User login
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
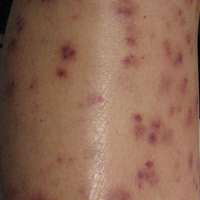
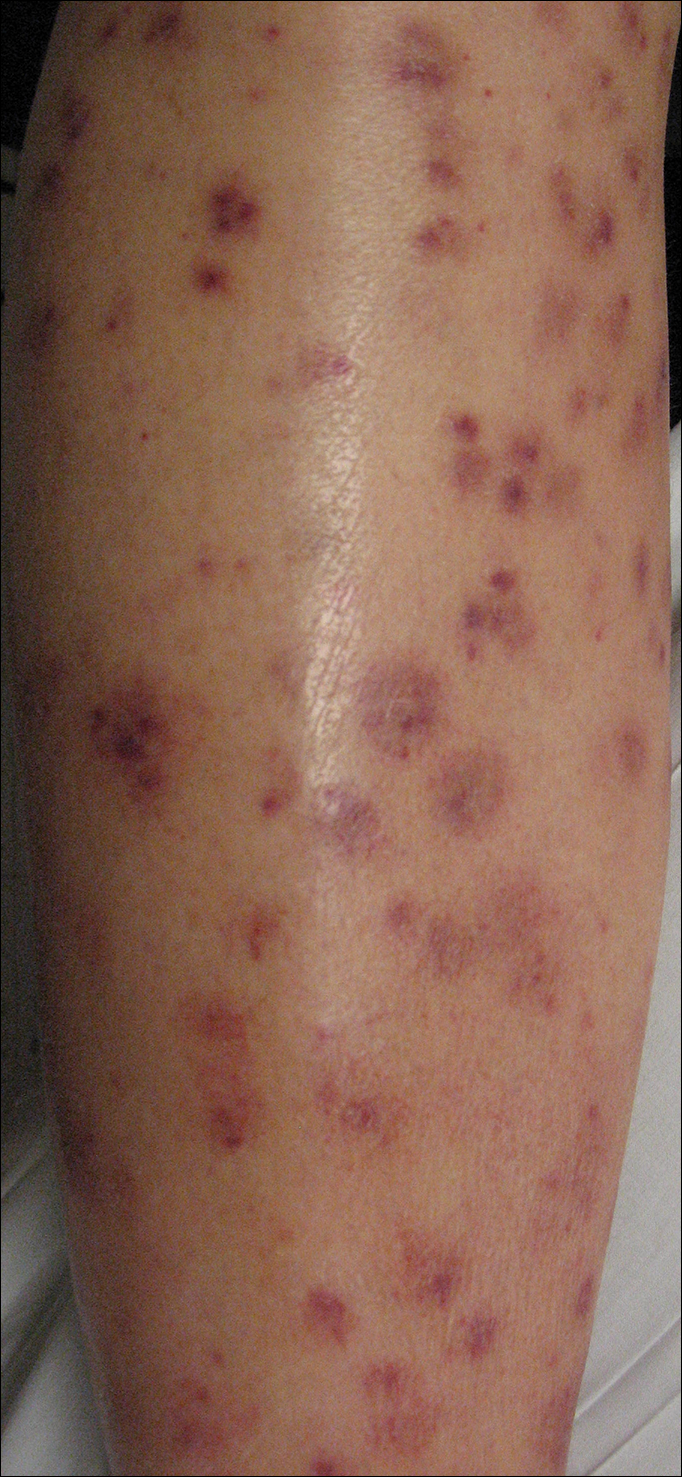
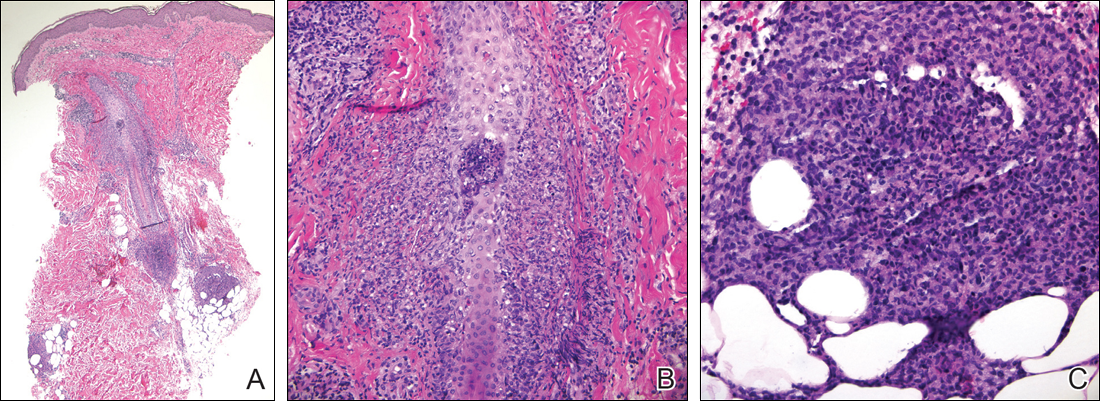
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.
In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.


In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.


In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
Practice Point
- Sterile mixed inflammatory skin reactions reminiscent of neutrophilic dermatoses may occur during neutrophil recovery in patients undergoing therapy for leukemias and need to be considered as part of the differential diagnosis.