User login
Acute exertional rhabdomyolysis (AER) is the breakdown/destruction of muscle tissue from extreme physical exertion. Risks that lead to AER include exercise in hot and humid conditions, improper hydration, inadequate recovery between bouts of exercise, intense physical training, and inadequate fitness levels. Other risk factors include sickle cell trait, ingestion of performance enhancing agents, anabolic steroids, and previous history of AER. This article, describes 3 cases of AER after a vigorous, upper body, organized, and supervised training session.
Rhabdomyolysis is not uncommon in competitive athletics,1-3 military training,4-8 and individual training.9-12 It is more common in the lower extremities after intense training or marathons. Creatine kinase (CK) levels rise within 12 hours of muscle injury, peak in 24 to 36 hours, and decrease at a rate of 30% to 40% per day.13 The serum half-life of CK is about 36 hours. The CK levels decline 3 to 5 days after resolution of muscle injury.14 Failure of CK levels to decrease suggests ongoing muscle injury or development of a compartment syndrome. The peak CK level, especially when it is > 15,000 U/L, may be predictive of renal failure.15
Total CK elevation is a sensitive but nonspecific marker for rhabdomyolysis. A CK level that is 1.5 × above the reference range suggests rhabdomyolysis, although CK levels in rhabdomyolysis often are as high as 100 times the reference range or more.12 Health care providers (HCPs) should suspect early rhabdomyolysis and initiate a full laboratory workup for patients with serum CK levels > 2 × the reference range and risk factors for rhabdomyolysis. Because the total CK may increase from the initial values, draw it is important to repeat total CK levels every 6 to 12 hours until a peak level is established.
Case Presentations
An upper body physical training session was conducted with a group of trainees (men and women, aged 24-35 years) 12 weeks into a 21-week program. The training session consisted of 125 push-ups and 85 pull-ups (assisted) performed within a 12-minute period in an indoor, climate-controlled facility. Liberal hydration with water or sports drinks was not allowed. On day 3 following the training session, 1 woman, and on day 4, 2 additional women presented to the clinic with extreme bilateral upper extremity muscle weakness, pain, and marked swelling of their upper-extremities from the shoulder to the forearm. None had firm compartments of the arm or forearm. Further, none had signs of compartment syndrome. There were trace blood and protein in their urine, and their serum CK ranged from 10,000 to 78,000 U/L.
Case 1
The first case to present to the clinic was a 26-year-old white female without any underlying disease. She was taking oral contraceptive medication. She usually exercised by running 10 to 12 miles/week with upper body workouts as required by the training program and was in excellent physical health at the halfway point in the training program.
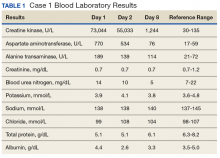
Following the workout of 125 push-ups and 85 pull-ups, the patient experienced muscle soreness and fatigue and took ibuprofen 800 mg that evening. Over the next 2 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 3 after the workout, she presented to the clinic in moderate distress, unable to raise her arms above chest level. Her urine showed trace blood and protein, and her serum CK level was 73,044 U/L (Table 1).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 8 days, and had no sequelae.
Case 2
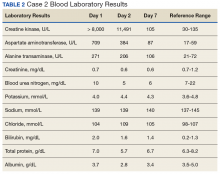
The second case involved a 27-year-old white female without any underlying disease who was not taking any medications and was in excellent physical health. Following the workout of 125 push-ups and 85 pull-ups, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was > 8,000 U/L (the hospital stopped dilutions when the CK level exceeded 8,000 U/L) (Table 2).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Case 3
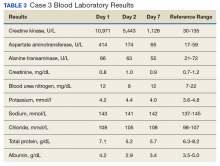
The third case involved a 33-year-old white female without any underlying disease who was taking oral contraceptive medication and was in excellent physical health. Following the 125 push-ups and 85 pull-ups workout, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was 10,971 U/L (Table 3).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Clinical Observation
The importance of brown urine is stressed as a key factor in the diagnosis of AER in much of the literature on the condition.1-10 It is suggested that brown urine is pathognomonic for this condition. However, the urine for the individuals presented here with significant AER was not brown or even tinged. In a field evaluation, an inexperienced HCP might miss an AER diagnosis in the absence of urine findings. In addition, given that AER can occur in stages, it is essential that the HCP have a situational awareness of this condition in today’s culture of fitness and exercise.
