User login
Schwannomas are benign tumors exclusively composed of Schwann cells that arise from the peripheral nerve sheath; these tumors theoretically can present anywhere in the body where nerves reside. They tend to occur in the head and neck region (classically an acoustic neuroma) but also occur in other locations, including the retroperitoneal space and the extremities, particularly flexural surfaces. Patients with cutaneous schwannomas are most likely to present to their primary care provider’s office reporting skin findings or localized pain, and providers should be aware of schwannomas on the differential for painful nodular growths.
Case Presentation
A 70-year-old man with type 2 diabetes mellitus presented to the primary care clinic for intermittent, sharp, localized left lower quadrant abdominal wall pain that was gradually progressive over the previous few months. The patient noticed the development of a small nodule 7 to 8 months prior to the visit, at which time the pain was less frequent and less severe. He reported no postprandial association of the pain, nausea, vomiting, diarrhea, constipation, or other gastrointestinal symptoms.
Ten months prior to the presentation, he was involved in a low-impact motor vehicle collision as a pedestrian in which he fell face-first onto the hood of an oncoming car. At that time, he did not note any abdominal trauma or pain. Evaluation at a local emergency department did not reveal any major injuries. In the interim, he had self-administered insulin in his abdominal region, as he had without incident for the previous 2 years. He reported that he was not injecting near the site of the nodule since it had formed. He could not recall whether the location was a previous insulin administration site.
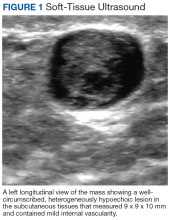
On examination, the patient’s vital signs were normal as were the cardiac and respiratory examinations. An abdominal exam revealed normal bowel sounds and no overlying skin changes or discoloration. Palpation revealed a 1.5 x 1 cm rubbery-to-firm, well-circumscribed subcutaneous nodule along his mid-left abdomen, about 7 cm lateral to the umbilicus. The nodule was sensitive to both light touch and deep pressure. It was firmer than expected for an abdominal wall lipoma. There was no central puncta or pore to suggest an epidermal inclusion cyst. There was no surrounding erythema or induration to suggest an abscess.
The patient was referred for surgery and underwent excisional biopsy of the mass. Pathology revealed a well-circumscribed vascular/spindle-cell lesion consistent with a schwannoma. His postoperative course was uncomplicated. At 4-week follow-up the incision had healed completely and the patient was pain free.
Discussion
Soft-tissue nodules are common—about two-thirds of soft-tissue tumors are classified into 7 diagnostic categories: lipoma and lipoma variants (16%), fibrous histiocytoma (13%), nodular fasciitis (11%), hemangioma (8%), fibromatosis (7%), neurofibroma (5%), and schwannoma (5%).1 Peripheral nerve tumors (schwannomas, neurofibromas) can be associated with pain or paresthesias, and less commonly, neurologic deficits, such as motor weakness. Peripheral nerve tumors have several classifications, such as nonneoplastic vs neoplastic, benign vs malignant, and sheath vs nonsheath origins. Schwannomas are considered part of the neoplastic subset due to their growth; otherwise, they are benign with a sheath origin. In contrast to neurofibromas, benign schwannomas have a slower rate of progression, lower association with pain, and fewer neurologic symptoms.2
The neural sheath is made up of 3 types of cells: the fibroblast, the Schwann cell, and the perineural cell, which lacks a basement membrane. It is the Schwann cell that can give rise to the 3 main types of cutaneous nerve tumors: neuromas, neurofibromas, and schwannomas.3 A nerve that is both entering and exiting a mass is a classic presentation for a peripheral nerve sheath tumor. If the nerve is eccentric to the lesion, then it is consistent with a schwannoma (not a neurofibroma).4 Schwannomas are made exclusively of Schwann cells that arise from the nerve sheath, whereas neurofibromas are made up of all the different cell types that constitute a nerve. Bilateral vestibular schwannomas (acoustic neuromas) are virtually pathognomonic of neurofibromatosis 2 (NF-2), which can manifest as hearing loss, tinnitus, and equilibrium problems. In contrast, neurofibromatosis 1 (NF-1) is more common, characterized by multiple café au lait spots, freckling in the axillary and groin regions, increased risk of cancers overall, and development of pedunculated skin growths, brain, or organ-based neurofibromas.
