User login
Several major gastroenterological and surgical societies have issued guidelines on how to manage acute pancreatitis, based on evidence from high-quality randomized trials and nonrandomized studies as well as on expert opinion.1–3 Information is limited on how well physicians in the United States comply with these guidelines, but compliance is suboptimal in other developed countries, according to several studies,4–8 and we suspect that many US physicians are not following the guidelines either.
Acute pancreatitis is a frequent inpatient diagnosis that internists, gastroenterologists, and surgeons all confront. The most common causes are gallstones and heavy alcohol intake. Its management is typically straightforward: intravenous fluids, analgesia, and nothing by mouth. However, treatment of severe cases can be quite complex, particularly if multiple organ systems are involved or if there are local complications.
The primary aim of this article is to raise awareness of recognized deviations from established recommendations that may lead to adverse patient outcomes.
MEASURING ENZYME LEVELS DAILY ADDS COST BUT LITTLE BENEFIT
Problem: Serum amylase and lipase levels are often needlessly measured every day.
Measuring the serum amylase and lipase levels is useful in diagnosing acute pancreatitis, which requires two of the following three features1:
- Characteristic abdominal pain
- Levels of serum amylase or serum lipase, or both, that are three or more times the upper limit of normal
- Findings of acute pancreatitis on computed tomography (CT).
However, the magnitude or duration of the serum enzyme elevation does not correlate with the severity of the attack. Further, we have noticed that physicians at our hospital often order daily serum amylase and lipase levels in patients admitted with acute pancreatitis.
The American College of Gastroenterology (ACG) guidelines1 state that daily monitoring of amylase and lipase has limited value in managing acute pancreatitis. Rechecking these concentrations may be reasonable if pain fails to resolve or worsens during a prolonged hospitalization, as this may suggest a recurrent attack of acute pancreatitis or a developing pseudocyst. But in most cases of acute pancreatitis, daily serum enzyme measurements add cost but little benefit.
REGULAR ASSESSMENT IS IMPORTANT
Problem: Often, severity assessments are not performed regularly or acted on.
Most cases of acute pancreatitis are mild, with rapid recovery and excellent prognosis. However, 15% to 20% are severe and may result in a prolonged hospitalization, systemic inflammatory response syndrome (SIRS), multiorgan system failure, and death.
In severe acute pancreatitis, as pancreatic enzymes and inflammatory cytokines damage the blood vessels, a vast amount of fluid leaks out into the interstitial (“third”) space. This fluid extravasation leads to decreased effective circulating volume, local pancreatic necrosis, hemodynamic instability, and end-organ failure.
It is important to recognize severe acute pancreatitis early because the patient needs to be transferred to a step-down unit or intensive care unit to receive optimal fluid resuscitation and supportive care for organ dysfunction. After 48 to 72 hours, a prediction of severe acute pancreatitis should also prompt the physician to order CT to detect pancreatic necrosis, and also to initiate nutritional support.
Assessment of severity begins in the emergency room or on admission to the hospital. Older age, obesity, organ failure, and pulmonary infiltrates or pleural effusions are initial indicators of poor prognosis. Signs of SIRS (high or low core body temperature, tachycardia, tachypnea, low or high peripheral white blood cell count) or organ failure (eg, elevated serum creatinine) are present on admission in 21% of patients with acute pancreatitis.9
Hemoconcentration is a marker of decreased effective circulating volume in severe acute pancreatitis. A hematocrit higher than 44% at admission or that rises in the first 24 to 48 hours of admission predicts necrosis.10,11 However, a more robust marker of organ failure may be the blood urea nitrogen level.12
Clinical scoring systems
Several clinical scoring systems have been studied for assessing severity.
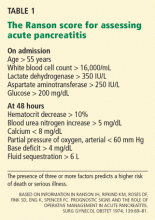
Limitations of the Ranson score are that it can only be completed after 48 hours, all the data points are not always obtained, and it cannot be repeated on a daily basis. Owing to these limitations and its less-than-optimal predictive value, the Ranson score has fallen into disuse.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) score is more versatile. It is based on multiple clinical and laboratory values, and it correlates very well with the risk of death in acute pancreatitis. Death rates are less than 4% when the APACHE II score is less than 8, and 11% to 18% when it is 8 or higher.1 The trajectory of the APACHE II score in the first 48 hours is also an accurate prognostic indicator.
Previous limitations of the APACHE II score were that it was complicated and timeconsuming to calculate and required arterial blood gas measurements. Easy-to-use online calculators are now available (eg, www.globalrph.com/apacheii.htm), and the venous bicarbonate level and the oxygen saturation can be substituted for the arterial pH and oxygen partial pressure.
BISAP, a new five-point scoring system,15 was recently prospectively validated.12 “BISAP” is an acronym for the five markers it is based on, each of which has been shown to predict severe illness in acute pancreatitis:
- Blood urea nitrogen level > 25 mg/dL
- Impaired mental status
- SIRS
- Age > 60 years
- Pleural effusion.
The presence of three or more of these factors correlates with higher risk of death, organ failure, and pancreatic necrosis.12
Compared with APACHE II, BISAP has similar accuracy and is easier to calculate. Also, BISAP was specifically developed for acute pancreatitis, whereas APACHE II is a generic score for all critically ill patients.
