User login
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

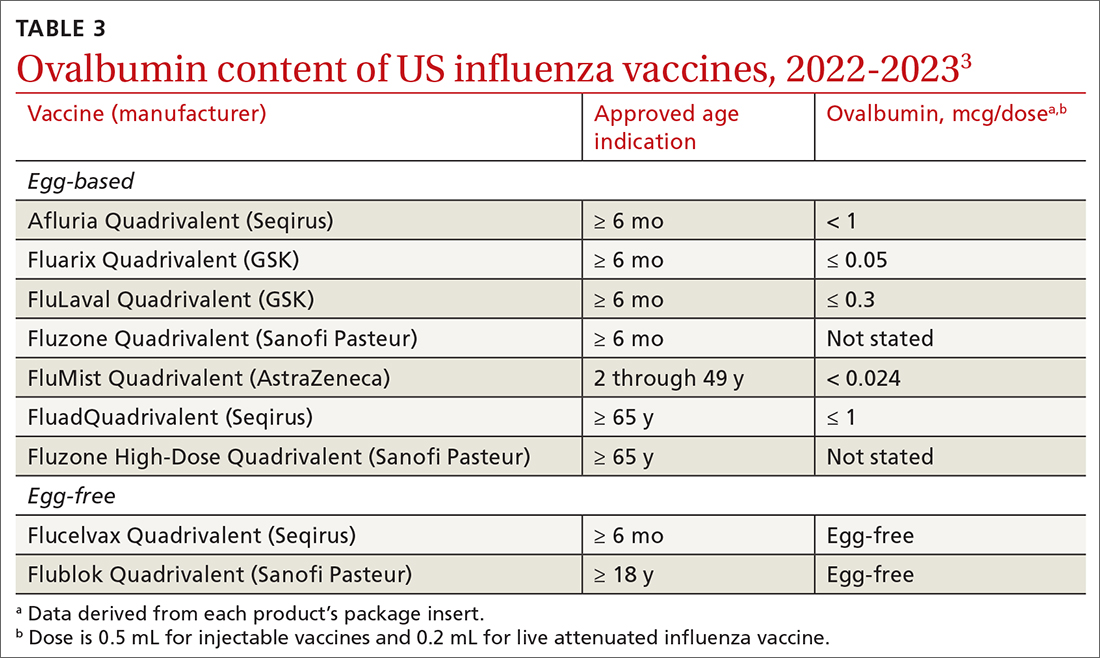
Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.
Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.

Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.

Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html