Other Causes
Cocaine is a common cause of rhabdomyolysis, namely, in urban patient populations. Prolonged vasoconstriction of intramuscular arteries may result in muscle ischemia and acute rhabdomyolysis, but there also is a direct toxic effect that can produce acute skeletal myofibrillar degeneration.
A number of prescription drugs have been implicated in cases of rhabdomyolysis, including colchicine, zidovudine, isoniazid, benzodiazepines, opiates, corticosteroids, statins, and fibric acid derivatives. One particular interaction that is clinically significant is the interaction between statins and fibrates.16
The pathogenesis of rhabdomyolysis precipitated by infections (whether bacterial, viral, or fungal) is thought to be the result of direct cell invasion of striated muscle and cellular degeneration by the pathogen. Substantial morbidity (57% of cases with acute renal failure) and mortality (death in almost 40% of cases) are linked to bacterial causes of rhabdomyolysis.
In adult patients, Legionella species most often are associated with rhabdomyolysis. Other bacteria linked to rhabdomyolysis include group A β-hemolytic streptococci, Salmonella species, Francisella tularensis and Escherichia coli. Viruses, such as influenza, parainfluenza, coxsackievirus, Epstein-Barr virus, adenovirus, HIV, and cytomegalovirus also have been associated with this condition.
Rhabdomyolysis also can be observed in septic patients without direct muscle infection when the damage is caused by a toxin, associated fever, dehydration, and rigors. Electrolyte disorders, such as hyponatremia or hypernatremia, hypokalemia, and hypophosphatemia can result in rhabdomyolysis, distorting the permeability and the functions of sarcolemma in the muscles. Some endocrine disorders (ie, pheochromocytoma and thyrotoxicosis) also are able to potentiate rhabdomyolysis due to hypermetabolism.
Prevention
A workout program should progress gradually according to the individual’s current level of fitness, whether it’s cardiovascular, circuit training, or weight training. Fluid intake should be monitored, particularly when the workout is long, intense, or hot and especially when the workout meets all 3 conditions. Fluid replacement is important, and for strenuous and longer training evolutions, electrolyte replacement should be considered. Hard exercise while maintaining a low-calorie diet or after long fasting periods should be avoided. Sufficient caloric and fluid intake to allow muscles to work efficiently during strenuous workout period is needed. Recreational drugs, including alcohol should be limited before exercise, and illicit recreational or performance-enhancing drugs should be avoided.
Conclusion
Acute exertional rhabdomyolysis is more common in the lower extremities and in males. Extremely rigorous upper-extremity training can result in AER. The presentation usually is clear with an inciting event and muscle pain in the extremity. Besides identifying associated risk factors and performing a thorough examination, the patient should be examined for compartment syndrome. Early diagnosis and comprehensive management are crucial to ensure full recovery and avoidance of complications, such as acute tubular necrosis, renal failure, cardiac arrhythmias from hyperkalemia, and death. As in most cases with early diagnosis and aggressive management, these patients fully recovered and experienced no sequelae at 8 weeks postevent.
1. Galvez R, Stacy J, Howley A. Exertional rhabdomyolysis in seven division-1 swimming athletes. Clin J Sport Med. 2008;18(4):366-368.
2. Smoot MK, Amendola A, Cramer E, et al. A cluster of exertional rhabdomyolysis affecting a division 1 football team. Clin J Sport Med. 2013;23(5):365-372.
3. Kahanov L, Eberman LE, Wasik M, Alvey T. Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. J Athl Train. 2012;47(2):228-232.
4. Aizawa H, Morita K, Minami H, Sasaki N, Tobise K. Exertional rhabdomyolysis as a result of strenuous military training. J Neurol Sci. 1995;132(2):239-240.
5. Tietjen DP, Guzzi LM. Exertional rhabdomyolysis and acute renal failure following the Army Physical Fitness Test. Mil Med. 154(1):23-25.
6. Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 159(2):160-163.
7. Armed Forces Health Surveillance Center (AFHSC). Exertional rhabdomyolysis among U.S. military members, 2004-2007. MSMR. 2008;15(2):8-11.
8. Gitin EL, Demos MA. Acute exertional rhabdomyolysis: a syndrome of increasing importance to the military physician. Mil Med.1974;139(1):33-36.
9. Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc. 2003;35(9):1499-1502.
10. Lin A, Lin C, Wang T, Leu J. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39(1):e3.
11. Hamer R. When exercise goes awry: exertional rhabdomyolysis. South Med J. 1997;90(5):548-551.
12. Soni SN, McDonald E, Marino C. Rhabdomyolysis after exercise. Postgrad Med. 1993;94(6):128-132.
13. Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212-2215.
14. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
15. Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871-874.
16. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G. Rhabdomyolysis updated. Hippokratia. 2007;11(3):129-137.
Acute exertional rhabdomyolysis (AER) is the breakdown/destruction of muscle tissue from extreme physical exertion. Risks that lead to AER include exercise in hot and humid conditions, improper hydration, inadequate recovery between bouts of exercise, intense physical training, and inadequate fitness levels. Other risk factors include sickle cell trait, ingestion of performance enhancing agents, anabolic steroids, and previous history of AER. This article, describes 3 cases of AER after a vigorous, upper body, organized, and supervised training session.
Rhabdomyolysis is not uncommon in competitive athletics,1-3 military training,4-8 and individual training.9-12 It is more common in the lower extremities after intense training or marathons. Creatine kinase (CK) levels rise within 12 hours of muscle injury, peak in 24 to 36 hours, and decrease at a rate of 30% to 40% per day.13 The serum half-life of CK is about 36 hours. The CK levels decline 3 to 5 days after resolution of muscle injury.14 Failure of CK levels to decrease suggests ongoing muscle injury or development of a compartment syndrome. The peak CK level, especially when it is > 15,000 U/L, may be predictive of renal failure.15
Total CK elevation is a sensitive but nonspecific marker for rhabdomyolysis. A CK level that is 1.5 × above the reference range suggests rhabdomyolysis, although CK levels in rhabdomyolysis often are as high as 100 times the reference range or more.12 Health care providers (HCPs) should suspect early rhabdomyolysis and initiate a full laboratory workup for patients with serum CK levels > 2 × the reference range and risk factors for rhabdomyolysis. Because the total CK may increase from the initial values, draw it is important to repeat total CK levels every 6 to 12 hours until a peak level is established.
Case Presentations
An upper body physical training session was conducted with a group of trainees (men and women, aged 24-35 years) 12 weeks into a 21-week program. The training session consisted of 125 push-ups and 85 pull-ups (assisted) performed within a 12-minute period in an indoor, climate-controlled facility. Liberal hydration with water or sports drinks was not allowed. On day 3 following the training session, 1 woman, and on day 4, 2 additional women presented to the clinic with extreme bilateral upper extremity muscle weakness, pain, and marked swelling of their upper-extremities from the shoulder to the forearm. None had firm compartments of the arm or forearm. Further, none had signs of compartment syndrome. There were trace blood and protein in their urine, and their serum CK ranged from 10,000 to 78,000 U/L.
Case 1
The first case to present to the clinic was a 26-year-old white female without any underlying disease. She was taking oral contraceptive medication. She usually exercised by running 10 to 12 miles/week with upper body workouts as required by the training program and was in excellent physical health at the halfway point in the training program.
Following the workout of 125 push-ups and 85 pull-ups, the patient experienced muscle soreness and fatigue and took ibuprofen 800 mg that evening. Over the next 2 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 3 after the workout, she presented to the clinic in moderate distress, unable to raise her arms above chest level. Her urine showed trace blood and protein, and her serum CK level was 73,044 U/L (Table 1).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 8 days, and had no sequelae.
Case 2
The second case involved a 27-year-old white female without any underlying disease who was not taking any medications and was in excellent physical health. Following the workout of 125 push-ups and 85 pull-ups, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was > 8,000 U/L (the hospital stopped dilutions when the CK level exceeded 8,000 U/L) (Table 2).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Case 3
The third case involved a 33-year-old white female without any underlying disease who was taking oral contraceptive medication and was in excellent physical health. Following the 125 push-ups and 85 pull-ups workout, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was 10,971 U/L (Table 3).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Clinical Observation
The importance of brown urine is stressed as a key factor in the diagnosis of AER in much of the literature on the condition.1-10 It is suggested that brown urine is pathognomonic for this condition. However, the urine for the individuals presented here with significant AER was not brown or even tinged. In a field evaluation, an inexperienced HCP might miss an AER diagnosis in the absence of urine findings. In addition, given that AER can occur in stages, it is essential that the HCP have a situational awareness of this condition in today’s culture of fitness and exercise.
Other Causes
Cocaine is a common cause of rhabdomyolysis, namely, in urban patient populations. Prolonged vasoconstriction of intramuscular arteries may result in muscle ischemia and acute rhabdomyolysis, but there also is a direct toxic effect that can produce acute skeletal myofibrillar degeneration.