Diagnosis
A workup generally includes a thorough history and examination as well as imaging. In cases of superficial subcutaneous lesions, an ultrasound is often the imaging modality of choice. However, magnetic resonance imaging (MRI) and computed tomography (CT) scans are frequently used for more deep-seated lesions. There can be significant differences between malignant and benign neural lesions on MRI and CT in terms of contrast-uptake and heterogeneity of tissue, but the visual features are not consistent. Best estimates for MRI suggest 61% sensitivity and 90% specificity for the diagnosis of high-grade malignant peripheral nerve sheath tumors based on imaging alone.5
Definitive diagnosis requires surgical excision. Fine-needle aspiration can be used to diagnose subcutaneous nodules, but there is a possibility that degenerative changes and nuclear atypia seen on a smaller sample may be confused with a more aggressive sarcoma. For example, long-standing schwannomas are often called ancient, meaning that they break down over time, and the atypia they display is a regressive phenomenon.6 Therefore, a small or limited tissue sampling may not be representative of the entire lesion.7 As such, patients will likely need referral for surgical removal to determine the exact nature of the growth.
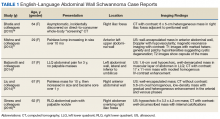
Although schwannomas are uncommon overall, the highest incidence is in the fourth decade of life with a slight predominance in females. They are often incidentally found as a palpable mass but can be symptomatic with paresthesias, pain, or neurologic changes—particularly when identified in the retroperitoneum or along joints. Schwannomas are most commonly found in the retroperitoneum (32%), mediastinum (23%), head and neck (18%), and extremities (16%).8 The majority of cases (about 90%) are sporadic; whereas 2% are related to NF-2.9 The abdominal wall schwannoma is rare. Our review of English-language literature in PubMed and EMBASE found only 5 other case reports (Table 1).
On physical examination, superficial lesions are freely movable except for a single point of attachment, which is generally along the long axis of the nerve.
Pathology
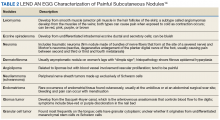
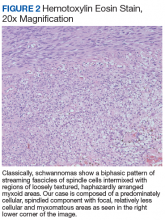
On gross pathology examination, schwannomas have a well-circumscribed smooth external surface. On microscopy, schwannomas are truly encapsulated, uninodular, spindle-cell proliferations arranged in a streaming pattern within a background of thick, hyalinized blood vessels. Classic schwannomas typically exhibit a biphasic pattern of alternating areas of high and low cellularity and are named for Swedish neurologist Nils Antoni. The more cellular regions are referred to as Antoni A areas and consist of streaming fascicles of compact spindle cells that often palisade around acellular eosinophilic areas of fibrillary processes known as Verocay bodies.
In contrast, the lower cellularity regions (Antoni B areas) consist of multipolar, loosely textured cells with abundant cytoplasm, haphazardly arranged processes, and an overall myxoid appearance.11 Schwannomas are known to have widely variable proportions of Antoni A and Antoni B areas; in this case, the excised specimen was noted to have predominately Antoni A areas without well-defined Verocay bodies and only scattered foci showing some suggestion of the hypocellular Antoni B architecture (Figure 2).9,12
Immunohistochemical stains for S100 and SOX10 (used to identify cells derived from a neural crest lineage) were strongly positive, which is characteristic of schwannomas.13 Although there have only been rare reports of extracranial schwannomas undergoing malignant transformation, it is critical to rule out the possibility of a de novo malignant peripheral nerve sheath tumor (MPNST).13 In general, MPNSTs tend to be more cellular, have brisk mitotic activity, areas of necrosis, hyperchromatic nuclei, and conspicuous pleomorphism. Mitotic figures, which can be concerning for malignant potential if present in high number, were noted occasionally in our patient; however, occasional mitosis may be seen in classic schwannomas. Clinically, MPNSTs have a poor prognosis. Based on case reports, disease-specific survival at 10 years is 31.6% for localized disease and only 7.5% for metastatic disease.14 In this case, there was no evidence of any of the high-grade features of a malignant peripheral nerve sheath tumor, thus supporting the diagnosis of schwannoma (neurilemmoma).
Treatment
Schwannomas are exclusively treated by excision. Prognosis is good with low recurrence rates. It is unknown what the recurrence rates are for completely resected abdominal wall schwannomas since there are so few reports in the literature. For other well-known entities, such as vestibular schwannoma (acoustic neuromas), the recurrence rates are generally 2% to 3%.15 Transformation of schwannomas into MPNSTs are so unusual that they are only described in single case reports.
Conclusion
Soft-tissue masses are a common complaint. Most are benign and do not require excision unless it interferes with the quality of life of the patient or if the diagnosis is uncertain. It is important to be aware of schwannomas in the differential diagnosis of soft-tissue masses. Diagnosis may be achieved through the combination of imaging and biopsy, but the definitive diagnosis is made on complete excision of the mass.
