User login
A 49-year-old woman presents with a cough that has persisted for 3 weeks.
Two weeks ago, she was seen in the outpatient clinic for a nonproductive cough, rhinorrhea, sneezing, and a sore throat. At that time, she described coughing spells that were occasionally accompanied by posttussive chest pain and vomiting. The cough was worse at night and was occasionally associated with wheezing. She reported no fevers, chills, rigors, night sweats, or dyspnea. She said she has tried over-the-counter cough suppressants, antihistamines, and decongestants, but they provided no relief. Since she had a history of well-controlled asthma, she was diagnosed with an asthma exacerbation and was given prednisone 20 mg to take orally every day for 5 days, to be followed by an inhaled corticosteroid until her symptoms resolved.
Now, she has returned because her symptoms have persisted despite treatment, and she is seeking a second medical opinion. Her paroxysmal cough has become more frequent and more severe.
In addition to asthma, she has a history of allergic rhinitis. Her current medications include the over-the-counter histamine H1 antagonist cetirizine (Zyrtec), a fluticasone-salmeterol inhaler (Advair), and an albuterol inhaler (Proventil HFA). She reports having had mild asthma exacerbations in the past during the winter, which were managed well with her albuterol inhaler.
She has never smoked; she drinks alcohol socially. She has not traveled outside the United States during the past several months. She is married and has two children, ages 25 and 23. She lives at home with only her husband, and he has not been sick. However, she works at a greeting card store, and two of her coworkers have similar upper respiratory symptoms, although they have only a mild cough.
Her immunizations are not up-to-date. She last received the tetanus-diphtheria toxoid (Td) vaccine 12 years ago, and she never received the pediatric tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. She generally receives the influenza vaccine annually, and she received it about 6 weeks before this presentation.
She is not in distress, but she has paroxysms of severe coughing throughout her examination. Her pulse is 100 beats per minute, respiratory rate 18, and blood pressure 130/86 mm Hg. Her oropharynx is clear. The pulmonary examination reveals poor inspiratory effort due to coughing but is otherwise normal. The rest of the examination is normal, as is her chest radiograph.
WHAT DOES SHE HAVE?
1. Which of the following would best explain her symptoms?
- Asthma
- Postviral cough
- Pertussis
- Chronic bronchitis
- Pneumonia
- Gastroesophageal reflux disease
Asthma is a reasonable consideration, given her medical history, her occasional wheezing, and her nonproductive cough that is worse at night. However, asthma typically responds well to corticosteroid therapy. She has already received a course of prednisone, but her symptoms have not improved.
Postviral cough could also be considered in this patient. However, postviral cough does not typically occur in paroxysms, nor does it lead to posttussive vomiting. It is also generally regarded as a diagnosis of exclusion.
Pertussis (whooping cough) should be suspected in this patient, given the time course of her symptoms, the paroxysmal cough, and the posttussive vomiting. In addition, at her job she interacts with hundreds of people a day, increasing her risk of exposure to respiratory tract pathogens, including Bordetella pertussis.
Chronic bronchitis is defined by cough (typically productive) lasting at least 3 months per year for at least 2 consecutive years, which does not fit the time course for this patient. It is vastly more common in smokers.
Pneumonia typically presents with a cough that can be productive or nonproductive, but also with fever, chills, and radiologic evidence of a pulmonary infiltrate or consolidation. This woman has none of these.
Gastroesophageal reflux disease is one of the most common causes of chronic cough, with symptoms typically worse at night. However, it is generally associated with symptoms such as heartburn, a sour taste in the mouth, or regurgitation, which our patient did not report.
Thus, pertussis is the most likely diagnosis.
PERTUSSIS IS ON THE RISE
Pertussis is an acute and highly contagious disease caused by infection of the respiratory tract by B pertussis, a small, aerobic, gramnegative, pleomorphic coccobacillus that produces a number of antigenic and biologically active products, including pertussis toxin, filamentous hemagglutinin, agglutinogens, and tracheal cytotoxin. Transmitted by aerosolized droplets, it attaches to the ciliated epithelial cells of the lower respiratory tract, paralyzes the cilia via toxins, and causes inflammation, thus interfering with the clearing of respiratory secretions.
The incidence of pertussis is on the rise. In 2005, 25,827 cases were reported in the United States, the highest number since 1959.1 Pertussis is now epidemic in California. At the time of this writing, the number of confirmed, probable, and suspected cases in California was 9,477 (including 10 infant deaths) for the year 2010—the most cases reported in the past 65 years.2,3
In 2010, outbreaks were also reported in Michigan, Texas, Ohio, upstate New York, and Arizona.4 The overall incidence of pertussis is likely even higher than what is reported, since many cases go unrecognized or unreported.
Highly contagious
Pertussis is transmitted person-to-person, primarily through aerosolized droplets from coughing or sneezing or by direct contact with secretions from the respiratory tract of infected persons. It is highly contagious, with secondary attack rates of up to 80% in susceptible people.
A three-stage clinical course
The clinical definition of pertussis used by the US Centers for Disease Control and Prevention (CDC) and the Council of State and Territorial Epidemiologists is an acute cough illness lasting at least 2 weeks, with paroxysms of coughing, an inspiratory “whoop,” or posttussive vomiting without another apparent cause.5
The clinical course of the illness is traditionally divided into three stages:
The catarrhal phase typically lasts 1 to 2 weeks and is clinically indistinguishable from a viral upper respiratory infection. It is characterized by the insidious onset of malaise, coryza, sneezing, low-grade fever, and a mild cough that gradually becomes severe.6
The paroxysmal phase normally lasts 1 to 6 weeks but may persist for up to 10 weeks. The diagnosis of pertussis is usually suspected during this phase. The classic features of this phase are bursts or paroxysms of numerous, rapid coughs. These are followed by a long inspiratory effort usually accompanied by a characteristic high-pitched whoop, most notably observed in infants and children. Infants and children may appear very ill and distressed during this time and may become cyanotic, but cyanosis is uncommon in adults and adolescents. The paroxysms may also be followed by exhaustion and posttussive vomiting. In some cases, the cough is not paroxysmal, but rather simply persistent. The coughing attacks tend to occur more often at night, with an average of 15 attacks per 24 hours. During the first 1 to 2 weeks of this stage, the attacks generally increase in frequency, remain at the same intensity level for 2 to 3 weeks, and then gradually decrease over 1 to 2 weeks.1,7
The convalescent phase can have a variable course, ranging from weeks to months, with an average duration of 2 to 3 weeks. During this stage, the paroxysms of coughing become less frequent and gradually resolve. Paroxysms often recur with subsequent respiratory infections.
In infants and young children, pertussis tends to follow these stages in a predictable sequence. Adolescents and adults, however, tend to go through the stages without being as ill and typically do not exhibit the characteristic whoop.
TESTING FOR PERTUSSIS
2. Which would be the test of choice to confirm pertussis in this patient?
- Bacterial culture of nasopharyngeal secretions
- Polymerase chain reaction (PCR) testing of nasopharyngeal secretions
- Direct fluorescent antibody testing of nasopharyngeal secretions
- Enzyme-linked immunosorbent assay (ELISA) serologic testing
Establishing the diagnosis of pertussis is often rather challenging.
Bacterial culture: Very specific, but slow and not so sensitive
Bacterial culture is still the gold standard for diagnosing pertussis, as a positive culture for B pertussis is 100% specific.5
However, this test has drawbacks. Its sensitivity has a wide range (15% to 80%) and depends very much on the time from the onset of symptoms to the time the culture specimen is collected. The yield drops off significantly after 1 week, and after 3 weeks the test has a sensitivity of only 1% to 3%.8 Therefore, for our patient, who has had symptoms for 3 weeks already, bacterial culture would not be the best test. In addition, the results are usually not known for 7 to 14 days, which is too slow to be useful in managing acute cases.
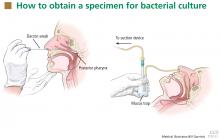
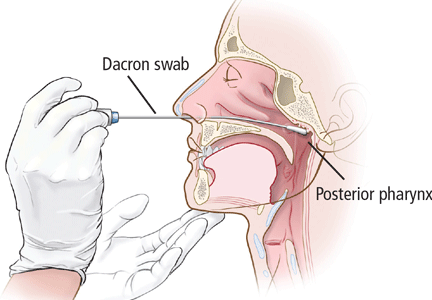
For swabbing, a Dacron swab is inserted through the nostril to the posterior pharynx and is left in place for 10 seconds to maximize the yield of the specimen. Recovery rates for B pertussis are low if the throat or the anterior nasal passage is swabbed instead of the posterior pharynx.9
Nasopharyngeal aspiration is a more complicated procedure, requiring a suction device to trap the mucus, but it may provide higher yields than swabbing.10 In this method, the specimen is obtained by inserting a small tube (eg, an infant feeding tube) connected to a mucus trap into the nostril back to the posterior pharynx.
Often, direct inoculation of medium for B pertussis is not possible. In such cases, clinical specimens are placed in Regan Lowe transport medium (half-strength charcoal agar supplemented with horse blood and cephalexin).11,12
Polymerase chain reaction testing: Faster, more sensitive, but less specific
PCR testing of nasopharyngeal specimens is now being used instead of bacterial culture to diagnose pertussis in many situations. Alternatively, nasopharyngeal aspirate (or secretions collected with two Dacron swabs) can be obtained and divided at the time of collection and the specimens sent for both culture and PCR testing. Because bacterial culture is time-consuming and has poor sensitivity, the CDC states that a positive PCR test, along with the clinical symptoms and epidemiologic information, is sufficient for diagnosis.5
PCR testing can detect B pertussis with greater sensitivity and more rapidly than bacterial culture.12–14 Its sensitivity ranges from 61% to 99%, its specificity ranges from 88% to 98%,12,15,16 and its results can be available in 2 to 24 hours.12
PCR testing’s advantage in terms of sensitivity is especially pronounced in the later stages of the disease (as in our patient), when clinical suspicion of pertussis typically arises. It can be used effectively for up to 4 weeks from the onset of cough.14 Our patient, who presented nearly 3 weeks after the onset of symptoms, underwent nasopharyngeal sampling for PCR testing.
However, PCR testing is not as specific for B pertussis as is bacterial culture, since other Bordetella species can cause positive results on PCR testing. Also, as with culture, a negative test does not reliably rule out the disease, especially if the sample is collected late in the course.