A number of prescription drugs have been implicated in cases of rhabdomyolysis, including colchicine, zidovudine, isoniazid, benzodiazepines, opiates, corticosteroids, statins, and fibric acid derivatives. One particular interaction that is clinically significant is the interaction between statins and fibrates.16
The pathogenesis of rhabdomyolysis precipitated by infections (whether bacterial, viral, or fungal) is thought to be the result of direct cell invasion of striated muscle and cellular degeneration by the pathogen. Substantial morbidity (57% of cases with acute renal failure) and mortality (death in almost 40% of cases) are linked to bacterial causes of rhabdomyolysis.
In adult patients, Legionella species most often are associated with rhabdomyolysis. Other bacteria linked to rhabdomyolysis include group A β-hemolytic streptococci, Salmonella species, Francisella tularensis and Escherichia coli. Viruses, such as influenza, parainfluenza, coxsackievirus, Epstein-Barr virus, adenovirus, HIV, and cytomegalovirus also have been associated with this condition.
Rhabdomyolysis also can be observed in septic patients without direct muscle infection when the damage is caused by a toxin, associated fever, dehydration, and rigors. Electrolyte disorders, such as hyponatremia or hypernatremia, hypokalemia, and hypophosphatemia can result in rhabdomyolysis, distorting the permeability and the functions of sarcolemma in the muscles. Some endocrine disorders (ie, pheochromocytoma and thyrotoxicosis) also are able to potentiate rhabdomyolysis due to hypermetabolism.
Prevention
A workout program should progress gradually according to the individual’s current level of fitness, whether it’s cardiovascular, circuit training, or weight training. Fluid intake should be monitored, particularly when the workout is long, intense, or hot and especially when the workout meets all 3 conditions. Fluid replacement is important, and for strenuous and longer training evolutions, electrolyte replacement should be considered. Hard exercise while maintaining a low-calorie diet or after long fasting periods should be avoided. Sufficient caloric and fluid intake to allow muscles to work efficiently during strenuous workout period is needed. Recreational drugs, including alcohol should be limited before exercise, and illicit recreational or performance-enhancing drugs should be avoided.
Conclusion
Acute exertional rhabdomyolysis is more common in the lower extremities and in males. Extremely rigorous upper-extremity training can result in AER. The presentation usually is clear with an inciting event and muscle pain in the extremity. Besides identifying associated risk factors and performing a thorough examination, the patient should be examined for compartment syndrome. Early diagnosis and comprehensive management are crucial to ensure full recovery and avoidance of complications, such as acute tubular necrosis, renal failure, cardiac arrhythmias from hyperkalemia, and death. As in most cases with early diagnosis and aggressive management, these patients fully recovered and experienced no sequelae at 8 weeks postevent.
Acute exertional rhabdomyolysis (AER) is the breakdown/destruction of muscle tissue from extreme physical exertion. Risks that lead to AER include exercise in hot and humid conditions, improper hydration, inadequate recovery between bouts of exercise, intense physical training, and inadequate fitness levels. Other risk factors include sickle cell trait, ingestion of performance enhancing agents, anabolic steroids, and previous history of AER. This article, describes 3 cases of AER after a vigorous, upper body, organized, and supervised training session.
Rhabdomyolysis is not uncommon in competitive athletics,1-3 military training,4-8 and individual training.9-12 It is more common in the lower extremities after intense training or marathons. Creatine kinase (CK) levels rise within 12 hours of muscle injury, peak in 24 to 36 hours, and decrease at a rate of 30% to 40% per day.13 The serum half-life of CK is about 36 hours. The CK levels decline 3 to 5 days after resolution of muscle injury.14 Failure of CK levels to decrease suggests ongoing muscle injury or development of a compartment syndrome. The peak CK level, especially when it is > 15,000 U/L, may be predictive of renal failure.15
Total CK elevation is a sensitive but nonspecific marker for rhabdomyolysis. A CK level that is 1.5 × above the reference range suggests rhabdomyolysis, although CK levels in rhabdomyolysis often are as high as 100 times the reference range or more.12 Health care providers (HCPs) should suspect early rhabdomyolysis and initiate a full laboratory workup for patients with serum CK levels > 2 × the reference range and risk factors for rhabdomyolysis. Because the total CK may increase from the initial values, draw it is important to repeat total CK levels every 6 to 12 hours until a peak level is established.