The Atlanta criteria16 define severe acute pancreatitis as one or more of the following:
- A Ranson score of 3 or higher during the first 48 hours
- An APACHE II score of 8 or higher at any time
- Failure of one or more organs
- One or more local complications (eg, necrosis, pseudocysts, abscesses).
Recommendation: Assess severity at least daily
A severity assessment should be performed at admission and at least every day thereafter. Clinical guidelines recognize the importance of severity assessment but vary in their specific recommendations.
The ACG advises calculating the APACHE II score within 3 days of admission and measuring the hematocrit at admission, at 12 hours, and at 24 hours. The level of evidence is III, ie, “from published well-designed trials without randomization, single group prepost, cohort, time series, or matched case controlled studies”.1
The American Gastroenterological Association (AGA) provides a more generalized recommendation, that “clinical judgment” should take into account the presence of risk factors (eg, age, obesity), presence or absence of SIRS, routine laboratory values (eg, hematocrit, serum creatinine), and APACHE II score when assessing severity and making decisions.2
In a German survey, only 32% of gastroenterologists used the APACHE II score for assessing risk in acute pancreatitis, in spite of national guidelines emphasizing its importance.7 Also, not all patients with severe acute pancreatitis are transferred to a step-down unit or intensive care unit as recommended. In a British study,4 only 8 (17%) of 46 patients with predicted severe acute pancreatitis were transferred, and 8 of the 38 patients who were not transferred died.
FLUID MUST BE AGGRESSIVELY REPLACED AND MONITORED
Problem: Often, not enough fluid is replaced, or fluid status is not adequately monitored.
Fluid must be aggressively replaced to balance the massive third-space fluid losses that occur in the early inflammatory phase of acute pancreatitis. Intravascular volume depletion can develop rapidly and result in tachycardia, hypotension, and renal failure. It may also impair the blood flow to the pancreas and worsen necrosis.
Animal studies show that aggressive fluid replacement supports the pancreatic microcirculation and prevents necrosis.17 It may also support the intestinal microcirculation and gut barrier, preventing bacterial translocation.
In humans, no controlled trials have been done to test the efficacy of aggressive fluid resuscitation in acute pancreatitis. However, the notion that intravascular fluid loss contributes to poor outcomes is inferred from human studies showing more necrosis and deaths in patients with hemoconcentration. In one study, patients who received inadequate fluid replacement (evidenced by a rise in hematocrit at 24 hours) were more likely to develop necrotizing pancreatitis.18
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
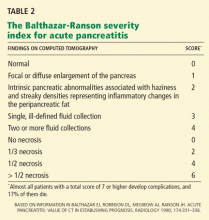
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4
SUSPECTED INFECTED NECROSIS
Problem: Fine-needle aspiration is not done in many cases of suspected infected necrosis.
Approximately one-third of patients with necrotizing pancreatitis develop infected necrosis. The death rate for patients with infected pancreatic necrosis is high—30%, compared with 12% in those with sterile necrosis.1 Differentiating sterile and infected necrosis is therefore essential.
Clinical signs such as fever are poor predictors of infection. Signs of SIRS can be present in both sterile and infected necrotizing pancreatitis.
Recommendation: Fine-needle aspiration of necrosis
For the reasons given above, the findings of necrosis on CT and persistent SIRS should prompt consideration of fine-needle aspiration with Gram stain and culture to differentiate sterile and infected necrosis (ACG guideline, level of evidence III).1 If infection is confirmed, surgical debridement should be strongly considered. Other less-invasive approaches such as endoscopic debridement can be used in selected cases.
Fine-needle aspiration of necrosis is too often neglected. In a cohort of German surgeons, only 55% complied with International Association of Pancreatology recommendations to perform biopsy to differentiate sterile from infected necrosis in patients with signs of sepsis.29
BROAD-SPECTRUM ANTIBIOTICS
Problem: Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Antibiotics are not indicated in mild acute pancreatitis. A limited course of antibiotics is typically indicated in severe cases with suspected or proven infected necrosis (in conjunction with surgical necrosectomy). However, the use of antibiotics in sterile necrosis has been very controversial.
At least six small, nonblinded, randomized trials have evaluated the benefit of giving antibiotics prophylactically for presumed sterile necrosis. A recent Cochrane analysis of five of these trials (294 patients) suggested that patients who got antibiotics had a lower risk of death (odds ratio 0.37, 95% confidence interval [CI] 0.17–0.83) but no difference in the rates of pancreatic infection or surgery.30 These paradoxical results suggest that antibiotics may prevent death by preventing nonpancreatic infections (eg, pneumonia, line infections) rather than by preventing infection of necrotic pancreatic tissue. The five trials in the meta-analysis are limited by significant methodologic heterogeneity and by lack of double-blinding.
In spite of the overall lower death rate observed in the meta-analysis, the prophylactic use of antibiotics in sterile necrosis remains controversial. One concern is that patients given long prophylactic courses of antibiotics may develop resistant bacterial or fungal infections. However, the Cochrane and other meta-analyses have not shown a higher rate of fungal infections in those given antibiotics.31
Recommendation: No routine antibiotics for mild cases
The AGA guidelines recommend against routinely giving antibiotics in mild acute pancreatitis and do not provide strict recommendations for prophylactic antibiotic use in necrotizing acute pancreatitis.2 The guidelines state that antibiotics can be used “on demand” based on clinical signs of infection (eg, high fevers, rising leukocytosis, hypotension) or worsening organ failure.