Therefore, basing the diagnosis on PCR testing alone without the proper clinical context is not advised: pertussis outbreaks have been mistakenly declared on the basis of false-positive PCR test results. Three so-called “pertussis outbreaks” in three different states from 2004 to 200617 were largely the result of overdiagnosis based on equivocal or false-positive PCR test results without the appropriate clinical circumstances. Retrospective review of these pseudo-outbreaks revealed that few cases actually met the CDC’s diagnostic criteria.17 Many patients were not tested (by any method) for pertussis and were treated as having probable cases of pertussis on the basis of their symptoms. Patients who were tested and who had a positive PCR test did not meet the clinical definition of pertussis according to the Council of State and Territorial Epidemiologists.17
Since PCR testing varies in sensitivity and specificity, obtaining culture confirmation of pertussis for at least one suspicious case is recommended any time an outbreak is suspected. This is necessary for monitoring for continued presence of the agent among cases of disease, recruitment of isolates for epidemiologic studies, and surveillance for antibiotic resistance.
Direct fluorescence antibody testing
The CDC does not recommend direct fluorescence antibody testing to diagnose pertussis. This test is commercially available and is sometimes used to screen patients for B pertussis infection, but it lacks sensitivity and specificity for this organism. Cross-reaction with normal nasopharyngeal flora can lead to a false-positive result.18 In addition, the interpretation of the test is subjective, so the sensitivity and specificity are quite variable: the sensitivity is reported as 52% to 65%, while the specificity can vary from 15% to 99%.
Enzyme-linked immunosorbent assay
ELISA testing has been used in epidemiologic studies to measure serum antibodies to B pertussis. Many serologic tests exist, but none is commercially available. Many of these tests are used by the CDC and state health departments to help confirm the diagnosis, especially during outbreaks. Generally, serologic tests are more useful for diagnosis in later phases of the disease. Currently used ELISA tests use both paired and single serology techniques measuring elevated immunoglobulin G serum antibody concentrations against an array of antigens, including pertussis toxin, filamentous hemagglutinin, pertactin, and fimbrae. As a result, a range of sensitivities (33%–95%) and specificities (72%–100%) has been reported.12,14,19
TREATING PERTUSSIS
Our patient’s PCR test result comes back positive. In view of her symptoms and this result, we decide to treat her empirically for pertussis, even though she has had no known contact with anyone with the disease and there is currently no outbreak of it in the community.
3. According to the most recent evidence, which of the following would be the treatment of choice for pertussis in this patient?
- Azithromycin (Zithromax)
- Amoxicillin (Moxatag)
- Levofloxacin (Levaquin)
- Sulfamethoxazole-trimethoprim (Bactrim)
- Supportive measures (hydration, humidifier, antitussives, antihistamines, decongestants)
Azithromycin and the other macrolide antibiotics erythromycin and clarithromycin are first-line therapies for pertussis in adolescents and adults. If given during the catarrhal phase, they can reduce the duration and severity of symptoms and lessen the period of communicability.20,21 After the catarrhal phase, however, it is uncertain whether antibiotics change the clinical course of pertussis, as the data are conflicting.20–22
Factors to consider when selecting a macrolide antibiotic are tolerability, the potential for adverse events and drug interactions, ease of compliance, and cost. All three macrolides are equally effective against pertussis, but azithromycin and clarithromycin are generally better tolerated and are associated with milder and less frequent side effects than erythromycin, including lower rates of gastrointestinal side effects.
Erythromycin and clarithromycin inhibit the cytochrome P450 enzyme system, specifically CYP3A4, and can interact with a great many commonly prescribed drugs metabolized by this enzyme. Therefore, azithromycin may be a better choice for patients already taking other medications, like our patient.
Azithromycin and clarithromycin have longer half-lives and achieve higher tissue concentrations than erythromycin, allowing for less-frequent dosing (daily for azithromycin and twice daily for clarithromycin) and shorter treatment duration (5 days for azithromycin and 7 days for clarithromycin).
An advantage of erythromycin, though, is its lower cost. The cost of a recommended course of erythromycin treatment for pertussis (ie, 500 mg every 6 hours for 14 days) is roughly $20, compared with $75 for azithromycin.
Amoxicillin is not effective in clearing B pertussis from the nasopharynx and thus is not a reasonable option for the treatment of pertussis.23
Levofloxacin is also not recommended for the treatment of pertussis.
Sulfamethoxazole-trimethoprim is a second-line agent for pertussis. It is effective in eradicating B pertussis from the nasopharynx20 and is generally used as an alternative to the macrolide agents in patients who cannot tolerate or have contraindications to macrolides. Sulfamethoxazole-trimethoprim can also be an option for patients infected with rare macrolide-resistant strains of B pertussis.
Supportive measures by themselves are reasonable for patients with pertussis beyond the catarrhal phase, since antibiotics are typically not effective at that stage of the disease.
From 80% to 90% of patients with untreated pertussis spontaneously clear the bacteria from the nasopharynx within 3 to 4 weeks from the onset of cough symptoms.20 However, supportive measures, including antitussives (both over-the-counter and prescription), tend to have very little effect on the severity or duration of the illness, especially when used past the early stage of the illness.
POSTEXPOSURE CHEMOPROPHYLAXIS FOR CLOSE CONTACTS
Postexposure chemoprophylaxis should be given to close contacts of patients who have pertussis to help prevent secondary cases.22 The CDC defines a close contact as someone who has had face-to-face exposure within 3 feet of a symptomatic patient within 21 days after the onset of symptoms in the patient. Close contacts should be treated with antibiotic regimens similar to those used in confirmed cases of pertussis.
In our patient’s case, the diagnosis of pertussis was reported to the Ohio Department of Health. Shortly afterward, the department contacted the patient and obtained information about her close contacts. These people were then contacted and encouraged to complete a course of antibiotics for postexposure chemoprophylaxis, given the high secondary attack rates.
PERTUSSIS VACCINES
4. Which of the following vaccines could have reduced our patient’s chance of contracting the disease or reduced the severity or time course of the illness?
- DTaP
- Tdap
- Whole-cell pertussis vaccine
- No vaccine would have reduced her risk
It is important to prevent pertussis, given its associated morbidities and its generally poor response to drug therapy. Continued vigilance is imperative to maintain high levels of vaccine coverage, including the timely completion of the pertussis vaccination schedule.
The two vaccines in current use in the United States to produce immunity to pertussis—DTaP and Tdap—also confer immunity to diphtheria and tetanus. DTaP is used for children under 7 years of age, and Tdap is for ages 10 to 64. Thus, our patient should have received a series of DTaP injections as an infant and small child, and a Tdap booster at age 11 or 12 years and every 10 years after that.
The upper case “D,” “T,” and “P” in the abbreviations signifies full-strength doses and the lower case “d,” “t,” and “p” indicate that the doses of those components have been reduced. The “a” in both vaccines stands for “acellular”: ie, the pertussis component does not contain cellular elements.
DTaP for initial pertussis vaccination
The current recommendation for initial pertussis vaccination consists of a primary series of DTaP. DTaP vaccination is recommended for infants at 2 months of age, then again at 4 months of age, and again at 6 months of age. A fourth dose is given between the ages of 15 and 18 months, and a fifth dose is given between the ages of 4 to 6 years. If the fourth dose was given after age 4, then no fifth dose is needed.20
Tdap as a booster
The booster vaccine for adolescents and adults is Tdap. In 2005, two Tdap vaccines were licensed in the United States: Adacel for people ages 11 to 64 years, and Boostrix for people ages 10 to 18 years.
The CDC’s Advisory Committee on Immunization Practices (ACIP) recommends a booster dose of Tdap at age 11 or 12 years. Every 10 years thereafter, a booster of tetanus and diphtheria toxoid (Td) vaccine is recommended, except that one of the Td doses can be replaced by Tdap if the patient hasn’t received Tdap yet.
For adults ages 19 to 64, the ACIP currently recommends routine use of a single booster dose of Tdap to replace a single dose of Td if they received the last dose of toxoid vaccine 10 or more years earlier. If the previous dose of Td was given within the past 10 years, a single dose of Tdap is appropriate to protect patients against pertussis. This is especially true for patients at increased risk of pertussis or its complications, as well as for health care professionals and adults who have close contact with infants, such as new parents, grandparents, and child-care providers. The minimum interval since the last Td vaccination is ideally 2 years, although shorter intervals can be used for control of pertussis outbreaks and for those who have close contact with infants.24
In 2010, the ACIP decided that, for those ages 65 and older, a single dose of Tdap vaccine may be given in place of Td if the patient has not previously received Tdap, regardless of how much time has elapsed since the last vaccination with a Td-containing vaccine.25 Data from the Vaccine Adverse Event Reporting System suggest that Tdap vaccine in this age group is as safe as the Td vaccine.25
Subsequent tetanus vaccine doses, in the form of Td, should be given at 10-year intervals throughout adulthood. Administration of Tdap at 10-year intervals appears to be highly immunogenic and well tolerated,25 suggesting that it is possible that Tdap will become part of routine booster dosing instead of Td, pending further study.
Tdap is not contraindicated in pregnant women. Ideally, women should be vaccinated with Tdap before becoming pregnant if they have not previously received it. If the pregnant woman is not at risk of acquiring or transmitting pertussis during pregnancy, the ACIP recommends deferring Tdap vaccination until the immediate postpartum period.
Adults who require a vaccine containing tetanus toxoid for wound management should receive Tdap instead of Td if they have never received Tdap. Adults who have never received vaccine containing tetanus and diphtheria toxoid should receive a series of three vaccinations. The preferred schedule is a dose of Tdap, followed by a dose of Td more than 4 weeks later, and a second dose of Td 6 to 12 months later, though Tdap can be substituted for Td for any one of the three doses in the series. Adults with a history of pertussis generally should receive Tdap according to routine recommendations.
Tdap is contraindicated in people with a history of serious allergic reaction to any component of the Tdap vaccine or with a history of encephalopathy not attributable to an identifiable cause within 7 days of receiving a pertussis vaccine. Tdap is relatively contraindicated and should be deferred in people with current moderate to severe acute illness, current unstable neurologic condition, or a history of Arthus hypersensitivity reaction to a tetanus-toxoid-containing vaccine within the past 10 years, and in people who have developed Guillain-Barré syndrome, within 6 weeks of receiving a tetanus-toxoid–containing vaccine.