Acknowledgments
Contributors: Michael Lewis, MD, Department of Pathology, VA Greater Los Angeles Healthcare System. Written permission also was obtained from the patient.
1. Kransdorf MJ. Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(2):395-402.
2. Valeyrie-Allanore L, Ismaili N, Bastuji-Garin S, et al. Symptoms associated with malignancy of peripheral nerve sheath tumors: a retrospective study of 69 patients with neurofibromatosis 1. Br J Dermatol. 2005;153(1):79-82.
3. Patterson JW. Neural and neuroendocrine tumors. In: Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1042-1049.
4. Balzarotti R, Rondelli F, Barizzi J, Cartolari R. Symptomatic schwannoma of the abdominal wall: a case report and review of the literature. Oncol Lett. 2015;9(3):1095-1098.
5. Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568-1574.
6. Dodd LG, Marom EM, Dash RC, Matthews MR, McLendon RE. Fine-needle aspiration cytology of “ancient” schwannoma. Diagn Cytopathol. 1999;20(5):307-311.
7. Powers CN, Berardo MD, Frable WJ. Fine-needle aspiration biopsy: pitfalls in the diagnosis of spindle-cell lesions. Diagn Cytopathol. 1994;10(3):232-240; discussion 241.
8. White W, Shiu MH, Rosenblum MK, Erlandson RA, Woodruff JM. Cellular schwannoma: a clinicopathologic study of 57 patients and 58 tumors. Cancer. 1990;66(6):1266-1275.
9. Goldblum JR, Weiss SW, Folpe AL. Benign tumors of peripheral nerves. In: Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Elsevier; 2014:813-828.
10. Naversen DN, Trask DM, Watson FH, Burket JM. Painful tumors of the skin: “LEND AN EGG.” J Am Acad Deramatol. 1993;28(2, pt 2):298-300.
11. Burger PC, Scheithauer BW. Diagnostic Pathology: Neuropathology. 1st ed. Salt Lake City, UT: Amirsys; 2012.
12. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. World Health Organization Histological Classification of Tumours of the Central Nervous System. Vol. 1. Paris, France: International Agency for Research on Cancer; 2016.
13. Woodruff JM, Selig AM, Crowley K, Allen PW. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol. 1994;18(9)82-895.
14. Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014-1022.
15. Ahmad RA, Sivalingam S, Topsakal V, Russo A, Taibah A, Sanna M. Rate of recurrent vestibular schwannoma after total removal via different surgical approaches. Ann Otol Rhinol Laryngol. 2012;121(3):156-161.
16. Bhatia RK, Banerjea A, Ram M, Lovett BE. Benign ancient schwannoma of the abdominal wall: an unwanted birthday present. BMC Surg. 2010;10:1-5.
17. Mishra A, Hamadto M, Azzabi M, Elfagieh M. Abdominal wall schwannoma: case report and review of the literature. Case Rep Radiol. 2013;2013:456863.
18. Liu Y, Chen X, Wang T, Wang Z. Imaging observations of a schwannoma of low malignant potential in the anterior abdominal wall: a case report. Oncol Lett. 2014;8(3):1159-1162.
19. Ginesu GC, Puledda M, Feo CF et al. Abdominal wall schwannoma. J Gastrointest Surg. 2016;20(10):1781-1783.
Schwannomas are benign tumors exclusively composed of Schwann cells that arise from the peripheral nerve sheath; these tumors theoretically can present anywhere in the body where nerves reside. They tend to occur in the head and neck region (classically an acoustic neuroma) but also occur in other locations, including the retroperitoneal space and the extremities, particularly flexural surfaces. Patients with cutaneous schwannomas are most likely to present to their primary care provider’s office reporting skin findings or localized pain, and providers should be aware of schwannomas on the differential for painful nodular growths.
Case Presentation
A 70-year-old man with type 2 diabetes mellitus presented to the primary care clinic for intermittent, sharp, localized left lower quadrant abdominal wall pain that was gradually progressive over the previous few months. The patient noticed the development of a small nodule 7 to 8 months prior to the visit, at which time the pain was less frequent and less severe. He reported no postprandial association of the pain, nausea, vomiting, diarrhea, constipation, or other gastrointestinal symptoms.
Ten months prior to the presentation, he was involved in a low-impact motor vehicle collision as a pedestrian in which he fell face-first onto the hood of an oncoming car. At that time, he did not note any abdominal trauma or pain. Evaluation at a local emergency department did not reveal any major injuries. In the interim, he had self-administered insulin in his abdominal region, as he had without incident for the previous 2 years. He reported that he was not injecting near the site of the nodule since it had formed. He could not recall whether the location was a previous insulin administration site.