If a purely prophylactic strategy is used, only patients at high risk of developing infection (eg, those with necrosis in more than 30% of the pancreas) should receive antibiotics. Antibiotics with high tissue-penetration should be used, such as imipenem-cilastin (Primaxin IV) or ciprofloxacin (Cipro) plus metronidazole (Flagyl).
Adherence to these guidelines is not optimal. For example, in an Italian multicenter study, 9% of patients with mild acute pancreatitis were treated with antibiotics.19 Moreover, many patients with proven infected necrosis received antibiotics that do not penetrate the pancreatic tissue very well.
ERCP IN SEVERE BILIARY ACUTE PANCREATITIS
Problem: Endoscopic retrograde cholangiopancreatography (ERCP) often is performed inappropriately in mild biliary acute pancreatitis or is not performed urgently in severe cases.
In most cases of mild biliary pancreatitis, the stones pass spontaneously, as verified by cholangiography done during laparoscopic cholecystectomy. Ongoing ampullary obstruction by impacted biliary stones can perpetuate pancreatic inflammation and delay recovery.
Two early randomized trials showed a benefit from early ERCP (within 72 hours) with sphincterotomy and stone extraction, primarily in those with severe biliary acute pancreatitis or ascending cholangitis,32,33 but a third trial failed to reveal a benefit.34 A Cochrane metaanalysis of these three trials failed to show a lower death rate with ERCP in mild or severe biliary pancreatitis.35 However, early ERCP did prevent complications in severe biliary pancreatitis (odds ratio 0.27, 95% CI 0.14–0.53).
Later, a fourth randomized trial was restricted to patients with suspected biliary pancreatitis, evidence of biliary obstruction, and no signs of cholangitis36: 103 patients were randomized to undergo either ERCP within 72 hours or conservative management. No difference was observed in rates of death or organ failure or in the CT severity index.
Recommendation: ER CP for suspected retained stones
ERCP has a limited role in patients with biliary pancreatitis, being used to clear retained bile duct stones or to relieve ongoing biliary obstruction.
The decision to perform ERCP before surgery should be based on how strongly one suspects retained stones. ERCP is most appropriate if the suspicion of retained stones and the likelihood of therapeutic intervention are high (eg, if the serum bilirubin and alkaline phosphatase levels are rising and ultrasonography shows a dilated bile duct). If there is moderate suspicion, a safer and less-invasive imaging study such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography can be done to screen for bile duct stones before proceeding to ERCP.
The ACG guidelines suggest urgent ERCP (preferably within 24 hours) for those with severe biliary pancreatitis complicated by organ failure or those with suspicion of cholangitis. The level of evidence is I, ie, “strong evidence from at least one published systematic review of multiple well-designed randomized controlled trials.”1
Elective ERCP is recommended for those who are poor surgical candidates. ERCP is also recommended for those with rising liver enzyme values or imaging findings suggesting a retained common bile duct stone (including intraoperative cholangiography). Endoscopic ultrasonography or MRCP is recommended for those with slow clinical resolution, who are pregnant, or in whom uncertainty exists regarding the biliary etiology of pancreatitis.
Compliance rates with these and similar guidelines are not adequate. In an audit of adherence to the British Society of Gastroenterology guidelines, early ERCP was performed in only 25% of patients with severe biliary acute pancreatitis.6
LAPAROSCOPIC CHOLECYSTECTOMY FOR MILD BILIARY PANCREATITIS
Problem: Laparoscopic cholecystectomy is not done at admission or within 2 weeks in many patients with mild biliary pancreatitis.
If the gallbladder is not removed, biliary pancreatitis may recur in up to 61% of patients within 6 weeks of hospital discharge.37 This is the basis for guideline recommendations for surgery (or a confirmation of a surgery date) prior to hospital discharge.
The International Association of Pancreatology recommends early cholecystectomy (preferably during the same hospitalization) for patients with mild gallstone-associated acute pancreatitis.38 In severe gallstone-associated acute pancreatitis, cholecystectomy should be delayed until there is sufficient resolution of the inflammatory response and clinical recovery. The AGA guidelines advocate cholecystectomy as soon as possible and in no case later than 4 weeks after discharge to prevent relapse. ERCP with biliary sphinc-terotomy may also protect against relapse in those who are not fit to undergo surgery.
Recommendations for definitive management of gallstones (laparoscopic cholecystectomy or ERCP, or both) are not always followed. For example, a British study showed 70% compliance with this recommendation.4 A similar compliance audit in Germany revealed that cholecystectomy was performed during the initial hospital stay in only 23% of cases.7 In a New Zealand study, a regular compliance audit with feedback to surgeons resulted in an increase in the early cholecystectomy rate from 54% to 80%.8
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007; 132:2022–2044.
- United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42(suppl 2):S1–S13.
- Norton SA, Cheruvu CV, Collins J, Dix FP, Eyre-Brook IA. An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 2001; 83:399–405.
- Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg 2004; 74:218–221.
- Barnard J, Siriwardena AK. Variations in implementation of current national guidelines for the treatment of acute pancreatitis: implications for acute surgical service provision. Ann R Coll Surg Engl 2002; 84:79–81.
- Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis in Germany [letter]. Pancreatology 2005; 5:591–593.
- Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J 2008; 121:19–25.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction, and death in acute pancreatitis. Br J Surg 2006; 93:738–744.
- Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000; 20:367–372.
- Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96:2081–2085.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Larvin M. Assessment of clinical severity and prognosis. In:Beger HG, Warshaw AL, Buchler MW, et al, editors. The Pancreas. Blackwell Science: New York, 1998:489–502.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993, 128:586–590.
- Forgacs B, Eible G, Faulhaber J, Kahrau S, Buhr H, Foitzik T. Effect of fluid resuscitation with and without endothelin A receptor blockade on hemoconcentration and organ function in experimental pancreatitis. Eur Surg Res 2000; 32:162–168.
- Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2:104–107.
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007; 132:1127–1151.
- Pezzilli R, Uomo G, Gabbrielli A, et al; ProInf-AISP Study Group. A prospective multicenter survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis 2007; 39:838–846.
- Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008; 6:1070–1076.
- Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther 2008; 28:704–712.
- Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004; 328:1407.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Foitzik T, Bassi DG, Schmidt J, et al. Intravenous contrast medium accentuates the severity of acute necrotizing pancreatitis in the rat. Gastroenterology 1994; 106:207–214.
- Carmona-Sanchez R, Uscanga L, Bezaury-Rivas P, Robles-Díaz G, Suazo-Barahona J, Vargas-Vorácková F. Potential harmful effect of iodinated intravenous contrast medium on the clinical course of mild acute pancreatitis. Arch Surg 2000; 135:1280–1284.
- Uhl W, Roggo A, Kirschstein T, et al. Influence of contrast-enhanced computed tomography on couse and outcome in patients with acute pancreatitis. Pancreas 2002; 24:191–197.
- Foitzik T, Klar E. Non-compliance with guidelines for the management of severe acute pancreatitis among German surgeons. Pancreatology 2007; 7:80–85.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006;CD002941.
- Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg 2006; 243:154–168.
- Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988; 2:979–983.
- Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993; 328:228–232.
- Folsch UR, Nitsche R, Ludtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997; 336:237–242.
- Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone associated pancreatitis. Cochrane Database Syst Rev 2004;CD003630
- Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. A randomized clinical trial. Ann Surg 2007; 245:10–17.
- Frei GJ, Frei VT, Thirlby RC, McClelland RN. Biliary pancreatitis: clinical presentation and surgical management. Am J Surg 1986; 151:170–175.
- Uhl W, Warshaw A, Imrie C, et al; International Association of Pancreatology. IAP guidelines on the surgical management of acute pancreatitis. Pancreatology 2002; 2:565–573.
Several major gastroenterological and surgical societies have issued guidelines on how to manage acute pancreatitis, based on evidence from high-quality randomized trials and nonrandomized studies as well as on expert opinion.1–3 Information is limited on how well physicians in the United States comply with these guidelines, but compliance is suboptimal in other developed countries, according to several studies,4–8 and we suspect that many US physicians are not following the guidelines either.
Acute pancreatitis is a frequent inpatient diagnosis that internists, gastroenterologists, and surgeons all confront. The most common causes are gallstones and heavy alcohol intake. Its management is typically straightforward: intravenous fluids, analgesia, and nothing by mouth. However, treatment of severe cases can be quite complex, particularly if multiple organ systems are involved or if there are local complications.
The primary aim of this article is to raise awareness of recognized deviations from established recommendations that may lead to adverse patient outcomes.
MEASURING ENZYME LEVELS DAILY ADDS COST BUT LITTLE BENEFIT
Problem: Serum amylase and lipase levels are often needlessly measured every day.
Measuring the serum amylase and lipase levels is useful in diagnosing acute pancreatitis, which requires two of the following three features1:
- Characteristic abdominal pain
- Levels of serum amylase or serum lipase, or both, that are three or more times the upper limit of normal
- Findings of acute pancreatitis on computed tomography (CT).
However, the magnitude or duration of the serum enzyme elevation does not correlate with the severity of the attack. Further, we have noticed that physicians at our hospital often order daily serum amylase and lipase levels in patients admitted with acute pancreatitis.
The American College of Gastroenterology (ACG) guidelines1 state that daily monitoring of amylase and lipase has limited value in managing acute pancreatitis. Rechecking these concentrations may be reasonable if pain fails to resolve or worsens during a prolonged hospitalization, as this may suggest a recurrent attack of acute pancreatitis or a developing pseudocyst. But in most cases of acute pancreatitis, daily serum enzyme measurements add cost but little benefit.
REGULAR ASSESSMENT IS IMPORTANT
Problem: Often, severity assessments are not performed regularly or acted on.
Most cases of acute pancreatitis are mild, with rapid recovery and excellent prognosis. However, 15% to 20% are severe and may result in a prolonged hospitalization, systemic inflammatory response syndrome (SIRS), multiorgan system failure, and death.
In severe acute pancreatitis, as pancreatic enzymes and inflammatory cytokines damage the blood vessels, a vast amount of fluid leaks out into the interstitial (“third”) space. This fluid extravasation leads to decreased effective circulating volume, local pancreatic necrosis, hemodynamic instability, and end-organ failure.
It is important to recognize severe acute pancreatitis early because the patient needs to be transferred to a step-down unit or intensive care unit to receive optimal fluid resuscitation and supportive care for organ dysfunction. After 48 to 72 hours, a prediction of severe acute pancreatitis should also prompt the physician to order CT to detect pancreatic necrosis, and also to initiate nutritional support.