Tdap is generally well tolerated. Adverse effects are typically mild and may include localized pain, redness, and swelling; low-grade fever; headache; fatigue; and, less commonly, gastrointestinal upset, myalgia, arthralgia, rash, and swollen glands.
Whole-cell pertussis vaccine is no longer available in the United States
Whole-cell pertussis vaccine provides good protection against pertussis, with 70% to 90% efficacy after three doses. It is less expensive-than acellular formulations and therefore is used in many parts of the world where cost is an issue. It is no longer available in the United States, however, due to high rates of local reactions such as redness, swelling, and pain at the injection site.
The importance of staying up-to-date with booster shots
Booster vaccination for pertussis in adolescents and adults is critical, since the largest recent outbreaks have occurred in these groups.21 The high rate of outbreaks is presumably the result of waning immunity from childhood immunizations and of high interpersonal contact rates. Vaccination has been shown to reduce the chance of contracting the disease and to reduce the severity and time course of the illness.21
Adolescents and adults are an important reservoir for potentially serious infections in infants who are either unvaccinated or whose vaccination schedule has not been completed. These infants are at risk of severe illness, including pneumonia, seizures, encephalopathy, and apnea, or even death. Adults and teens can also suffer complications from pertussis, although these tend to be less serious, especially in those who have been vaccinated. Complications in teens and adults are often caused by malaise and the cough itself, including weight loss (33%), urinary stress incontinence (28%), syncope (6%), rib fractures from severe coughing (4%), and pneumonia (2%).26 Thus, it is important that adolescents and adults stay up-to-date with pertussis vaccination.
CASE CONTINUED
Our patient was treated with a short (5-day) course of azithromycin 500 mg daily. It did not improve her symptoms very much, but this was not unexpected, given her late presentation and duration of symptoms. Her cough persisted for about 2 months afterwards, but it improved with time and with supportive care at home.
CONTINUED CHALLENGES
Pertussis is a reemerging disease with an increased incidence over the past 30 years, and even more so over the past 10 years. Unfortunately, treatments are not very effective, especially since the disease is often diagnosed late in the course.
We are fortunate to have a vaccine that can prevent pertussis, yet pertussis persists, in large part because of waning immunity from childhood vaccination. The duration of immunity from childhood vaccination is not yet clear. Many adolescents and adults do not follow up on these booster vaccines, thus increasing their susceptibility to pertussis. Consequently, they can transmit the disease to children who are not fully immunized. Prevention by maintaining active immunity is the key to controlling this terrible disease.
- Centers for Disease Control and Prevention. Pertussis. National Immunization Program, 2005. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf. Accessed July 6, 2011.
- California Department of Public Health. Pertussis report. www.cdph.ca.gov/programs/immunize/Documents/PertussisReport2011-01-07.pdf. Accessed July 6, 2011.
- Centers for Disease Control and Prevention. Pertussis (whooping cough). www.cdc.gov/pertussis/outbreaks.html. Accessed July 3, 2011.
- Centers for Disease Control and Prevention. Notifiable diseases and mortality tables. MMWR Morb Mortal Wkly Rep 2010; 59:847–861. http://www.cdc.gov/mmwr/PDF/wk/mm5927.pdf. Accessed July 1, 2011.
- Centers for Disease Control and Prevention. Pertussis. Vaccines and preventable diseases: pertussis (whooping cough) vaccination, 2010. http://www.cdc.gov/vaccines/vpd-vac/pertussis/default.htm. Accessed July 6, 2011.
- Hewlett EL, Edwards KM. Clinical practice. Pertussis—not just for kids. N Engl J Med 2005; 352:1215–1222.
- Hewlett E. Bordetella species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 5th ed, Philadelphia, PA: Churchill Livingstone; 2000:2701.
- Viljanen MK, Ruuskanen O, Granberg C, Salmi TT. Serological diagnosis of pertussis: IgM, IgA and IgG antibodies against Bordetella pertussis measured by enzyme-linked immunosorbent assay (ELISA). Scand J Infect Dis 1982; 14:117–122.
- Bejuk D, Begovac J, Bace A, Kuzmanovic-Sterk N, Aleraj B. Culture of Bordetella pertussis from three upper respiratory tract specimens. Pediatr Infect Dis J 1995; 14:64–65.
- Hallander HO, Reizenstein E, Renemar B, Rasmuson G, Mardin L, Olin P. Comparison of nasopharyngeal aspirates with swabs for culture of Bordetella pertussis. J Clin Microbiol 1993; 31:50–52.
- Regan J, Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol 1977; 6:303–309.
- World Health Organization. Laboratory manual for the diagnosis of whooping cough caused by Bordetella pertussis/Bordetella para-pertussis. Department of Immunization, Vaccines and Biologicals. Printed 2004. Revised 2007. www.who.int/vaccines-documents/. Accessed July 6, 2011.
- Meade BD, Bollen A. Recommendations for use of the polymerase chain reaction in the diagnosis of Bordetella pertussis infections. J Med Microbiol 1994; 41:51–55.
- Wendelboe AM, Van Rie A. Diagnosis of pertussis: a historical review and recent developments. Expert Rev Mol Diagn 2006; 6:857–864.
- Knorr L, Fox JD, Tilley PA, Ahmed-Bentley J. Evaluation of real-time PCR for diagnosis of Bordetella pertussis infection. BMC Infect Dis 2006; 6:62.
- Sotir MJ, Cappozzo DL, Warshauer DM, et al. Evaluation of polymerase chain reaction and culture for diagnosis of pertussis in the control of a county-wide outbreak focused among adolescents and adults. Clin Infect Dis 2007; 44:1216–1219.
- Centers for Disease Control and Prevention (CDC). Outbreaks of respiratory illness mistakenly attributed to pertussis—New Hampshire, Massachusetts, and Tennessee, 2004–2006. MMWR Morb Mortal Wkly Rep 2007; 56:837–842.
- Ewanowich CA, Chui LW, Paranchych MG, Peppler MS, Marusyk RG, Albritton WL. Major outbreak of pertussis in northern Alberta, Canada: analysis of discrepant direct fluorescent-antibody and culture results by using polymerase chain reaction methodology. J Clin Microbiol 1993; 31:1715–1725.
- Müller FM, Hoppe JE, Wirsing von König CH. Laboratory diagnosis of pertussis: state of the art in 1997. J Clin Microbiol 1997; 35:2435–2443.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep 2005; 54:1–16.
- Wirsing von König CH, Postels-Multani S, Bock HL, Schmitt HJ. Pertussis in adults: frequency of transmission after household exposure. Lancet 1995; 346:1326–1329.
- von König CH. Use of antibiotics in the prevention and treatment of pertussis. Pediatr Infect Dis J 2005; 24(suppl 5):S66–S68.
- Trollfors B. Effect of erythromycin and amoxycillin on Bordetella pertussis in the nasopharynx. Infection 1978; 6:228–230.
- Broder KR, Cortese MM, Iskander JK, et al; Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–34.
- Centers for Disease Control and Prevention. Recommendations and Guidelines. ACIP presentation slides: October 2010 meeting. http://www.cdc.gov/vaccines/recs/acip/slides-oct10.htm. Accessed July 6, 2011.
- Cortese MM, Bisgard KM. Pertussis. In:Wallace RB, Kohatsu N, Last JM, editors. Wallace/Maxcy-Rosenau-Last Public Health & Preventive Medicine. 15th ed. New York, NY: McGraw-Hill Medical, 2008:111–114.
A 49-year-old woman presents with a cough that has persisted for 3 weeks.
Two weeks ago, she was seen in the outpatient clinic for a nonproductive cough, rhinorrhea, sneezing, and a sore throat. At that time, she described coughing spells that were occasionally accompanied by posttussive chest pain and vomiting. The cough was worse at night and was occasionally associated with wheezing. She reported no fevers, chills, rigors, night sweats, or dyspnea. She said she has tried over-the-counter cough suppressants, antihistamines, and decongestants, but they provided no relief. Since she had a history of well-controlled asthma, she was diagnosed with an asthma exacerbation and was given prednisone 20 mg to take orally every day for 5 days, to be followed by an inhaled corticosteroid until her symptoms resolved.
Now, she has returned because her symptoms have persisted despite treatment, and she is seeking a second medical opinion. Her paroxysmal cough has become more frequent and more severe.
In addition to asthma, she has a history of allergic rhinitis. Her current medications include the over-the-counter histamine H1 antagonist cetirizine (Zyrtec), a fluticasone-salmeterol inhaler (Advair), and an albuterol inhaler (Proventil HFA). She reports having had mild asthma exacerbations in the past during the winter, which were managed well with her albuterol inhaler.
She has never smoked; she drinks alcohol socially. She has not traveled outside the United States during the past several months. She is married and has two children, ages 25 and 23. She lives at home with only her husband, and he has not been sick. However, she works at a greeting card store, and two of her coworkers have similar upper respiratory symptoms, although they have only a mild cough.
Her immunizations are not up-to-date. She last received the tetanus-diphtheria toxoid (Td) vaccine 12 years ago, and she never received the pediatric tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. She generally receives the influenza vaccine annually, and she received it about 6 weeks before this presentation.
She is not in distress, but she has paroxysms of severe coughing throughout her examination. Her pulse is 100 beats per minute, respiratory rate 18, and blood pressure 130/86 mm Hg. Her oropharynx is clear. The pulmonary examination reveals poor inspiratory effort due to coughing but is otherwise normal. The rest of the examination is normal, as is her chest radiograph.
WHAT DOES SHE HAVE?
1. Which of the following would best explain her symptoms?
- Asthma
- Postviral cough
- Pertussis
- Chronic bronchitis
- Pneumonia
- Gastroesophageal reflux disease
Asthma is a reasonable consideration, given her medical history, her occasional wheezing, and her nonproductive cough that is worse at night. However, asthma typically responds well to corticosteroid therapy. She has already received a course of prednisone, but her symptoms have not improved.
Postviral cough could also be considered in this patient. However, postviral cough does not typically occur in paroxysms, nor does it lead to posttussive vomiting. It is also generally regarded as a diagnosis of exclusion.
Pertussis (whooping cough) should be suspected in this patient, given the time course of her symptoms, the paroxysmal cough, and the posttussive vomiting. In addition, at her job she interacts with hundreds of people a day, increasing her risk of exposure to respiratory tract pathogens, including Bordetella pertussis.
Chronic bronchitis is defined by cough (typically productive) lasting at least 3 months per year for at least 2 consecutive years, which does not fit the time course for this patient. It is vastly more common in smokers.