On examination, the patient’s vital signs were normal as were the cardiac and respiratory examinations. An abdominal exam revealed normal bowel sounds and no overlying skin changes or discoloration. Palpation revealed a 1.5 x 1 cm rubbery-to-firm, well-circumscribed subcutaneous nodule along his mid-left abdomen, about 7 cm lateral to the umbilicus. The nodule was sensitive to both light touch and deep pressure. It was firmer than expected for an abdominal wall lipoma. There was no central puncta or pore to suggest an epidermal inclusion cyst. There was no surrounding erythema or induration to suggest an abscess.
The patient was referred for surgery and underwent excisional biopsy of the mass. Pathology revealed a well-circumscribed vascular/spindle-cell lesion consistent with a schwannoma. His postoperative course was uncomplicated. At 4-week follow-up the incision had healed completely and the patient was pain free.
Discussion
Soft-tissue nodules are common—about two-thirds of soft-tissue tumors are classified into 7 diagnostic categories: lipoma and lipoma variants (16%), fibrous histiocytoma (13%), nodular fasciitis (11%), hemangioma (8%), fibromatosis (7%), neurofibroma (5%), and schwannoma (5%).1 Peripheral nerve tumors (schwannomas, neurofibromas) can be associated with pain or paresthesias, and less commonly, neurologic deficits, such as motor weakness. Peripheral nerve tumors have several classifications, such as nonneoplastic vs neoplastic, benign vs malignant, and sheath vs nonsheath origins. Schwannomas are considered part of the neoplastic subset due to their growth; otherwise, they are benign with a sheath origin. In contrast to neurofibromas, benign schwannomas have a slower rate of progression, lower association with pain, and fewer neurologic symptoms.2
The neural sheath is made up of 3 types of cells: the fibroblast, the Schwann cell, and the perineural cell, which lacks a basement membrane. It is the Schwann cell that can give rise to the 3 main types of cutaneous nerve tumors: neuromas, neurofibromas, and schwannomas.3 A nerve that is both entering and exiting a mass is a classic presentation for a peripheral nerve sheath tumor. If the nerve is eccentric to the lesion, then it is consistent with a schwannoma (not a neurofibroma).4 Schwannomas are made exclusively of Schwann cells that arise from the nerve sheath, whereas neurofibromas are made up of all the different cell types that constitute a nerve. Bilateral vestibular schwannomas (acoustic neuromas) are virtually pathognomonic of neurofibromatosis 2 (NF-2), which can manifest as hearing loss, tinnitus, and equilibrium problems. In contrast, neurofibromatosis 1 (NF-1) is more common, characterized by multiple café au lait spots, freckling in the axillary and groin regions, increased risk of cancers overall, and development of pedunculated skin growths, brain, or organ-based neurofibromas.
Diagnosis
A workup generally includes a thorough history and examination as well as imaging. In cases of superficial subcutaneous lesions, an ultrasound is often the imaging modality of choice. However, magnetic resonance imaging (MRI) and computed tomography (CT) scans are frequently used for more deep-seated lesions. There can be significant differences between malignant and benign neural lesions on MRI and CT in terms of contrast-uptake and heterogeneity of tissue, but the visual features are not consistent. Best estimates for MRI suggest 61% sensitivity and 90% specificity for the diagnosis of high-grade malignant peripheral nerve sheath tumors based on imaging alone.5
Definitive diagnosis requires surgical excision. Fine-needle aspiration can be used to diagnose subcutaneous nodules, but there is a possibility that degenerative changes and nuclear atypia seen on a smaller sample may be confused with a more aggressive sarcoma. For example, long-standing schwannomas are often called ancient, meaning that they break down over time, and the atypia they display is a regressive phenomenon.6 Therefore, a small or limited tissue sampling may not be representative of the entire lesion.7 As such, patients will likely need referral for surgical removal to determine the exact nature of the growth.
Although schwannomas are uncommon overall, the highest incidence is in the fourth decade of life with a slight predominance in females. They are often incidentally found as a palpable mass but can be symptomatic with paresthesias, pain, or neurologic changes—particularly when identified in the retroperitoneum or along joints. Schwannomas are most commonly found in the retroperitoneum (32%), mediastinum (23%), head and neck (18%), and extremities (16%).8 The majority of cases (about 90%) are sporadic; whereas 2% are related to NF-2.9 The abdominal wall schwannoma is rare. Our review of English-language literature in PubMed and EMBASE found only 5 other case reports (Table 1).