Assessment of severity begins in the emergency room or on admission to the hospital. Older age, obesity, organ failure, and pulmonary infiltrates or pleural effusions are initial indicators of poor prognosis. Signs of SIRS (high or low core body temperature, tachycardia, tachypnea, low or high peripheral white blood cell count) or organ failure (eg, elevated serum creatinine) are present on admission in 21% of patients with acute pancreatitis.9
Hemoconcentration is a marker of decreased effective circulating volume in severe acute pancreatitis. A hematocrit higher than 44% at admission or that rises in the first 24 to 48 hours of admission predicts necrosis.10,11 However, a more robust marker of organ failure may be the blood urea nitrogen level.12
Clinical scoring systems
Several clinical scoring systems have been studied for assessing severity.
Limitations of the Ranson score are that it can only be completed after 48 hours, all the data points are not always obtained, and it cannot be repeated on a daily basis. Owing to these limitations and its less-than-optimal predictive value, the Ranson score has fallen into disuse.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) score is more versatile. It is based on multiple clinical and laboratory values, and it correlates very well with the risk of death in acute pancreatitis. Death rates are less than 4% when the APACHE II score is less than 8, and 11% to 18% when it is 8 or higher.1 The trajectory of the APACHE II score in the first 48 hours is also an accurate prognostic indicator.
Previous limitations of the APACHE II score were that it was complicated and timeconsuming to calculate and required arterial blood gas measurements. Easy-to-use online calculators are now available (eg, www.globalrph.com/apacheii.htm), and the venous bicarbonate level and the oxygen saturation can be substituted for the arterial pH and oxygen partial pressure.
BISAP, a new five-point scoring system,15 was recently prospectively validated.12 “BISAP” is an acronym for the five markers it is based on, each of which has been shown to predict severe illness in acute pancreatitis:
- Blood urea nitrogen level > 25 mg/dL
- Impaired mental status
- SIRS
- Age > 60 years
- Pleural effusion.
The presence of three or more of these factors correlates with higher risk of death, organ failure, and pancreatic necrosis.12
Compared with APACHE II, BISAP has similar accuracy and is easier to calculate. Also, BISAP was specifically developed for acute pancreatitis, whereas APACHE II is a generic score for all critically ill patients.
The Atlanta criteria16 define severe acute pancreatitis as one or more of the following:
- A Ranson score of 3 or higher during the first 48 hours
- An APACHE II score of 8 or higher at any time
- Failure of one or more organs
- One or more local complications (eg, necrosis, pseudocysts, abscesses).
Recommendation: Assess severity at least daily
A severity assessment should be performed at admission and at least every day thereafter. Clinical guidelines recognize the importance of severity assessment but vary in their specific recommendations.
The ACG advises calculating the APACHE II score within 3 days of admission and measuring the hematocrit at admission, at 12 hours, and at 24 hours. The level of evidence is III, ie, “from published well-designed trials without randomization, single group prepost, cohort, time series, or matched case controlled studies”.1
The American Gastroenterological Association (AGA) provides a more generalized recommendation, that “clinical judgment” should take into account the presence of risk factors (eg, age, obesity), presence or absence of SIRS, routine laboratory values (eg, hematocrit, serum creatinine), and APACHE II score when assessing severity and making decisions.2
In a German survey, only 32% of gastroenterologists used the APACHE II score for assessing risk in acute pancreatitis, in spite of national guidelines emphasizing its importance.7 Also, not all patients with severe acute pancreatitis are transferred to a step-down unit or intensive care unit as recommended. In a British study,4 only 8 (17%) of 46 patients with predicted severe acute pancreatitis were transferred, and 8 of the 38 patients who were not transferred died.
FLUID MUST BE AGGRESSIVELY REPLACED AND MONITORED
Problem: Often, not enough fluid is replaced, or fluid status is not adequately monitored.
Fluid must be aggressively replaced to balance the massive third-space fluid losses that occur in the early inflammatory phase of acute pancreatitis. Intravascular volume depletion can develop rapidly and result in tachycardia, hypotension, and renal failure. It may also impair the blood flow to the pancreas and worsen necrosis.
Animal studies show that aggressive fluid replacement supports the pancreatic microcirculation and prevents necrosis.17 It may also support the intestinal microcirculation and gut barrier, preventing bacterial translocation.
In humans, no controlled trials have been done to test the efficacy of aggressive fluid resuscitation in acute pancreatitis. However, the notion that intravascular fluid loss contributes to poor outcomes is inferred from human studies showing more necrosis and deaths in patients with hemoconcentration. In one study, patients who received inadequate fluid replacement (evidenced by a rise in hematocrit at 24 hours) were more likely to develop necrotizing pancreatitis.18
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4
SUSPECTED INFECTED NECROSIS
Problem: Fine-needle aspiration is not done in many cases of suspected infected necrosis.
Approximately one-third of patients with necrotizing pancreatitis develop infected necrosis. The death rate for patients with infected pancreatic necrosis is high—30%, compared with 12% in those with sterile necrosis.1 Differentiating sterile and infected necrosis is therefore essential.
Clinical signs such as fever are poor predictors of infection. Signs of SIRS can be present in both sterile and infected necrotizing pancreatitis.
