVM) anatomy of 30 female cadavers was analyzed via 3D translabial ultrasonography to confirm LMA. The cadavers were then dissected to assess the finding anatomically. Da Silva and colleagues found LMA on imaging in 11 (36.7%) cadavers. LMA was unilateral in 10 (33.3%) cadavers and bilateral in 1 (3.3%). However, no LMA was found at dissection.
When an additional 39 cadavers were dissected, no LMA was identified.
On ultrasound, LMA is strongly associated with a narrower PVM insertion depth (mean of 4.79 mm vs 6.32 mm; P = .001). Da Silva and colleagues concluded that “there is a clear difference between anatomical and ultrasonographic findings. The imaged appearance of an ‘avulsion’ does not represent a true anatomical ‘avulsion’ as confirmed on dissection.”
What this EVIDENCE means for practice
Before we prematurely adopt ultrasound evidence of LMA as a significant morbidity, we need to learn more about its true etiology, pathophysiology, and epidemiology. We don’t yet know enough to say that it’s such a bad injury, when imaged via ultrasound, that it warrants cesarean delivery to avoid it.
When deciding between cesarean and forceps, keep the risks of second-stage cesarean in mind
Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
This expert systematic review and meta-analysis summarizes the morbidity of second-stage cesarean delivery. When an obstetrician has a patient who is arrested at persistent occiput posterior position, say, and is trying to decide on cesarean delivery versus Kielland’s rotation or other forceps delivery, it is necessary to balance the risks and benefits of the 2 options. And as all clinicians are aware, when cesarean delivery is performed late in labor and the patient has been pushing for a prolonged period of time in the second stage—cesarean can be a challenging procedure. Moreover, these late cesareans are associated with much greater risks than cesarean deliveries performed earlier in labor.
Details of the review
Pergialiotis and colleagues selected 10 studies comparing maternal and neonatal morbidity and mortality between cesarean delivery at full dilatation and cesarean delivery prior to full dilatation. These studies involved 23,104 women with a singleton fetus who underwent cesarean delivery in the first (n = 18,160) or second (n = 4,944) stage of labor.
They found that second-stage cesarean was associated with a higher rate of maternal death (OR, 7.96; 95% CI, 1.61–39.39), a higher rate of maternal admission to the intensive care unit (OR, 7.41; 95% CI, 2.47–22.5), and a higher maternal transfusion rate (OR, 2.60; 95% CI, 1.49–2.54).
The rate of neonatal death also was higher among second-stage cesareans (OR, 5.20; 95% CI, 2.49–10.85), as was admission to the neonatal intensive care unit (OR, 1.63; 95% CI, 0.91–2.91), and the 5-minute Apgar score was more likely to be less than 7 (OR, 2.77; 95% CI, 1.02–7.50).
According to the authors, this study is the “first systematic review and meta-analysis that investigates the impact of the stage of labor on maternal and neonatal outcomes among women delivering by cesarean section.” The findings demonstrate with authority that second-stage cesareans can be a risky undertaking.
What this EVIDENCE means for practice
Cesareans performed late in the second stage of labor are distinct from those performed in the first stage, carrying much higher risks, especially for the mother. When deciding whether to proceed with cesarean, vacuum, or forceps, the added risk of second-stage cesarean is an important aspect of both the consent conversation and clinical decision making.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Danforth DN, Ellis AH. Midforceps delivery—a vanishing art? Am J Obstet Gynecol. 1963;86:29–37.
2. Tan KH, Sim R, Yam KL. Kielland’s forceps delivery: Is it a dying art? Singapore Med J. 1992;33(4):380–382.
3. Bofill JA. Operative obstetrics: a lost art? Obstet Gynecol Surv. 2000;55(7):405–406.
4. Dietz HP. Forceps: towards obsolescence or revival? Acta Obstet Gynecol Scand. 2015;94(4):347–351.
5. Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Surtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):9.
1. Danforth DN, Ellis AH. Midforceps delivery—a vanishing art? Am J Obstet Gynecol. 1963;86:29–37.
2. Tan KH, Sim R, Yam KL. Kielland’s forceps delivery: Is it a dying art? Singapore Med J. 1992;33(4):380–382.
3. Bofill JA. Operative obstetrics: a lost art? Obstet Gynecol Surv. 2000;55(7):405–406.
4. Dietz HP. Forceps: towards obsolescence or revival? Acta Obstet Gynecol Scand. 2015;94(4):347–351.
5. Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Surtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):9.
In this Article
- Rate of suboptimal instrument placement
- Are we educating ourselves in the most productive manner?
- Keep the risks of second-stage cesarean in mind