On physical examination, superficial lesions are freely movable except for a single point of attachment, which is generally along the long axis of the nerve.
Pathology
On gross pathology examination, schwannomas have a well-circumscribed smooth external surface. On microscopy, schwannomas are truly encapsulated, uninodular, spindle-cell proliferations arranged in a streaming pattern within a background of thick, hyalinized blood vessels. Classic schwannomas typically exhibit a biphasic pattern of alternating areas of high and low cellularity and are named for Swedish neurologist Nils Antoni. The more cellular regions are referred to as Antoni A areas and consist of streaming fascicles of compact spindle cells that often palisade around acellular eosinophilic areas of fibrillary processes known as Verocay bodies.
In contrast, the lower cellularity regions (Antoni B areas) consist of multipolar, loosely textured cells with abundant cytoplasm, haphazardly arranged processes, and an overall myxoid appearance.11 Schwannomas are known to have widely variable proportions of Antoni A and Antoni B areas; in this case, the excised specimen was noted to have predominately Antoni A areas without well-defined Verocay bodies and only scattered foci showing some suggestion of the hypocellular Antoni B architecture (Figure 2).9,12
Immunohistochemical stains for S100 and SOX10 (used to identify cells derived from a neural crest lineage) were strongly positive, which is characteristic of schwannomas.13 Although there have only been rare reports of extracranial schwannomas undergoing malignant transformation, it is critical to rule out the possibility of a de novo malignant peripheral nerve sheath tumor (MPNST).13 In general, MPNSTs tend to be more cellular, have brisk mitotic activity, areas of necrosis, hyperchromatic nuclei, and conspicuous pleomorphism. Mitotic figures, which can be concerning for malignant potential if present in high number, were noted occasionally in our patient; however, occasional mitosis may be seen in classic schwannomas. Clinically, MPNSTs have a poor prognosis. Based on case reports, disease-specific survival at 10 years is 31.6% for localized disease and only 7.5% for metastatic disease.14 In this case, there was no evidence of any of the high-grade features of a malignant peripheral nerve sheath tumor, thus supporting the diagnosis of schwannoma (neurilemmoma).
Treatment
Schwannomas are exclusively treated by excision. Prognosis is good with low recurrence rates. It is unknown what the recurrence rates are for completely resected abdominal wall schwannomas since there are so few reports in the literature. For other well-known entities, such as vestibular schwannoma (acoustic neuromas), the recurrence rates are generally 2% to 3%.15 Transformation of schwannomas into MPNSTs are so unusual that they are only described in single case reports.
Conclusion
Soft-tissue masses are a common complaint. Most are benign and do not require excision unless it interferes with the quality of life of the patient or if the diagnosis is uncertain. It is important to be aware of schwannomas in the differential diagnosis of soft-tissue masses. Diagnosis may be achieved through the combination of imaging and biopsy, but the definitive diagnosis is made on complete excision of the mass.
Acknowledgments
Contributors: Michael Lewis, MD, Department of Pathology, VA Greater Los Angeles Healthcare System. Written permission also was obtained from the patient.
Schwannomas are benign tumors exclusively composed of Schwann cells that arise from the peripheral nerve sheath; these tumors theoretically can present anywhere in the body where nerves reside. They tend to occur in the head and neck region (classically an acoustic neuroma) but also occur in other locations, including the retroperitoneal space and the extremities, particularly flexural surfaces. Patients with cutaneous schwannomas are most likely to present to their primary care provider’s office reporting skin findings or localized pain, and providers should be aware of schwannomas on the differential for painful nodular growths.
Case Presentation
A 70-year-old man with type 2 diabetes mellitus presented to the primary care clinic for intermittent, sharp, localized left lower quadrant abdominal wall pain that was gradually progressive over the previous few months. The patient noticed the development of a small nodule 7 to 8 months prior to the visit, at which time the pain was less frequent and less severe. He reported no postprandial association of the pain, nausea, vomiting, diarrhea, constipation, or other gastrointestinal symptoms.
Ten months prior to the presentation, he was involved in a low-impact motor vehicle collision as a pedestrian in which he fell face-first onto the hood of an oncoming car. At that time, he did not note any abdominal trauma or pain. Evaluation at a local emergency department did not reveal any major injuries. In the interim, he had self-administered insulin in his abdominal region, as he had without incident for the previous 2 years. He reported that he was not injecting near the site of the nodule since it had formed. He could not recall whether the location was a previous insulin administration site.