Recommendation: Fine-needle aspiration of necrosis
For the reasons given above, the findings of necrosis on CT and persistent SIRS should prompt consideration of fine-needle aspiration with Gram stain and culture to differentiate sterile and infected necrosis (ACG guideline, level of evidence III).1 If infection is confirmed, surgical debridement should be strongly considered. Other less-invasive approaches such as endoscopic debridement can be used in selected cases.
Fine-needle aspiration of necrosis is too often neglected. In a cohort of German surgeons, only 55% complied with International Association of Pancreatology recommendations to perform biopsy to differentiate sterile from infected necrosis in patients with signs of sepsis.29
BROAD-SPECTRUM ANTIBIOTICS
Problem: Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Antibiotics are not indicated in mild acute pancreatitis. A limited course of antibiotics is typically indicated in severe cases with suspected or proven infected necrosis (in conjunction with surgical necrosectomy). However, the use of antibiotics in sterile necrosis has been very controversial.
At least six small, nonblinded, randomized trials have evaluated the benefit of giving antibiotics prophylactically for presumed sterile necrosis. A recent Cochrane analysis of five of these trials (294 patients) suggested that patients who got antibiotics had a lower risk of death (odds ratio 0.37, 95% confidence interval [CI] 0.17–0.83) but no difference in the rates of pancreatic infection or surgery.30 These paradoxical results suggest that antibiotics may prevent death by preventing nonpancreatic infections (eg, pneumonia, line infections) rather than by preventing infection of necrotic pancreatic tissue. The five trials in the meta-analysis are limited by significant methodologic heterogeneity and by lack of double-blinding.
In spite of the overall lower death rate observed in the meta-analysis, the prophylactic use of antibiotics in sterile necrosis remains controversial. One concern is that patients given long prophylactic courses of antibiotics may develop resistant bacterial or fungal infections. However, the Cochrane and other meta-analyses have not shown a higher rate of fungal infections in those given antibiotics.31
Recommendation: No routine antibiotics for mild cases
The AGA guidelines recommend against routinely giving antibiotics in mild acute pancreatitis and do not provide strict recommendations for prophylactic antibiotic use in necrotizing acute pancreatitis.2 The guidelines state that antibiotics can be used “on demand” based on clinical signs of infection (eg, high fevers, rising leukocytosis, hypotension) or worsening organ failure.
If a purely prophylactic strategy is used, only patients at high risk of developing infection (eg, those with necrosis in more than 30% of the pancreas) should receive antibiotics. Antibiotics with high tissue-penetration should be used, such as imipenem-cilastin (Primaxin IV) or ciprofloxacin (Cipro) plus metronidazole (Flagyl).
Adherence to these guidelines is not optimal. For example, in an Italian multicenter study, 9% of patients with mild acute pancreatitis were treated with antibiotics.19 Moreover, many patients with proven infected necrosis received antibiotics that do not penetrate the pancreatic tissue very well.
ERCP IN SEVERE BILIARY ACUTE PANCREATITIS
Problem: Endoscopic retrograde cholangiopancreatography (ERCP) often is performed inappropriately in mild biliary acute pancreatitis or is not performed urgently in severe cases.
In most cases of mild biliary pancreatitis, the stones pass spontaneously, as verified by cholangiography done during laparoscopic cholecystectomy. Ongoing ampullary obstruction by impacted biliary stones can perpetuate pancreatic inflammation and delay recovery.
Two early randomized trials showed a benefit from early ERCP (within 72 hours) with sphincterotomy and stone extraction, primarily in those with severe biliary acute pancreatitis or ascending cholangitis,32,33 but a third trial failed to reveal a benefit.34 A Cochrane metaanalysis of these three trials failed to show a lower death rate with ERCP in mild or severe biliary pancreatitis.35 However, early ERCP did prevent complications in severe biliary pancreatitis (odds ratio 0.27, 95% CI 0.14–0.53).
Later, a fourth randomized trial was restricted to patients with suspected biliary pancreatitis, evidence of biliary obstruction, and no signs of cholangitis36: 103 patients were randomized to undergo either ERCP within 72 hours or conservative management. No difference was observed in rates of death or organ failure or in the CT severity index.
Recommendation: ER CP for suspected retained stones
ERCP has a limited role in patients with biliary pancreatitis, being used to clear retained bile duct stones or to relieve ongoing biliary obstruction.
The decision to perform ERCP before surgery should be based on how strongly one suspects retained stones. ERCP is most appropriate if the suspicion of retained stones and the likelihood of therapeutic intervention are high (eg, if the serum bilirubin and alkaline phosphatase levels are rising and ultrasonography shows a dilated bile duct). If there is moderate suspicion, a safer and less-invasive imaging study such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography can be done to screen for bile duct stones before proceeding to ERCP.
The ACG guidelines suggest urgent ERCP (preferably within 24 hours) for those with severe biliary pancreatitis complicated by organ failure or those with suspicion of cholangitis. The level of evidence is I, ie, “strong evidence from at least one published systematic review of multiple well-designed randomized controlled trials.”1
Elective ERCP is recommended for those who are poor surgical candidates. ERCP is also recommended for those with rising liver enzyme values or imaging findings suggesting a retained common bile duct stone (including intraoperative cholangiography). Endoscopic ultrasonography or MRCP is recommended for those with slow clinical resolution, who are pregnant, or in whom uncertainty exists regarding the biliary etiology of pancreatitis.