Pneumonia typically presents with a cough that can be productive or nonproductive, but also with fever, chills, and radiologic evidence of a pulmonary infiltrate or consolidation. This woman has none of these.
Gastroesophageal reflux disease is one of the most common causes of chronic cough, with symptoms typically worse at night. However, it is generally associated with symptoms such as heartburn, a sour taste in the mouth, or regurgitation, which our patient did not report.
Thus, pertussis is the most likely diagnosis.
PERTUSSIS IS ON THE RISE
Pertussis is an acute and highly contagious disease caused by infection of the respiratory tract by B pertussis, a small, aerobic, gramnegative, pleomorphic coccobacillus that produces a number of antigenic and biologically active products, including pertussis toxin, filamentous hemagglutinin, agglutinogens, and tracheal cytotoxin. Transmitted by aerosolized droplets, it attaches to the ciliated epithelial cells of the lower respiratory tract, paralyzes the cilia via toxins, and causes inflammation, thus interfering with the clearing of respiratory secretions.
The incidence of pertussis is on the rise. In 2005, 25,827 cases were reported in the United States, the highest number since 1959.1 Pertussis is now epidemic in California. At the time of this writing, the number of confirmed, probable, and suspected cases in California was 9,477 (including 10 infant deaths) for the year 2010—the most cases reported in the past 65 years.2,3
In 2010, outbreaks were also reported in Michigan, Texas, Ohio, upstate New York, and Arizona.4 The overall incidence of pertussis is likely even higher than what is reported, since many cases go unrecognized or unreported.
Highly contagious
Pertussis is transmitted person-to-person, primarily through aerosolized droplets from coughing or sneezing or by direct contact with secretions from the respiratory tract of infected persons. It is highly contagious, with secondary attack rates of up to 80% in susceptible people.
A three-stage clinical course
The clinical definition of pertussis used by the US Centers for Disease Control and Prevention (CDC) and the Council of State and Territorial Epidemiologists is an acute cough illness lasting at least 2 weeks, with paroxysms of coughing, an inspiratory “whoop,” or posttussive vomiting without another apparent cause.5
The clinical course of the illness is traditionally divided into three stages:
The catarrhal phase typically lasts 1 to 2 weeks and is clinically indistinguishable from a viral upper respiratory infection. It is characterized by the insidious onset of malaise, coryza, sneezing, low-grade fever, and a mild cough that gradually becomes severe.6
The paroxysmal phase normally lasts 1 to 6 weeks but may persist for up to 10 weeks. The diagnosis of pertussis is usually suspected during this phase. The classic features of this phase are bursts or paroxysms of numerous, rapid coughs. These are followed by a long inspiratory effort usually accompanied by a characteristic high-pitched whoop, most notably observed in infants and children. Infants and children may appear very ill and distressed during this time and may become cyanotic, but cyanosis is uncommon in adults and adolescents. The paroxysms may also be followed by exhaustion and posttussive vomiting. In some cases, the cough is not paroxysmal, but rather simply persistent. The coughing attacks tend to occur more often at night, with an average of 15 attacks per 24 hours. During the first 1 to 2 weeks of this stage, the attacks generally increase in frequency, remain at the same intensity level for 2 to 3 weeks, and then gradually decrease over 1 to 2 weeks.1,7
The convalescent phase can have a variable course, ranging from weeks to months, with an average duration of 2 to 3 weeks. During this stage, the paroxysms of coughing become less frequent and gradually resolve. Paroxysms often recur with subsequent respiratory infections.
In infants and young children, pertussis tends to follow these stages in a predictable sequence. Adolescents and adults, however, tend to go through the stages without being as ill and typically do not exhibit the characteristic whoop.
TESTING FOR PERTUSSIS
2. Which would be the test of choice to confirm pertussis in this patient?
- Bacterial culture of nasopharyngeal secretions
- Polymerase chain reaction (PCR) testing of nasopharyngeal secretions
- Direct fluorescent antibody testing of nasopharyngeal secretions
- Enzyme-linked immunosorbent assay (ELISA) serologic testing
Establishing the diagnosis of pertussis is often rather challenging.
Bacterial culture: Very specific, but slow and not so sensitive
Bacterial culture is still the gold standard for diagnosing pertussis, as a positive culture for B pertussis is 100% specific.5
However, this test has drawbacks. Its sensitivity has a wide range (15% to 80%) and depends very much on the time from the onset of symptoms to the time the culture specimen is collected. The yield drops off significantly after 1 week, and after 3 weeks the test has a sensitivity of only 1% to 3%.8 Therefore, for our patient, who has had symptoms for 3 weeks already, bacterial culture would not be the best test. In addition, the results are usually not known for 7 to 14 days, which is too slow to be useful in managing acute cases.
For swabbing, a Dacron swab is inserted through the nostril to the posterior pharynx and is left in place for 10 seconds to maximize the yield of the specimen. Recovery rates for B pertussis are low if the throat or the anterior nasal passage is swabbed instead of the posterior pharynx.9
Nasopharyngeal aspiration is a more complicated procedure, requiring a suction device to trap the mucus, but it may provide higher yields than swabbing.10 In this method, the specimen is obtained by inserting a small tube (eg, an infant feeding tube) connected to a mucus trap into the nostril back to the posterior pharynx.
Often, direct inoculation of medium for B pertussis is not possible. In such cases, clinical specimens are placed in Regan Lowe transport medium (half-strength charcoal agar supplemented with horse blood and cephalexin).11,12
Polymerase chain reaction testing: Faster, more sensitive, but less specific
PCR testing of nasopharyngeal specimens is now being used instead of bacterial culture to diagnose pertussis in many situations. Alternatively, nasopharyngeal aspirate (or secretions collected with two Dacron swabs) can be obtained and divided at the time of collection and the specimens sent for both culture and PCR testing. Because bacterial culture is time-consuming and has poor sensitivity, the CDC states that a positive PCR test, along with the clinical symptoms and epidemiologic information, is sufficient for diagnosis.5
PCR testing can detect B pertussis with greater sensitivity and more rapidly than bacterial culture.12–14 Its sensitivity ranges from 61% to 99%, its specificity ranges from 88% to 98%,12,15,16 and its results can be available in 2 to 24 hours.12
PCR testing’s advantage in terms of sensitivity is especially pronounced in the later stages of the disease (as in our patient), when clinical suspicion of pertussis typically arises. It can be used effectively for up to 4 weeks from the onset of cough.14 Our patient, who presented nearly 3 weeks after the onset of symptoms, underwent nasopharyngeal sampling for PCR testing.
However, PCR testing is not as specific for B pertussis as is bacterial culture, since other Bordetella species can cause positive results on PCR testing. Also, as with culture, a negative test does not reliably rule out the disease, especially if the sample is collected late in the course.
Therefore, basing the diagnosis on PCR testing alone without the proper clinical context is not advised: pertussis outbreaks have been mistakenly declared on the basis of false-positive PCR test results. Three so-called “pertussis outbreaks” in three different states from 2004 to 200617 were largely the result of overdiagnosis based on equivocal or false-positive PCR test results without the appropriate clinical circumstances. Retrospective review of these pseudo-outbreaks revealed that few cases actually met the CDC’s diagnostic criteria.17 Many patients were not tested (by any method) for pertussis and were treated as having probable cases of pertussis on the basis of their symptoms. Patients who were tested and who had a positive PCR test did not meet the clinical definition of pertussis according to the Council of State and Territorial Epidemiologists.17
Since PCR testing varies in sensitivity and specificity, obtaining culture confirmation of pertussis for at least one suspicious case is recommended any time an outbreak is suspected. This is necessary for monitoring for continued presence of the agent among cases of disease, recruitment of isolates for epidemiologic studies, and surveillance for antibiotic resistance.
Direct fluorescence antibody testing
The CDC does not recommend direct fluorescence antibody testing to diagnose pertussis. This test is commercially available and is sometimes used to screen patients for B pertussis infection, but it lacks sensitivity and specificity for this organism. Cross-reaction with normal nasopharyngeal flora can lead to a false-positive result.18 In addition, the interpretation of the test is subjective, so the sensitivity and specificity are quite variable: the sensitivity is reported as 52% to 65%, while the specificity can vary from 15% to 99%.
Enzyme-linked immunosorbent assay
ELISA testing has been used in epidemiologic studies to measure serum antibodies to B pertussis. Many serologic tests exist, but none is commercially available. Many of these tests are used by the CDC and state health departments to help confirm the diagnosis, especially during outbreaks. Generally, serologic tests are more useful for diagnosis in later phases of the disease. Currently used ELISA tests use both paired and single serology techniques measuring elevated immunoglobulin G serum antibody concentrations against an array of antigens, including pertussis toxin, filamentous hemagglutinin, pertactin, and fimbrae. As a result, a range of sensitivities (33%–95%) and specificities (72%–100%) has been reported.12,14,19
TREATING PERTUSSIS
Our patient’s PCR test result comes back positive. In view of her symptoms and this result, we decide to treat her empirically for pertussis, even though she has had no known contact with anyone with the disease and there is currently no outbreak of it in the community.
3. According to the most recent evidence, which of the following would be the treatment of choice for pertussis in this patient?
- Azithromycin (Zithromax)
- Amoxicillin (Moxatag)
- Levofloxacin (Levaquin)
- Sulfamethoxazole-trimethoprim (Bactrim)
- Supportive measures (hydration, humidifier, antitussives, antihistamines, decongestants)
Azithromycin and the other macrolide antibiotics erythromycin and clarithromycin are first-line therapies for pertussis in adolescents and adults. If given during the catarrhal phase, they can reduce the duration and severity of symptoms and lessen the period of communicability.20,21 After the catarrhal phase, however, it is uncertain whether antibiotics change the clinical course of pertussis, as the data are conflicting.20–22
Factors to consider when selecting a macrolide antibiotic are tolerability, the potential for adverse events and drug interactions, ease of compliance, and cost. All three macrolides are equally effective against pertussis, but azithromycin and clarithromycin are generally better tolerated and are associated with milder and less frequent side effects than erythromycin, including lower rates of gastrointestinal side effects.
Erythromycin and clarithromycin inhibit the cytochrome P450 enzyme system, specifically CYP3A4, and can interact with a great many commonly prescribed drugs metabolized by this enzyme. Therefore, azithromycin may be a better choice for patients already taking other medications, like our patient.