On examination, the patient’s vital signs were normal as were the cardiac and respiratory examinations. An abdominal exam revealed normal bowel sounds and no overlying skin changes or discoloration. Palpation revealed a 1.5 x 1 cm rubbery-to-firm, well-circumscribed subcutaneous nodule along his mid-left abdomen, about 7 cm lateral to the umbilicus. The nodule was sensitive to both light touch and deep pressure. It was firmer than expected for an abdominal wall lipoma. There was no central puncta or pore to suggest an epidermal inclusion cyst. There was no surrounding erythema or induration to suggest an abscess.
The patient was referred for surgery and underwent excisional biopsy of the mass. Pathology revealed a well-circumscribed vascular/spindle-cell lesion consistent with a schwannoma. His postoperative course was uncomplicated. At 4-week follow-up the incision had healed completely and the patient was pain free.
Discussion
Soft-tissue nodules are common—about two-thirds of soft-tissue tumors are classified into 7 diagnostic categories: lipoma and lipoma variants (16%), fibrous histiocytoma (13%), nodular fasciitis (11%), hemangioma (8%), fibromatosis (7%), neurofibroma (5%), and schwannoma (5%).1 Peripheral nerve tumors (schwannomas, neurofibromas) can be associated with pain or paresthesias, and less commonly, neurologic deficits, such as motor weakness. Peripheral nerve tumors have several classifications, such as nonneoplastic vs neoplastic, benign vs malignant, and sheath vs nonsheath origins. Schwannomas are considered part of the neoplastic subset due to their growth; otherwise, they are benign with a sheath origin. In contrast to neurofibromas, benign schwannomas have a slower rate of progression, lower association with pain, and fewer neurologic symptoms.2
The neural sheath is made up of 3 types of cells: the fibroblast, the Schwann cell, and the perineural cell, which lacks a basement membrane. It is the Schwann cell that can give rise to the 3 main types of cutaneous nerve tumors: neuromas, neurofibromas, and schwannomas.3 A nerve that is both entering and exiting a mass is a classic presentation for a peripheral nerve sheath tumor. If the nerve is eccentric to the lesion, then it is consistent with a schwannoma (not a neurofibroma).4 Schwannomas are made exclusively of Schwann cells that arise from the nerve sheath, whereas neurofibromas are made up of all the different cell types that constitute a nerve. Bilateral vestibular schwannomas (acoustic neuromas) are virtually pathognomonic of neurofibromatosis 2 (NF-2), which can manifest as hearing loss, tinnitus, and equilibrium problems. In contrast, neurofibromatosis 1 (NF-1) is more common, characterized by multiple café au lait spots, freckling in the axillary and groin regions, increased risk of cancers overall, and development of pedunculated skin growths, brain, or organ-based neurofibromas.
Diagnosis
A workup generally includes a thorough history and examination as well as imaging. In cases of superficial subcutaneous lesions, an ultrasound is often the imaging modality of choice. However, magnetic resonance imaging (MRI) and computed tomography (CT) scans are frequently used for more deep-seated lesions. There can be significant differences between malignant and benign neural lesions on MRI and CT in terms of contrast-uptake and heterogeneity of tissue, but the visual features are not consistent. Best estimates for MRI suggest 61% sensitivity and 90% specificity for the diagnosis of high-grade malignant peripheral nerve sheath tumors based on imaging alone.5
Definitive diagnosis requires surgical excision. Fine-needle aspiration can be used to diagnose subcutaneous nodules, but there is a possibility that degenerative changes and nuclear atypia seen on a smaller sample may be confused with a more aggressive sarcoma. For example, long-standing schwannomas are often called ancient, meaning that they break down over time, and the atypia they display is a regressive phenomenon.6 Therefore, a small or limited tissue sampling may not be representative of the entire lesion.7 As such, patients will likely need referral for surgical removal to determine the exact nature of the growth.
Although schwannomas are uncommon overall, the highest incidence is in the fourth decade of life with a slight predominance in females. They are often incidentally found as a palpable mass but can be symptomatic with paresthesias, pain, or neurologic changes—particularly when identified in the retroperitoneum or along joints. Schwannomas are most commonly found in the retroperitoneum (32%), mediastinum (23%), head and neck (18%), and extremities (16%).8 The majority of cases (about 90%) are sporadic; whereas 2% are related to NF-2.9 The abdominal wall schwannoma is rare. Our review of English-language literature in PubMed and EMBASE found only 5 other case reports (Table 1).
On physical examination, superficial lesions are freely movable except for a single point of attachment, which is generally along the long axis of the nerve.