Case Presentations
An upper body physical training session was conducted with a group of trainees (men and women, aged 24-35 years) 12 weeks into a 21-week program. The training session consisted of 125 push-ups and 85 pull-ups (assisted) performed within a 12-minute period in an indoor, climate-controlled facility. Liberal hydration with water or sports drinks was not allowed. On day 3 following the training session, 1 woman, and on day 4, 2 additional women presented to the clinic with extreme bilateral upper extremity muscle weakness, pain, and marked swelling of their upper-extremities from the shoulder to the forearm. None had firm compartments of the arm or forearm. Further, none had signs of compartment syndrome. There were trace blood and protein in their urine, and their serum CK ranged from 10,000 to 78,000 U/L.
Case 1
The first case to present to the clinic was a 26-year-old white female without any underlying disease. She was taking oral contraceptive medication. She usually exercised by running 10 to 12 miles/week with upper body workouts as required by the training program and was in excellent physical health at the halfway point in the training program.
Following the workout of 125 push-ups and 85 pull-ups, the patient experienced muscle soreness and fatigue and took ibuprofen 800 mg that evening. Over the next 2 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 3 after the workout, she presented to the clinic in moderate distress, unable to raise her arms above chest level. Her urine showed trace blood and protein, and her serum CK level was 73,044 U/L (Table 1).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 8 days, and had no sequelae.
Case 2
The second case involved a 27-year-old white female without any underlying disease who was not taking any medications and was in excellent physical health. Following the workout of 125 push-ups and 85 pull-ups, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was > 8,000 U/L (the hospital stopped dilutions when the CK level exceeded 8,000 U/L) (Table 2).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Case 3
The third case involved a 33-year-old white female without any underlying disease who was taking oral contraceptive medication and was in excellent physical health. Following the 125 push-ups and 85 pull-ups workout, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was 10,971 U/L (Table 3).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Clinical Observation
The importance of brown urine is stressed as a key factor in the diagnosis of AER in much of the literature on the condition.1-10 It is suggested that brown urine is pathognomonic for this condition. However, the urine for the individuals presented here with significant AER was not brown or even tinged. In a field evaluation, an inexperienced HCP might miss an AER diagnosis in the absence of urine findings. In addition, given that AER can occur in stages, it is essential that the HCP have a situational awareness of this condition in today’s culture of fitness and exercise.
Other Causes
Cocaine is a common cause of rhabdomyolysis, namely, in urban patient populations. Prolonged vasoconstriction of intramuscular arteries may result in muscle ischemia and acute rhabdomyolysis, but there also is a direct toxic effect that can produce acute skeletal myofibrillar degeneration.
A number of prescription drugs have been implicated in cases of rhabdomyolysis, including colchicine, zidovudine, isoniazid, benzodiazepines, opiates, corticosteroids, statins, and fibric acid derivatives. One particular interaction that is clinically significant is the interaction between statins and fibrates.16
The pathogenesis of rhabdomyolysis precipitated by infections (whether bacterial, viral, or fungal) is thought to be the result of direct cell invasion of striated muscle and cellular degeneration by the pathogen. Substantial morbidity (57% of cases with acute renal failure) and mortality (death in almost 40% of cases) are linked to bacterial causes of rhabdomyolysis.
In adult patients, Legionella species most often are associated with rhabdomyolysis. Other bacteria linked to rhabdomyolysis include group A β-hemolytic streptococci, Salmonella species, Francisella tularensis and Escherichia coli. Viruses, such as influenza, parainfluenza, coxsackievirus, Epstein-Barr virus, adenovirus, HIV, and cytomegalovirus also have been associated with this condition.
Rhabdomyolysis also can be observed in septic patients without direct muscle infection when the damage is caused by a toxin, associated fever, dehydration, and rigors. Electrolyte disorders, such as hyponatremia or hypernatremia, hypokalemia, and hypophosphatemia can result in rhabdomyolysis, distorting the permeability and the functions of sarcolemma in the muscles. Some endocrine disorders (ie, pheochromocytoma and thyrotoxicosis) also are able to potentiate rhabdomyolysis due to hypermetabolism.
Prevention
A workout program should progress gradually according to the individual’s current level of fitness, whether it’s cardiovascular, circuit training, or weight training. Fluid intake should be monitored, particularly when the workout is long, intense, or hot and especially when the workout meets all 3 conditions. Fluid replacement is important, and for strenuous and longer training evolutions, electrolyte replacement should be considered. Hard exercise while maintaining a low-calorie diet or after long fasting periods should be avoided. Sufficient caloric and fluid intake to allow muscles to work efficiently during strenuous workout period is needed. Recreational drugs, including alcohol should be limited before exercise, and illicit recreational or performance-enhancing drugs should be avoided.