Compliance rates with these and similar guidelines are not adequate. In an audit of adherence to the British Society of Gastroenterology guidelines, early ERCP was performed in only 25% of patients with severe biliary acute pancreatitis.6
LAPAROSCOPIC CHOLECYSTECTOMY FOR MILD BILIARY PANCREATITIS
Problem: Laparoscopic cholecystectomy is not done at admission or within 2 weeks in many patients with mild biliary pancreatitis.
If the gallbladder is not removed, biliary pancreatitis may recur in up to 61% of patients within 6 weeks of hospital discharge.37 This is the basis for guideline recommendations for surgery (or a confirmation of a surgery date) prior to hospital discharge.
The International Association of Pancreatology recommends early cholecystectomy (preferably during the same hospitalization) for patients with mild gallstone-associated acute pancreatitis.38 In severe gallstone-associated acute pancreatitis, cholecystectomy should be delayed until there is sufficient resolution of the inflammatory response and clinical recovery. The AGA guidelines advocate cholecystectomy as soon as possible and in no case later than 4 weeks after discharge to prevent relapse. ERCP with biliary sphinc-terotomy may also protect against relapse in those who are not fit to undergo surgery.
Recommendations for definitive management of gallstones (laparoscopic cholecystectomy or ERCP, or both) are not always followed. For example, a British study showed 70% compliance with this recommendation.4 A similar compliance audit in Germany revealed that cholecystectomy was performed during the initial hospital stay in only 23% of cases.7 In a New Zealand study, a regular compliance audit with feedback to surgeons resulted in an increase in the early cholecystectomy rate from 54% to 80%.8
Several major gastroenterological and surgical societies have issued guidelines on how to manage acute pancreatitis, based on evidence from high-quality randomized trials and nonrandomized studies as well as on expert opinion.1–3 Information is limited on how well physicians in the United States comply with these guidelines, but compliance is suboptimal in other developed countries, according to several studies,4–8 and we suspect that many US physicians are not following the guidelines either.
Acute pancreatitis is a frequent inpatient diagnosis that internists, gastroenterologists, and surgeons all confront. The most common causes are gallstones and heavy alcohol intake. Its management is typically straightforward: intravenous fluids, analgesia, and nothing by mouth. However, treatment of severe cases can be quite complex, particularly if multiple organ systems are involved or if there are local complications.
The primary aim of this article is to raise awareness of recognized deviations from established recommendations that may lead to adverse patient outcomes.
MEASURING ENZYME LEVELS DAILY ADDS COST BUT LITTLE BENEFIT
Problem: Serum amylase and lipase levels are often needlessly measured every day.
Measuring the serum amylase and lipase levels is useful in diagnosing acute pancreatitis, which requires two of the following three features1:
- Characteristic abdominal pain
- Levels of serum amylase or serum lipase, or both, that are three or more times the upper limit of normal
- Findings of acute pancreatitis on computed tomography (CT).
However, the magnitude or duration of the serum enzyme elevation does not correlate with the severity of the attack. Further, we have noticed that physicians at our hospital often order daily serum amylase and lipase levels in patients admitted with acute pancreatitis.
The American College of Gastroenterology (ACG) guidelines1 state that daily monitoring of amylase and lipase has limited value in managing acute pancreatitis. Rechecking these concentrations may be reasonable if pain fails to resolve or worsens during a prolonged hospitalization, as this may suggest a recurrent attack of acute pancreatitis or a developing pseudocyst. But in most cases of acute pancreatitis, daily serum enzyme measurements add cost but little benefit.
REGULAR ASSESSMENT IS IMPORTANT
Problem: Often, severity assessments are not performed regularly or acted on.
Most cases of acute pancreatitis are mild, with rapid recovery and excellent prognosis. However, 15% to 20% are severe and may result in a prolonged hospitalization, systemic inflammatory response syndrome (SIRS), multiorgan system failure, and death.
In severe acute pancreatitis, as pancreatic enzymes and inflammatory cytokines damage the blood vessels, a vast amount of fluid leaks out into the interstitial (“third”) space. This fluid extravasation leads to decreased effective circulating volume, local pancreatic necrosis, hemodynamic instability, and end-organ failure.
It is important to recognize severe acute pancreatitis early because the patient needs to be transferred to a step-down unit or intensive care unit to receive optimal fluid resuscitation and supportive care for organ dysfunction. After 48 to 72 hours, a prediction of severe acute pancreatitis should also prompt the physician to order CT to detect pancreatic necrosis, and also to initiate nutritional support.
Assessment of severity begins in the emergency room or on admission to the hospital. Older age, obesity, organ failure, and pulmonary infiltrates or pleural effusions are initial indicators of poor prognosis. Signs of SIRS (high or low core body temperature, tachycardia, tachypnea, low or high peripheral white blood cell count) or organ failure (eg, elevated serum creatinine) are present on admission in 21% of patients with acute pancreatitis.9
Hemoconcentration is a marker of decreased effective circulating volume in severe acute pancreatitis. A hematocrit higher than 44% at admission or that rises in the first 24 to 48 hours of admission predicts necrosis.10,11 However, a more robust marker of organ failure may be the blood urea nitrogen level.12
Clinical scoring systems
Several clinical scoring systems have been studied for assessing severity.