Pathology
On gross pathology examination, schwannomas have a well-circumscribed smooth external surface. On microscopy, schwannomas are truly encapsulated, uninodular, spindle-cell proliferations arranged in a streaming pattern within a background of thick, hyalinized blood vessels. Classic schwannomas typically exhibit a biphasic pattern of alternating areas of high and low cellularity and are named for Swedish neurologist Nils Antoni. The more cellular regions are referred to as Antoni A areas and consist of streaming fascicles of compact spindle cells that often palisade around acellular eosinophilic areas of fibrillary processes known as Verocay bodies.
In contrast, the lower cellularity regions (Antoni B areas) consist of multipolar, loosely textured cells with abundant cytoplasm, haphazardly arranged processes, and an overall myxoid appearance.11 Schwannomas are known to have widely variable proportions of Antoni A and Antoni B areas; in this case, the excised specimen was noted to have predominately Antoni A areas without well-defined Verocay bodies and only scattered foci showing some suggestion of the hypocellular Antoni B architecture (Figure 2).9,12
Immunohistochemical stains for S100 and SOX10 (used to identify cells derived from a neural crest lineage) were strongly positive, which is characteristic of schwannomas.13 Although there have only been rare reports of extracranial schwannomas undergoing malignant transformation, it is critical to rule out the possibility of a de novo malignant peripheral nerve sheath tumor (MPNST).13 In general, MPNSTs tend to be more cellular, have brisk mitotic activity, areas of necrosis, hyperchromatic nuclei, and conspicuous pleomorphism. Mitotic figures, which can be concerning for malignant potential if present in high number, were noted occasionally in our patient; however, occasional mitosis may be seen in classic schwannomas. Clinically, MPNSTs have a poor prognosis. Based on case reports, disease-specific survival at 10 years is 31.6% for localized disease and only 7.5% for metastatic disease.14 In this case, there was no evidence of any of the high-grade features of a malignant peripheral nerve sheath tumor, thus supporting the diagnosis of schwannoma (neurilemmoma).
Treatment
Schwannomas are exclusively treated by excision. Prognosis is good with low recurrence rates. It is unknown what the recurrence rates are for completely resected abdominal wall schwannomas since there are so few reports in the literature. For other well-known entities, such as vestibular schwannoma (acoustic neuromas), the recurrence rates are generally 2% to 3%.15 Transformation of schwannomas into MPNSTs are so unusual that they are only described in single case reports.
Conclusion
Soft-tissue masses are a common complaint. Most are benign and do not require excision unless it interferes with the quality of life of the patient or if the diagnosis is uncertain. It is important to be aware of schwannomas in the differential diagnosis of soft-tissue masses. Diagnosis may be achieved through the combination of imaging and biopsy, but the definitive diagnosis is made on complete excision of the mass.
Acknowledgments
Contributors: Michael Lewis, MD, Department of Pathology, VA Greater Los Angeles Healthcare System. Written permission also was obtained from the patient.
1. Kransdorf MJ. Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(2):395-402.
2. Valeyrie-Allanore L, Ismaili N, Bastuji-Garin S, et al. Symptoms associated with malignancy of peripheral nerve sheath tumors: a retrospective study of 69 patients with neurofibromatosis 1. Br J Dermatol. 2005;153(1):79-82.
3. Patterson JW. Neural and neuroendocrine tumors. In: Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1042-1049.
4. Balzarotti R, Rondelli F, Barizzi J, Cartolari R. Symptomatic schwannoma of the abdominal wall: a case report and review of the literature. Oncol Lett. 2015;9(3):1095-1098.
5. Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568-1574.
6. Dodd LG, Marom EM, Dash RC, Matthews MR, McLendon RE. Fine-needle aspiration cytology of “ancient” schwannoma. Diagn Cytopathol. 1999;20(5):307-311.
7. Powers CN, Berardo MD, Frable WJ. Fine-needle aspiration biopsy: pitfalls in the diagnosis of spindle-cell lesions. Diagn Cytopathol. 1994;10(3):232-240; discussion 241.
8. White W, Shiu MH, Rosenblum MK, Erlandson RA, Woodruff JM. Cellular schwannoma: a clinicopathologic study of 57 patients and 58 tumors. Cancer. 1990;66(6):1266-1275.
9. Goldblum JR, Weiss SW, Folpe AL. Benign tumors of peripheral nerves. In: Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Elsevier; 2014:813-828.
10. Naversen DN, Trask DM, Watson FH, Burket JM. Painful tumors of the skin: “LEND AN EGG.” J Am Acad Deramatol. 1993;28(2, pt 2):298-300.