Azithromycin and clarithromycin have longer half-lives and achieve higher tissue concentrations than erythromycin, allowing for less-frequent dosing (daily for azithromycin and twice daily for clarithromycin) and shorter treatment duration (5 days for azithromycin and 7 days for clarithromycin).
An advantage of erythromycin, though, is its lower cost. The cost of a recommended course of erythromycin treatment for pertussis (ie, 500 mg every 6 hours for 14 days) is roughly $20, compared with $75 for azithromycin.
Amoxicillin is not effective in clearing B pertussis from the nasopharynx and thus is not a reasonable option for the treatment of pertussis.23
Levofloxacin is also not recommended for the treatment of pertussis.
Sulfamethoxazole-trimethoprim is a second-line agent for pertussis. It is effective in eradicating B pertussis from the nasopharynx20 and is generally used as an alternative to the macrolide agents in patients who cannot tolerate or have contraindications to macrolides. Sulfamethoxazole-trimethoprim can also be an option for patients infected with rare macrolide-resistant strains of B pertussis.
Supportive measures by themselves are reasonable for patients with pertussis beyond the catarrhal phase, since antibiotics are typically not effective at that stage of the disease.
From 80% to 90% of patients with untreated pertussis spontaneously clear the bacteria from the nasopharynx within 3 to 4 weeks from the onset of cough symptoms.20 However, supportive measures, including antitussives (both over-the-counter and prescription), tend to have very little effect on the severity or duration of the illness, especially when used past the early stage of the illness.
POSTEXPOSURE CHEMOPROPHYLAXIS FOR CLOSE CONTACTS
Postexposure chemoprophylaxis should be given to close contacts of patients who have pertussis to help prevent secondary cases.22 The CDC defines a close contact as someone who has had face-to-face exposure within 3 feet of a symptomatic patient within 21 days after the onset of symptoms in the patient. Close contacts should be treated with antibiotic regimens similar to those used in confirmed cases of pertussis.
In our patient’s case, the diagnosis of pertussis was reported to the Ohio Department of Health. Shortly afterward, the department contacted the patient and obtained information about her close contacts. These people were then contacted and encouraged to complete a course of antibiotics for postexposure chemoprophylaxis, given the high secondary attack rates.
PERTUSSIS VACCINES
4. Which of the following vaccines could have reduced our patient’s chance of contracting the disease or reduced the severity or time course of the illness?
- DTaP
- Tdap
- Whole-cell pertussis vaccine
- No vaccine would have reduced her risk
It is important to prevent pertussis, given its associated morbidities and its generally poor response to drug therapy. Continued vigilance is imperative to maintain high levels of vaccine coverage, including the timely completion of the pertussis vaccination schedule.
The two vaccines in current use in the United States to produce immunity to pertussis—DTaP and Tdap—also confer immunity to diphtheria and tetanus. DTaP is used for children under 7 years of age, and Tdap is for ages 10 to 64. Thus, our patient should have received a series of DTaP injections as an infant and small child, and a Tdap booster at age 11 or 12 years and every 10 years after that.
The upper case “D,” “T,” and “P” in the abbreviations signifies full-strength doses and the lower case “d,” “t,” and “p” indicate that the doses of those components have been reduced. The “a” in both vaccines stands for “acellular”: ie, the pertussis component does not contain cellular elements.
DTaP for initial pertussis vaccination
The current recommendation for initial pertussis vaccination consists of a primary series of DTaP. DTaP vaccination is recommended for infants at 2 months of age, then again at 4 months of age, and again at 6 months of age. A fourth dose is given between the ages of 15 and 18 months, and a fifth dose is given between the ages of 4 to 6 years. If the fourth dose was given after age 4, then no fifth dose is needed.20
Tdap as a booster
The booster vaccine for adolescents and adults is Tdap. In 2005, two Tdap vaccines were licensed in the United States: Adacel for people ages 11 to 64 years, and Boostrix for people ages 10 to 18 years.
The CDC’s Advisory Committee on Immunization Practices (ACIP) recommends a booster dose of Tdap at age 11 or 12 years. Every 10 years thereafter, a booster of tetanus and diphtheria toxoid (Td) vaccine is recommended, except that one of the Td doses can be replaced by Tdap if the patient hasn’t received Tdap yet.
For adults ages 19 to 64, the ACIP currently recommends routine use of a single booster dose of Tdap to replace a single dose of Td if they received the last dose of toxoid vaccine 10 or more years earlier. If the previous dose of Td was given within the past 10 years, a single dose of Tdap is appropriate to protect patients against pertussis. This is especially true for patients at increased risk of pertussis or its complications, as well as for health care professionals and adults who have close contact with infants, such as new parents, grandparents, and child-care providers. The minimum interval since the last Td vaccination is ideally 2 years, although shorter intervals can be used for control of pertussis outbreaks and for those who have close contact with infants.24
In 2010, the ACIP decided that, for those ages 65 and older, a single dose of Tdap vaccine may be given in place of Td if the patient has not previously received Tdap, regardless of how much time has elapsed since the last vaccination with a Td-containing vaccine.25 Data from the Vaccine Adverse Event Reporting System suggest that Tdap vaccine in this age group is as safe as the Td vaccine.25
Subsequent tetanus vaccine doses, in the form of Td, should be given at 10-year intervals throughout adulthood. Administration of Tdap at 10-year intervals appears to be highly immunogenic and well tolerated,25 suggesting that it is possible that Tdap will become part of routine booster dosing instead of Td, pending further study.
Tdap is not contraindicated in pregnant women. Ideally, women should be vaccinated with Tdap before becoming pregnant if they have not previously received it. If the pregnant woman is not at risk of acquiring or transmitting pertussis during pregnancy, the ACIP recommends deferring Tdap vaccination until the immediate postpartum period.
Adults who require a vaccine containing tetanus toxoid for wound management should receive Tdap instead of Td if they have never received Tdap. Adults who have never received vaccine containing tetanus and diphtheria toxoid should receive a series of three vaccinations. The preferred schedule is a dose of Tdap, followed by a dose of Td more than 4 weeks later, and a second dose of Td 6 to 12 months later, though Tdap can be substituted for Td for any one of the three doses in the series. Adults with a history of pertussis generally should receive Tdap according to routine recommendations.
Tdap is contraindicated in people with a history of serious allergic reaction to any component of the Tdap vaccine or with a history of encephalopathy not attributable to an identifiable cause within 7 days of receiving a pertussis vaccine. Tdap is relatively contraindicated and should be deferred in people with current moderate to severe acute illness, current unstable neurologic condition, or a history of Arthus hypersensitivity reaction to a tetanus-toxoid-containing vaccine within the past 10 years, and in people who have developed Guillain-Barré syndrome, within 6 weeks of receiving a tetanus-toxoid–containing vaccine.
Tdap is generally well tolerated. Adverse effects are typically mild and may include localized pain, redness, and swelling; low-grade fever; headache; fatigue; and, less commonly, gastrointestinal upset, myalgia, arthralgia, rash, and swollen glands.
Whole-cell pertussis vaccine is no longer available in the United States
Whole-cell pertussis vaccine provides good protection against pertussis, with 70% to 90% efficacy after three doses. It is less expensive-than acellular formulations and therefore is used in many parts of the world where cost is an issue. It is no longer available in the United States, however, due to high rates of local reactions such as redness, swelling, and pain at the injection site.
The importance of staying up-to-date with booster shots
Booster vaccination for pertussis in adolescents and adults is critical, since the largest recent outbreaks have occurred in these groups.21 The high rate of outbreaks is presumably the result of waning immunity from childhood immunizations and of high interpersonal contact rates. Vaccination has been shown to reduce the chance of contracting the disease and to reduce the severity and time course of the illness.21
Adolescents and adults are an important reservoir for potentially serious infections in infants who are either unvaccinated or whose vaccination schedule has not been completed. These infants are at risk of severe illness, including pneumonia, seizures, encephalopathy, and apnea, or even death. Adults and teens can also suffer complications from pertussis, although these tend to be less serious, especially in those who have been vaccinated. Complications in teens and adults are often caused by malaise and the cough itself, including weight loss (33%), urinary stress incontinence (28%), syncope (6%), rib fractures from severe coughing (4%), and pneumonia (2%).26 Thus, it is important that adolescents and adults stay up-to-date with pertussis vaccination.
CASE CONTINUED
Our patient was treated with a short (5-day) course of azithromycin 500 mg daily. It did not improve her symptoms very much, but this was not unexpected, given her late presentation and duration of symptoms. Her cough persisted for about 2 months afterwards, but it improved with time and with supportive care at home.
CONTINUED CHALLENGES
Pertussis is a reemerging disease with an increased incidence over the past 30 years, and even more so over the past 10 years. Unfortunately, treatments are not very effective, especially since the disease is often diagnosed late in the course.
We are fortunate to have a vaccine that can prevent pertussis, yet pertussis persists, in large part because of waning immunity from childhood vaccination. The duration of immunity from childhood vaccination is not yet clear. Many adolescents and adults do not follow up on these booster vaccines, thus increasing their susceptibility to pertussis. Consequently, they can transmit the disease to children who are not fully immunized. Prevention by maintaining active immunity is the key to controlling this terrible disease.
A 49-year-old woman presents with a cough that has persisted for 3 weeks.
Two weeks ago, she was seen in the outpatient clinic for a nonproductive cough, rhinorrhea, sneezing, and a sore throat. At that time, she described coughing spells that were occasionally accompanied by posttussive chest pain and vomiting. The cough was worse at night and was occasionally associated with wheezing. She reported no fevers, chills, rigors, night sweats, or dyspnea. She said she has tried over-the-counter cough suppressants, antihistamines, and decongestants, but they provided no relief. Since she had a history of well-controlled asthma, she was diagnosed with an asthma exacerbation and was given prednisone 20 mg to take orally every day for 5 days, to be followed by an inhaled corticosteroid until her symptoms resolved.
Now, she has returned because her symptoms have persisted despite treatment, and she is seeking a second medical opinion. Her paroxysmal cough has become more frequent and more severe.
In addition to asthma, she has a history of allergic rhinitis. Her current medications include the over-the-counter histamine H1 antagonist cetirizine (Zyrtec), a fluticasone-salmeterol inhaler (Advair), and an albuterol inhaler (Proventil HFA). She reports having had mild asthma exacerbations in the past during the winter, which were managed well with her albuterol inhaler.