Conclusion
Acute exertional rhabdomyolysis is more common in the lower extremities and in males. Extremely rigorous upper-extremity training can result in AER. The presentation usually is clear with an inciting event and muscle pain in the extremity. Besides identifying associated risk factors and performing a thorough examination, the patient should be examined for compartment syndrome. Early diagnosis and comprehensive management are crucial to ensure full recovery and avoidance of complications, such as acute tubular necrosis, renal failure, cardiac arrhythmias from hyperkalemia, and death. As in most cases with early diagnosis and aggressive management, these patients fully recovered and experienced no sequelae at 8 weeks postevent.
1. Galvez R, Stacy J, Howley A. Exertional rhabdomyolysis in seven division-1 swimming athletes. Clin J Sport Med. 2008;18(4):366-368.
2. Smoot MK, Amendola A, Cramer E, et al. A cluster of exertional rhabdomyolysis affecting a division 1 football team. Clin J Sport Med. 2013;23(5):365-372.
3. Kahanov L, Eberman LE, Wasik M, Alvey T. Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. J Athl Train. 2012;47(2):228-232.
4. Aizawa H, Morita K, Minami H, Sasaki N, Tobise K. Exertional rhabdomyolysis as a result of strenuous military training. J Neurol Sci. 1995;132(2):239-240.
5. Tietjen DP, Guzzi LM. Exertional rhabdomyolysis and acute renal failure following the Army Physical Fitness Test. Mil Med. 154(1):23-25.
6. Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 159(2):160-163.
7. Armed Forces Health Surveillance Center (AFHSC). Exertional rhabdomyolysis among U.S. military members, 2004-2007. MSMR. 2008;15(2):8-11.
8. Gitin EL, Demos MA. Acute exertional rhabdomyolysis: a syndrome of increasing importance to the military physician. Mil Med.1974;139(1):33-36.
9. Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc. 2003;35(9):1499-1502.
10. Lin A, Lin C, Wang T, Leu J. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39(1):e3.
11. Hamer R. When exercise goes awry: exertional rhabdomyolysis. South Med J. 1997;90(5):548-551.
12. Soni SN, McDonald E, Marino C. Rhabdomyolysis after exercise. Postgrad Med. 1993;94(6):128-132.
13. Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212-2215.
14. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
15. Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871-874.
16. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G. Rhabdomyolysis updated. Hippokratia. 2007;11(3):129-137.
1. Galvez R, Stacy J, Howley A. Exertional rhabdomyolysis in seven division-1 swimming athletes. Clin J Sport Med. 2008;18(4):366-368.
2. Smoot MK, Amendola A, Cramer E, et al. A cluster of exertional rhabdomyolysis affecting a division 1 football team. Clin J Sport Med. 2013;23(5):365-372.
3. Kahanov L, Eberman LE, Wasik M, Alvey T. Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. J Athl Train. 2012;47(2):228-232.
4. Aizawa H, Morita K, Minami H, Sasaki N, Tobise K. Exertional rhabdomyolysis as a result of strenuous military training. J Neurol Sci. 1995;132(2):239-240.
5. Tietjen DP, Guzzi LM. Exertional rhabdomyolysis and acute renal failure following the Army Physical Fitness Test. Mil Med. 154(1):23-25.
6. Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 159(2):160-163.
7. Armed Forces Health Surveillance Center (AFHSC). Exertional rhabdomyolysis among U.S. military members, 2004-2007. MSMR. 2008;15(2):8-11.
8. Gitin EL, Demos MA. Acute exertional rhabdomyolysis: a syndrome of increasing importance to the military physician. Mil Med.1974;139(1):33-36.
9. Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc. 2003;35(9):1499-1502.
10. Lin A, Lin C, Wang T, Leu J. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39(1):e3.
11. Hamer R. When exercise goes awry: exertional rhabdomyolysis. South Med J. 1997;90(5):548-551.
12. Soni SN, McDonald E, Marino C. Rhabdomyolysis after exercise. Postgrad Med. 1993;94(6):128-132.
13. Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212-2215.
14. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
15. Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871-874.
16. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G. Rhabdomyolysis updated. Hippokratia. 2007;11(3):129-137.