11. Burger PC, Scheithauer BW. Diagnostic Pathology: Neuropathology. 1st ed. Salt Lake City, UT: Amirsys; 2012.
12. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. World Health Organization Histological Classification of Tumours of the Central Nervous System. Vol. 1. Paris, France: International Agency for Research on Cancer; 2016.
13. Woodruff JM, Selig AM, Crowley K, Allen PW. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol. 1994;18(9)82-895.
14. Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014-1022.
15. Ahmad RA, Sivalingam S, Topsakal V, Russo A, Taibah A, Sanna M. Rate of recurrent vestibular schwannoma after total removal via different surgical approaches. Ann Otol Rhinol Laryngol. 2012;121(3):156-161.
16. Bhatia RK, Banerjea A, Ram M, Lovett BE. Benign ancient schwannoma of the abdominal wall: an unwanted birthday present. BMC Surg. 2010;10:1-5.
17. Mishra A, Hamadto M, Azzabi M, Elfagieh M. Abdominal wall schwannoma: case report and review of the literature. Case Rep Radiol. 2013;2013:456863.
18. Liu Y, Chen X, Wang T, Wang Z. Imaging observations of a schwannoma of low malignant potential in the anterior abdominal wall: a case report. Oncol Lett. 2014;8(3):1159-1162.
19. Ginesu GC, Puledda M, Feo CF et al. Abdominal wall schwannoma. J Gastrointest Surg. 2016;20(10):1781-1783.
1. Kransdorf MJ. Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(2):395-402.
2. Valeyrie-Allanore L, Ismaili N, Bastuji-Garin S, et al. Symptoms associated with malignancy of peripheral nerve sheath tumors: a retrospective study of 69 patients with neurofibromatosis 1. Br J Dermatol. 2005;153(1):79-82.
3. Patterson JW. Neural and neuroendocrine tumors. In: Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1042-1049.
4. Balzarotti R, Rondelli F, Barizzi J, Cartolari R. Symptomatic schwannoma of the abdominal wall: a case report and review of the literature. Oncol Lett. 2015;9(3):1095-1098.
5. Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568-1574.
6. Dodd LG, Marom EM, Dash RC, Matthews MR, McLendon RE. Fine-needle aspiration cytology of “ancient” schwannoma. Diagn Cytopathol. 1999;20(5):307-311.
7. Powers CN, Berardo MD, Frable WJ. Fine-needle aspiration biopsy: pitfalls in the diagnosis of spindle-cell lesions. Diagn Cytopathol. 1994;10(3):232-240; discussion 241.
8. White W, Shiu MH, Rosenblum MK, Erlandson RA, Woodruff JM. Cellular schwannoma: a clinicopathologic study of 57 patients and 58 tumors. Cancer. 1990;66(6):1266-1275.
9. Goldblum JR, Weiss SW, Folpe AL. Benign tumors of peripheral nerves. In: Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Elsevier; 2014:813-828.
10. Naversen DN, Trask DM, Watson FH, Burket JM. Painful tumors of the skin: “LEND AN EGG.” J Am Acad Deramatol. 1993;28(2, pt 2):298-300.
11. Burger PC, Scheithauer BW. Diagnostic Pathology: Neuropathology. 1st ed. Salt Lake City, UT: Amirsys; 2012.
12. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. World Health Organization Histological Classification of Tumours of the Central Nervous System. Vol. 1. Paris, France: International Agency for Research on Cancer; 2016.
13. Woodruff JM, Selig AM, Crowley K, Allen PW. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol. 1994;18(9)82-895.
14. Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014-1022.
15. Ahmad RA, Sivalingam S, Topsakal V, Russo A, Taibah A, Sanna M. Rate of recurrent vestibular schwannoma after total removal via different surgical approaches. Ann Otol Rhinol Laryngol. 2012;121(3):156-161.
16. Bhatia RK, Banerjea A, Ram M, Lovett BE. Benign ancient schwannoma of the abdominal wall: an unwanted birthday present. BMC Surg. 2010;10:1-5.
17. Mishra A, Hamadto M, Azzabi M, Elfagieh M. Abdominal wall schwannoma: case report and review of the literature. Case Rep Radiol. 2013;2013:456863.
18. Liu Y, Chen X, Wang T, Wang Z. Imaging observations of a schwannoma of low malignant potential in the anterior abdominal wall: a case report. Oncol Lett. 2014;8(3):1159-1162.
19. Ginesu GC, Puledda M, Feo CF et al. Abdominal wall schwannoma. J Gastrointest Surg. 2016;20(10):1781-1783.