She has never smoked; she drinks alcohol socially. She has not traveled outside the United States during the past several months. She is married and has two children, ages 25 and 23. She lives at home with only her husband, and he has not been sick. However, she works at a greeting card store, and two of her coworkers have similar upper respiratory symptoms, although they have only a mild cough.
Her immunizations are not up-to-date. She last received the tetanus-diphtheria toxoid (Td) vaccine 12 years ago, and she never received the pediatric tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. She generally receives the influenza vaccine annually, and she received it about 6 weeks before this presentation.
She is not in distress, but she has paroxysms of severe coughing throughout her examination. Her pulse is 100 beats per minute, respiratory rate 18, and blood pressure 130/86 mm Hg. Her oropharynx is clear. The pulmonary examination reveals poor inspiratory effort due to coughing but is otherwise normal. The rest of the examination is normal, as is her chest radiograph.
WHAT DOES SHE HAVE?
1. Which of the following would best explain her symptoms?
- Asthma
- Postviral cough
- Pertussis
- Chronic bronchitis
- Pneumonia
- Gastroesophageal reflux disease
Asthma is a reasonable consideration, given her medical history, her occasional wheezing, and her nonproductive cough that is worse at night. However, asthma typically responds well to corticosteroid therapy. She has already received a course of prednisone, but her symptoms have not improved.
Postviral cough could also be considered in this patient. However, postviral cough does not typically occur in paroxysms, nor does it lead to posttussive vomiting. It is also generally regarded as a diagnosis of exclusion.
Pertussis (whooping cough) should be suspected in this patient, given the time course of her symptoms, the paroxysmal cough, and the posttussive vomiting. In addition, at her job she interacts with hundreds of people a day, increasing her risk of exposure to respiratory tract pathogens, including Bordetella pertussis.
Chronic bronchitis is defined by cough (typically productive) lasting at least 3 months per year for at least 2 consecutive years, which does not fit the time course for this patient. It is vastly more common in smokers.
Pneumonia typically presents with a cough that can be productive or nonproductive, but also with fever, chills, and radiologic evidence of a pulmonary infiltrate or consolidation. This woman has none of these.
Gastroesophageal reflux disease is one of the most common causes of chronic cough, with symptoms typically worse at night. However, it is generally associated with symptoms such as heartburn, a sour taste in the mouth, or regurgitation, which our patient did not report.
Thus, pertussis is the most likely diagnosis.
PERTUSSIS IS ON THE RISE
Pertussis is an acute and highly contagious disease caused by infection of the respiratory tract by B pertussis, a small, aerobic, gramnegative, pleomorphic coccobacillus that produces a number of antigenic and biologically active products, including pertussis toxin, filamentous hemagglutinin, agglutinogens, and tracheal cytotoxin. Transmitted by aerosolized droplets, it attaches to the ciliated epithelial cells of the lower respiratory tract, paralyzes the cilia via toxins, and causes inflammation, thus interfering with the clearing of respiratory secretions.
The incidence of pertussis is on the rise. In 2005, 25,827 cases were reported in the United States, the highest number since 1959.1 Pertussis is now epidemic in California. At the time of this writing, the number of confirmed, probable, and suspected cases in California was 9,477 (including 10 infant deaths) for the year 2010—the most cases reported in the past 65 years.2,3
In 2010, outbreaks were also reported in Michigan, Texas, Ohio, upstate New York, and Arizona.4 The overall incidence of pertussis is likely even higher than what is reported, since many cases go unrecognized or unreported.
Highly contagious
Pertussis is transmitted person-to-person, primarily through aerosolized droplets from coughing or sneezing or by direct contact with secretions from the respiratory tract of infected persons. It is highly contagious, with secondary attack rates of up to 80% in susceptible people.
A three-stage clinical course
The clinical definition of pertussis used by the US Centers for Disease Control and Prevention (CDC) and the Council of State and Territorial Epidemiologists is an acute cough illness lasting at least 2 weeks, with paroxysms of coughing, an inspiratory “whoop,” or posttussive vomiting without another apparent cause.5
The clinical course of the illness is traditionally divided into three stages:
The catarrhal phase typically lasts 1 to 2 weeks and is clinically indistinguishable from a viral upper respiratory infection. It is characterized by the insidious onset of malaise, coryza, sneezing, low-grade fever, and a mild cough that gradually becomes severe.6
The paroxysmal phase normally lasts 1 to 6 weeks but may persist for up to 10 weeks. The diagnosis of pertussis is usually suspected during this phase. The classic features of this phase are bursts or paroxysms of numerous, rapid coughs. These are followed by a long inspiratory effort usually accompanied by a characteristic high-pitched whoop, most notably observed in infants and children. Infants and children may appear very ill and distressed during this time and may become cyanotic, but cyanosis is uncommon in adults and adolescents. The paroxysms may also be followed by exhaustion and posttussive vomiting. In some cases, the cough is not paroxysmal, but rather simply persistent. The coughing attacks tend to occur more often at night, with an average of 15 attacks per 24 hours. During the first 1 to 2 weeks of this stage, the attacks generally increase in frequency, remain at the same intensity level for 2 to 3 weeks, and then gradually decrease over 1 to 2 weeks.1,7
The convalescent phase can have a variable course, ranging from weeks to months, with an average duration of 2 to 3 weeks. During this stage, the paroxysms of coughing become less frequent and gradually resolve. Paroxysms often recur with subsequent respiratory infections.
In infants and young children, pertussis tends to follow these stages in a predictable sequence. Adolescents and adults, however, tend to go through the stages without being as ill and typically do not exhibit the characteristic whoop.
TESTING FOR PERTUSSIS
2. Which would be the test of choice to confirm pertussis in this patient?
- Bacterial culture of nasopharyngeal secretions
- Polymerase chain reaction (PCR) testing of nasopharyngeal secretions
- Direct fluorescent antibody testing of nasopharyngeal secretions
- Enzyme-linked immunosorbent assay (ELISA) serologic testing
Establishing the diagnosis of pertussis is often rather challenging.
Bacterial culture: Very specific, but slow and not so sensitive
Bacterial culture is still the gold standard for diagnosing pertussis, as a positive culture for B pertussis is 100% specific.5
However, this test has drawbacks. Its sensitivity has a wide range (15% to 80%) and depends very much on the time from the onset of symptoms to the time the culture specimen is collected. The yield drops off significantly after 1 week, and after 3 weeks the test has a sensitivity of only 1% to 3%.8 Therefore, for our patient, who has had symptoms for 3 weeks already, bacterial culture would not be the best test. In addition, the results are usually not known for 7 to 14 days, which is too slow to be useful in managing acute cases.
For swabbing, a Dacron swab is inserted through the nostril to the posterior pharynx and is left in place for 10 seconds to maximize the yield of the specimen. Recovery rates for B pertussis are low if the throat or the anterior nasal passage is swabbed instead of the posterior pharynx.9
Nasopharyngeal aspiration is a more complicated procedure, requiring a suction device to trap the mucus, but it may provide higher yields than swabbing.10 In this method, the specimen is obtained by inserting a small tube (eg, an infant feeding tube) connected to a mucus trap into the nostril back to the posterior pharynx.
Often, direct inoculation of medium for B pertussis is not possible. In such cases, clinical specimens are placed in Regan Lowe transport medium (half-strength charcoal agar supplemented with horse blood and cephalexin).11,12
Polymerase chain reaction testing: Faster, more sensitive, but less specific
PCR testing of nasopharyngeal specimens is now being used instead of bacterial culture to diagnose pertussis in many situations. Alternatively, nasopharyngeal aspirate (or secretions collected with two Dacron swabs) can be obtained and divided at the time of collection and the specimens sent for both culture and PCR testing. Because bacterial culture is time-consuming and has poor sensitivity, the CDC states that a positive PCR test, along with the clinical symptoms and epidemiologic information, is sufficient for diagnosis.5
PCR testing can detect B pertussis with greater sensitivity and more rapidly than bacterial culture.12–14 Its sensitivity ranges from 61% to 99%, its specificity ranges from 88% to 98%,12,15,16 and its results can be available in 2 to 24 hours.12
PCR testing’s advantage in terms of sensitivity is especially pronounced in the later stages of the disease (as in our patient), when clinical suspicion of pertussis typically arises. It can be used effectively for up to 4 weeks from the onset of cough.14 Our patient, who presented nearly 3 weeks after the onset of symptoms, underwent nasopharyngeal sampling for PCR testing.
However, PCR testing is not as specific for B pertussis as is bacterial culture, since other Bordetella species can cause positive results on PCR testing. Also, as with culture, a negative test does not reliably rule out the disease, especially if the sample is collected late in the course.
Therefore, basing the diagnosis on PCR testing alone without the proper clinical context is not advised: pertussis outbreaks have been mistakenly declared on the basis of false-positive PCR test results. Three so-called “pertussis outbreaks” in three different states from 2004 to 200617 were largely the result of overdiagnosis based on equivocal or false-positive PCR test results without the appropriate clinical circumstances. Retrospective review of these pseudo-outbreaks revealed that few cases actually met the CDC’s diagnostic criteria.17 Many patients were not tested (by any method) for pertussis and were treated as having probable cases of pertussis on the basis of their symptoms. Patients who were tested and who had a positive PCR test did not meet the clinical definition of pertussis according to the Council of State and Territorial Epidemiologists.17
Since PCR testing varies in sensitivity and specificity, obtaining culture confirmation of pertussis for at least one suspicious case is recommended any time an outbreak is suspected. This is necessary for monitoring for continued presence of the agent among cases of disease, recruitment of isolates for epidemiologic studies, and surveillance for antibiotic resistance.
Direct fluorescence antibody testing
The CDC does not recommend direct fluorescence antibody testing to diagnose pertussis. This test is commercially available and is sometimes used to screen patients for B pertussis infection, but it lacks sensitivity and specificity for this organism. Cross-reaction with normal nasopharyngeal flora can lead to a false-positive result.18 In addition, the interpretation of the test is subjective, so the sensitivity and specificity are quite variable: the sensitivity is reported as 52% to 65%, while the specificity can vary from 15% to 99%.
Enzyme-linked immunosorbent assay
ELISA testing has been used in epidemiologic studies to measure serum antibodies to B pertussis. Many serologic tests exist, but none is commercially available. Many of these tests are used by the CDC and state health departments to help confirm the diagnosis, especially during outbreaks. Generally, serologic tests are more useful for diagnosis in later phases of the disease. Currently used ELISA tests use both paired and single serology techniques measuring elevated immunoglobulin G serum antibody concentrations against an array of antigens, including pertussis toxin, filamentous hemagglutinin, pertactin, and fimbrae. As a result, a range of sensitivities (33%–95%) and specificities (72%–100%) has been reported.12,14,19
TREATING PERTUSSIS
Our patient’s PCR test result comes back positive. In view of her symptoms and this result, we decide to treat her empirically for pertussis, even though she has had no known contact with anyone with the disease and there is currently no outbreak of it in the community.
3. According to the most recent evidence, which of the following would be the treatment of choice for pertussis in this patient?
- Azithromycin (Zithromax)
- Amoxicillin (Moxatag)
- Levofloxacin (Levaquin)
- Sulfamethoxazole-trimethoprim (Bactrim)
- Supportive measures (hydration, humidifier, antitussives, antihistamines, decongestants)
Azithromycin and the other macrolide antibiotics erythromycin and clarithromycin are first-line therapies for pertussis in adolescents and adults. If given during the catarrhal phase, they can reduce the duration and severity of symptoms and lessen the period of communicability.20,21 After the catarrhal phase, however, it is uncertain whether antibiotics change the clinical course of pertussis, as the data are conflicting.20–22
Factors to consider when selecting a macrolide antibiotic are tolerability, the potential for adverse events and drug interactions, ease of compliance, and cost. All three macrolides are equally effective against pertussis, but azithromycin and clarithromycin are generally better tolerated and are associated with milder and less frequent side effects than erythromycin, including lower rates of gastrointestinal side effects.
Erythromycin and clarithromycin inhibit the cytochrome P450 enzyme system, specifically CYP3A4, and can interact with a great many commonly prescribed drugs metabolized by this enzyme. Therefore, azithromycin may be a better choice for patients already taking other medications, like our patient.
Azithromycin and clarithromycin have longer half-lives and achieve higher tissue concentrations than erythromycin, allowing for less-frequent dosing (daily for azithromycin and twice daily for clarithromycin) and shorter treatment duration (5 days for azithromycin and 7 days for clarithromycin).
An advantage of erythromycin, though, is its lower cost. The cost of a recommended course of erythromycin treatment for pertussis (ie, 500 mg every 6 hours for 14 days) is roughly $20, compared with $75 for azithromycin.
Amoxicillin is not effective in clearing B pertussis from the nasopharynx and thus is not a reasonable option for the treatment of pertussis.23
Levofloxacin is also not recommended for the treatment of pertussis.
Sulfamethoxazole-trimethoprim is a second-line agent for pertussis. It is effective in eradicating B pertussis from the nasopharynx20 and is generally used as an alternative to the macrolide agents in patients who cannot tolerate or have contraindications to macrolides. Sulfamethoxazole-trimethoprim can also be an option for patients infected with rare macrolide-resistant strains of B pertussis.
Supportive measures by themselves are reasonable for patients with pertussis beyond the catarrhal phase, since antibiotics are typically not effective at that stage of the disease.
From 80% to 90% of patients with untreated pertussis spontaneously clear the bacteria from the nasopharynx within 3 to 4 weeks from the onset of cough symptoms.20 However, supportive measures, including antitussives (both over-the-counter and prescription), tend to have very little effect on the severity or duration of the illness, especially when used past the early stage of the illness.
POSTEXPOSURE CHEMOPROPHYLAXIS FOR CLOSE CONTACTS
Postexposure chemoprophylaxis should be given to close contacts of patients who have pertussis to help prevent secondary cases.22 The CDC defines a close contact as someone who has had face-to-face exposure within 3 feet of a symptomatic patient within 21 days after the onset of symptoms in the patient. Close contacts should be treated with antibiotic regimens similar to those used in confirmed cases of pertussis.
In our patient’s case, the diagnosis of pertussis was reported to the Ohio Department of Health. Shortly afterward, the department contacted the patient and obtained information about her close contacts. These people were then contacted and encouraged to complete a course of antibiotics for postexposure chemoprophylaxis, given the high secondary attack rates.
PERTUSSIS VACCINES
4. Which of the following vaccines could have reduced our patient’s chance of contracting the disease or reduced the severity or time course of the illness?
- DTaP
- Tdap
- Whole-cell pertussis vaccine
- No vaccine would have reduced her risk
It is important to prevent pertussis, given its associated morbidities and its generally poor response to drug therapy. Continued vigilance is imperative to maintain high levels of vaccine coverage, including the timely completion of the pertussis vaccination schedule.
The two vaccines in current use in the United States to produce immunity to pertussis—DTaP and Tdap—also confer immunity to diphtheria and tetanus. DTaP is used for children under 7 years of age, and Tdap is for ages 10 to 64. Thus, our patient should have received a series of DTaP injections as an infant and small child, and a Tdap booster at age 11 or 12 years and every 10 years after that.
The upper case “D,” “T,” and “P” in the abbreviations signifies full-strength doses and the lower case “d,” “t,” and “p” indicate that the doses of those components have been reduced. The “a” in both vaccines stands for “acellular”: ie, the pertussis component does not contain cellular elements.
DTaP for initial pertussis vaccination
The current recommendation for initial pertussis vaccination consists of a primary series of DTaP. DTaP vaccination is recommended for infants at 2 months of age, then again at 4 months of age, and again at 6 months of age. A fourth dose is given between the ages of 15 and 18 months, and a fifth dose is given between the ages of 4 to 6 years. If the fourth dose was given after age 4, then no fifth dose is needed.20
Tdap as a booster
The booster vaccine for adolescents and adults is Tdap. In 2005, two Tdap vaccines were licensed in the United States: Adacel for people ages 11 to 64 years, and Boostrix for people ages 10 to 18 years.
The CDC’s Advisory Committee on Immunization Practices (ACIP) recommends a booster dose of Tdap at age 11 or 12 years. Every 10 years thereafter, a booster of tetanus and diphtheria toxoid (Td) vaccine is recommended, except that one of the Td doses can be replaced by Tdap if the patient hasn’t received Tdap yet.
For adults ages 19 to 64, the ACIP currently recommends routine use of a single booster dose of Tdap to replace a single dose of Td if they received the last dose of toxoid vaccine 10 or more years earlier. If the previous dose of Td was given within the past 10 years, a single dose of Tdap is appropriate to protect patients against pertussis. This is especially true for patients at increased risk of pertussis or its complications, as well as for health care professionals and adults who have close contact with infants, such as new parents, grandparents, and child-care providers. The minimum interval since the last Td vaccination is ideally 2 years, although shorter intervals can be used for control of pertussis outbreaks and for those who have close contact with infants.24
In 2010, the ACIP decided that, for those ages 65 and older, a single dose of Tdap vaccine may be given in place of Td if the patient has not previously received Tdap, regardless of how much time has elapsed since the last vaccination with a Td-containing vaccine.25 Data from the Vaccine Adverse Event Reporting System suggest that Tdap vaccine in this age group is as safe as the Td vaccine.25
Subsequent tetanus vaccine doses, in the form of Td, should be given at 10-year intervals throughout adulthood. Administration of Tdap at 10-year intervals appears to be highly immunogenic and well tolerated,25 suggesting that it is possible that Tdap will become part of routine booster dosing instead of Td, pending further study.
Tdap is not contraindicated in pregnant women. Ideally, women should be vaccinated with Tdap before becoming pregnant if they have not previously received it. If the pregnant woman is not at risk of acquiring or transmitting pertussis during pregnancy, the ACIP recommends deferring Tdap vaccination until the immediate postpartum period.
Adults who require a vaccine containing tetanus toxoid for wound management should receive Tdap instead of Td if they have never received Tdap. Adults who have never received vaccine containing tetanus and diphtheria toxoid should receive a series of three vaccinations. The preferred schedule is a dose of Tdap, followed by a dose of Td more than 4 weeks later, and a second dose of Td 6 to 12 months later, though Tdap can be substituted for Td for any one of the three doses in the series. Adults with a history of pertussis generally should receive Tdap according to routine recommendations.
Tdap is contraindicated in people with a history of serious allergic reaction to any component of the Tdap vaccine or with a history of encephalopathy not attributable to an identifiable cause within 7 days of receiving a pertussis vaccine. Tdap is relatively contraindicated and should be deferred in people with current moderate to severe acute illness, current unstable neurologic condition, or a history of Arthus hypersensitivity reaction to a tetanus-toxoid-containing vaccine within the past 10 years, and in people who have developed Guillain-Barré syndrome, within 6 weeks of receiving a tetanus-toxoid–containing vaccine.
Tdap is generally well tolerated. Adverse effects are typically mild and may include localized pain, redness, and swelling; low-grade fever; headache; fatigue; and, less commonly, gastrointestinal upset, myalgia, arthralgia, rash, and swollen glands.
Whole-cell pertussis vaccine is no longer available in the United States
Whole-cell pertussis vaccine provides good protection against pertussis, with 70% to 90% efficacy after three doses. It is less expensive-than acellular formulations and therefore is used in many parts of the world where cost is an issue. It is no longer available in the United States, however, due to high rates of local reactions such as redness, swelling, and pain at the injection site.
The importance of staying up-to-date with booster shots
Booster vaccination for pertussis in adolescents and adults is critical, since the largest recent outbreaks have occurred in these groups.21 The high rate of outbreaks is presumably the result of waning immunity from childhood immunizations and of high interpersonal contact rates. Vaccination has been shown to reduce the chance of contracting the disease and to reduce the severity and time course of the illness.21
Adolescents and adults are an important reservoir for potentially serious infections in infants who are either unvaccinated or whose vaccination schedule has not been completed. These infants are at risk of severe illness, including pneumonia, seizures, encephalopathy, and apnea, or even death. Adults and teens can also suffer complications from pertussis, although these tend to be less serious, especially in those who have been vaccinated. Complications in teens and adults are often caused by malaise and the cough itself, including weight loss (33%), urinary stress incontinence (28%), syncope (6%), rib fractures from severe coughing (4%), and pneumonia (2%).26 Thus, it is important that adolescents and adults stay up-to-date with pertussis vaccination.
CASE CONTINUED
Our patient was treated with a short (5-day) course of azithromycin 500 mg daily. It did not improve her symptoms very much, but this was not unexpected, given her late presentation and duration of symptoms. Her cough persisted for about 2 months afterwards, but it improved with time and with supportive care at home.
CONTINUED CHALLENGES
Pertussis is a reemerging disease with an increased incidence over the past 30 years, and even more so over the past 10 years. Unfortunately, treatments are not very effective, especially since the disease is often diagnosed late in the course.
We are fortunate to have a vaccine that can prevent pertussis, yet pertussis persists, in large part because of waning immunity from childhood vaccination. The duration of immunity from childhood vaccination is not yet clear. Many adolescents and adults do not follow up on these booster vaccines, thus increasing their susceptibility to pertussis. Consequently, they can transmit the disease to children who are not fully immunized. Prevention by maintaining active immunity is the key to controlling this terrible disease.
- Centers for Disease Control and Prevention. Pertussis. National Immunization Program, 2005. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf. Accessed July 6, 2011.
- California Department of Public Health. Pertussis report. www.cdph.ca.gov/programs/immunize/Documents/PertussisReport2011-01-07.pdf. Accessed July 6, 2011.
- Centers for Disease Control and Prevention. Pertussis (whooping cough). www.cdc.gov/pertussis/outbreaks.html. Accessed July 3, 2011.
- Centers for Disease Control and Prevention. Notifiable diseases and mortality tables. MMWR Morb Mortal Wkly Rep 2010; 59:847–861. http://www.cdc.gov/mmwr/PDF/wk/mm5927.pdf. Accessed July 1, 2011.
- Centers for Disease Control and Prevention. Pertussis. Vaccines and preventable diseases: pertussis (whooping cough) vaccination, 2010. http://www.cdc.gov/vaccines/vpd-vac/pertussis/default.htm. Accessed July 6, 2011.
- Hewlett EL, Edwards KM. Clinical practice. Pertussis—not just for kids. N Engl J Med 2005; 352:1215–1222.
- Hewlett E. Bordetella species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 5th ed, Philadelphia, PA: Churchill Livingstone; 2000:2701.
- Viljanen MK, Ruuskanen O, Granberg C, Salmi TT. Serological diagnosis of pertussis: IgM, IgA and IgG antibodies against Bordetella pertussis measured by enzyme-linked immunosorbent assay (ELISA). Scand J Infect Dis 1982; 14:117–122.
- Bejuk D, Begovac J, Bace A, Kuzmanovic-Sterk N, Aleraj B. Culture of Bordetella pertussis from three upper respiratory tract specimens. Pediatr Infect Dis J 1995; 14:64–65.
- Hallander HO, Reizenstein E, Renemar B, Rasmuson G, Mardin L, Olin P. Comparison of nasopharyngeal aspirates with swabs for culture of Bordetella pertussis. J Clin Microbiol 1993; 31:50–52.
- Regan J, Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol 1977; 6:303–309.
- World Health Organization. Laboratory manual for the diagnosis of whooping cough caused by Bordetella pertussis/Bordetella para-pertussis. Department of Immunization, Vaccines and Biologicals. Printed 2004. Revised 2007. www.who.int/vaccines-documents/. Accessed July 6, 2011.
- Meade BD, Bollen A. Recommendations for use of the polymerase chain reaction in the diagnosis of Bordetella pertussis infections. J Med Microbiol 1994; 41:51–55.
- Wendelboe AM, Van Rie A. Diagnosis of pertussis: a historical review and recent developments. Expert Rev Mol Diagn 2006; 6:857–864.
- Knorr L, Fox JD, Tilley PA, Ahmed-Bentley J. Evaluation of real-time PCR for diagnosis of Bordetella pertussis infection. BMC Infect Dis 2006; 6:62.
- Sotir MJ, Cappozzo DL, Warshauer DM, et al. Evaluation of polymerase chain reaction and culture for diagnosis of pertussis in the control of a county-wide outbreak focused among adolescents and adults. Clin Infect Dis 2007; 44:1216–1219.
- Centers for Disease Control and Prevention (CDC). Outbreaks of respiratory illness mistakenly attributed to pertussis—New Hampshire, Massachusetts, and Tennessee, 2004–2006. MMWR Morb Mortal Wkly Rep 2007; 56:837–842.
- Ewanowich CA, Chui LW, Paranchych MG, Peppler MS, Marusyk RG, Albritton WL. Major outbreak of pertussis in northern Alberta, Canada: analysis of discrepant direct fluorescent-antibody and culture results by using polymerase chain reaction methodology. J Clin Microbiol 1993; 31:1715–1725.
- Müller FM, Hoppe JE, Wirsing von König CH. Laboratory diagnosis of pertussis: state of the art in 1997. J Clin Microbiol 1997; 35:2435–2443.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep 2005; 54:1–16.
- Wirsing von König CH, Postels-Multani S, Bock HL, Schmitt HJ. Pertussis in adults: frequency of transmission after household exposure. Lancet 1995; 346:1326–1329.
- von König CH. Use of antibiotics in the prevention and treatment of pertussis. Pediatr Infect Dis J 2005; 24(suppl 5):S66–S68.
- Trollfors B. Effect of erythromycin and amoxycillin on Bordetella pertussis in the nasopharynx. Infection 1978; 6:228–230.
- Broder KR, Cortese MM, Iskander JK, et al; Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–34.
- Centers for Disease Control and Prevention. Recommendations and Guidelines. ACIP presentation slides: October 2010 meeting. http://www.cdc.gov/vaccines/recs/acip/slides-oct10.htm. Accessed July 6, 2011.
- Cortese MM, Bisgard KM. Pertussis. In:Wallace RB, Kohatsu N, Last JM, editors. Wallace/Maxcy-Rosenau-Last Public Health & Preventive Medicine. 15th ed. New York, NY: McGraw-Hill Medical, 2008:111–114.
- Centers for Disease Control and Prevention. Pertussis. National Immunization Program, 2005. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf. Accessed July 6, 2011.
- California Department of Public Health. Pertussis report. www.cdph.ca.gov/programs/immunize/Documents/PertussisReport2011-01-07.pdf. Accessed July 6, 2011.
- Centers for Disease Control and Prevention. Pertussis (whooping cough). www.cdc.gov/pertussis/outbreaks.html. Accessed July 3, 2011.
- Centers for Disease Control and Prevention. Notifiable diseases and mortality tables. MMWR Morb Mortal Wkly Rep 2010; 59:847–861. http://www.cdc.gov/mmwr/PDF/wk/mm5927.pdf. Accessed July 1, 2011.
- Centers for Disease Control and Prevention. Pertussis. Vaccines and preventable diseases: pertussis (whooping cough) vaccination, 2010. http://www.cdc.gov/vaccines/vpd-vac/pertussis/default.htm. Accessed July 6, 2011.
- Hewlett EL, Edwards KM. Clinical practice. Pertussis—not just for kids. N Engl J Med 2005; 352:1215–1222.
- Hewlett E. Bordetella species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 5th ed, Philadelphia, PA: Churchill Livingstone; 2000:2701.
- Viljanen MK, Ruuskanen O, Granberg C, Salmi TT. Serological diagnosis of pertussis: IgM, IgA and IgG antibodies against Bordetella pertussis measured by enzyme-linked immunosorbent assay (ELISA). Scand J Infect Dis 1982; 14:117–122.
- Bejuk D, Begovac J, Bace A, Kuzmanovic-Sterk N, Aleraj B. Culture of Bordetella pertussis from three upper respiratory tract specimens. Pediatr Infect Dis J 1995; 14:64–65.
- Hallander HO, Reizenstein E, Renemar B, Rasmuson G, Mardin L, Olin P. Comparison of nasopharyngeal aspirates with swabs for culture of Bordetella pertussis. J Clin Microbiol 1993; 31:50–52.
- Regan J, Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol 1977; 6:303–309.
- World Health Organization. Laboratory manual for the diagnosis of whooping cough caused by Bordetella pertussis/Bordetella para-pertussis. Department of Immunization, Vaccines and Biologicals. Printed 2004. Revised 2007. www.who.int/vaccines-documents/. Accessed July 6, 2011.
- Meade BD, Bollen A. Recommendations for use of the polymerase chain reaction in the diagnosis of Bordetella pertussis infections. J Med Microbiol 1994; 41:51–55.
- Wendelboe AM, Van Rie A. Diagnosis of pertussis: a historical review and recent developments. Expert Rev Mol Diagn 2006; 6:857–864.
- Knorr L, Fox JD, Tilley PA, Ahmed-Bentley J. Evaluation of real-time PCR for diagnosis of Bordetella pertussis infection. BMC Infect Dis 2006; 6:62.
- Sotir MJ, Cappozzo DL, Warshauer DM, et al. Evaluation of polymerase chain reaction and culture for diagnosis of pertussis in the control of a county-wide outbreak focused among adolescents and adults. Clin Infect Dis 2007; 44:1216–1219.
- Centers for Disease Control and Prevention (CDC). Outbreaks of respiratory illness mistakenly attributed to pertussis—New Hampshire, Massachusetts, and Tennessee, 2004–2006. MMWR Morb Mortal Wkly Rep 2007; 56:837–842.
- Ewanowich CA, Chui LW, Paranchych MG, Peppler MS, Marusyk RG, Albritton WL. Major outbreak of pertussis in northern Alberta, Canada: analysis of discrepant direct fluorescent-antibody and culture results by using polymerase chain reaction methodology. J Clin Microbiol 1993; 31:1715–1725.
- Müller FM, Hoppe JE, Wirsing von König CH. Laboratory diagnosis of pertussis: state of the art in 1997. J Clin Microbiol 1997; 35:2435–2443.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep 2005; 54:1–16.
- Wirsing von König CH, Postels-Multani S, Bock HL, Schmitt HJ. Pertussis in adults: frequency of transmission after household exposure. Lancet 1995; 346:1326–1329.
- von König CH. Use of antibiotics in the prevention and treatment of pertussis. Pediatr Infect Dis J 2005; 24(suppl 5):S66–S68.
- Trollfors B. Effect of erythromycin and amoxycillin on Bordetella pertussis in the nasopharynx. Infection 1978; 6:228–230.
- Broder KR, Cortese MM, Iskander JK, et al; Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–34.
- Centers for Disease Control and Prevention. Recommendations and Guidelines. ACIP presentation slides: October 2010 meeting. http://www.cdc.gov/vaccines/recs/acip/slides-oct10.htm. Accessed July 6, 2011.
- Cortese MM, Bisgard KM. Pertussis. In:Wallace RB, Kohatsu N, Last JM, editors. Wallace/Maxcy-Rosenau-Last Public Health & Preventive Medicine. 15th ed. New York, NY: McGraw-Hill Medical, 2008:111–114.