Limitations of the Ranson score are that it can only be completed after 48 hours, all the data points are not always obtained, and it cannot be repeated on a daily basis. Owing to these limitations and its less-than-optimal predictive value, the Ranson score has fallen into disuse.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) score is more versatile. It is based on multiple clinical and laboratory values, and it correlates very well with the risk of death in acute pancreatitis. Death rates are less than 4% when the APACHE II score is less than 8, and 11% to 18% when it is 8 or higher.1 The trajectory of the APACHE II score in the first 48 hours is also an accurate prognostic indicator.
Previous limitations of the APACHE II score were that it was complicated and timeconsuming to calculate and required arterial blood gas measurements. Easy-to-use online calculators are now available (eg, www.globalrph.com/apacheii.htm), and the venous bicarbonate level and the oxygen saturation can be substituted for the arterial pH and oxygen partial pressure.
BISAP, a new five-point scoring system,15 was recently prospectively validated.12 “BISAP” is an acronym for the five markers it is based on, each of which has been shown to predict severe illness in acute pancreatitis:
- Blood urea nitrogen level > 25 mg/dL
- Impaired mental status
- SIRS
- Age > 60 years
- Pleural effusion.
The presence of three or more of these factors correlates with higher risk of death, organ failure, and pancreatic necrosis.12
Compared with APACHE II, BISAP has similar accuracy and is easier to calculate. Also, BISAP was specifically developed for acute pancreatitis, whereas APACHE II is a generic score for all critically ill patients.
The Atlanta criteria16 define severe acute pancreatitis as one or more of the following:
- A Ranson score of 3 or higher during the first 48 hours
- An APACHE II score of 8 or higher at any time
- Failure of one or more organs
- One or more local complications (eg, necrosis, pseudocysts, abscesses).
Recommendation: Assess severity at least daily
A severity assessment should be performed at admission and at least every day thereafter. Clinical guidelines recognize the importance of severity assessment but vary in their specific recommendations.
The ACG advises calculating the APACHE II score within 3 days of admission and measuring the hematocrit at admission, at 12 hours, and at 24 hours. The level of evidence is III, ie, “from published well-designed trials without randomization, single group prepost, cohort, time series, or matched case controlled studies”.1
The American Gastroenterological Association (AGA) provides a more generalized recommendation, that “clinical judgment” should take into account the presence of risk factors (eg, age, obesity), presence or absence of SIRS, routine laboratory values (eg, hematocrit, serum creatinine), and APACHE II score when assessing severity and making decisions.2
In a German survey, only 32% of gastroenterologists used the APACHE II score for assessing risk in acute pancreatitis, in spite of national guidelines emphasizing its importance.7 Also, not all patients with severe acute pancreatitis are transferred to a step-down unit or intensive care unit as recommended. In a British study,4 only 8 (17%) of 46 patients with predicted severe acute pancreatitis were transferred, and 8 of the 38 patients who were not transferred died.
FLUID MUST BE AGGRESSIVELY REPLACED AND MONITORED
Problem: Often, not enough fluid is replaced, or fluid status is not adequately monitored.
Fluid must be aggressively replaced to balance the massive third-space fluid losses that occur in the early inflammatory phase of acute pancreatitis. Intravascular volume depletion can develop rapidly and result in tachycardia, hypotension, and renal failure. It may also impair the blood flow to the pancreas and worsen necrosis.
Animal studies show that aggressive fluid replacement supports the pancreatic microcirculation and prevents necrosis.17 It may also support the intestinal microcirculation and gut barrier, preventing bacterial translocation.
In humans, no controlled trials have been done to test the efficacy of aggressive fluid resuscitation in acute pancreatitis. However, the notion that intravascular fluid loss contributes to poor outcomes is inferred from human studies showing more necrosis and deaths in patients with hemoconcentration. In one study, patients who received inadequate fluid replacement (evidenced by a rise in hematocrit at 24 hours) were more likely to develop necrotizing pancreatitis.18
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4
SUSPECTED INFECTED NECROSIS
Problem: Fine-needle aspiration is not done in many cases of suspected infected necrosis.
Approximately one-third of patients with necrotizing pancreatitis develop infected necrosis. The death rate for patients with infected pancreatic necrosis is high—30%, compared with 12% in those with sterile necrosis.1 Differentiating sterile and infected necrosis is therefore essential.
Clinical signs such as fever are poor predictors of infection. Signs of SIRS can be present in both sterile and infected necrotizing pancreatitis.
Recommendation: Fine-needle aspiration of necrosis
For the reasons given above, the findings of necrosis on CT and persistent SIRS should prompt consideration of fine-needle aspiration with Gram stain and culture to differentiate sterile and infected necrosis (ACG guideline, level of evidence III).1 If infection is confirmed, surgical debridement should be strongly considered. Other less-invasive approaches such as endoscopic debridement can be used in selected cases.
Fine-needle aspiration of necrosis is too often neglected. In a cohort of German surgeons, only 55% complied with International Association of Pancreatology recommendations to perform biopsy to differentiate sterile from infected necrosis in patients with signs of sepsis.29
BROAD-SPECTRUM ANTIBIOTICS
Problem: Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Antibiotics are not indicated in mild acute pancreatitis. A limited course of antibiotics is typically indicated in severe cases with suspected or proven infected necrosis (in conjunction with surgical necrosectomy). However, the use of antibiotics in sterile necrosis has been very controversial.
At least six small, nonblinded, randomized trials have evaluated the benefit of giving antibiotics prophylactically for presumed sterile necrosis. A recent Cochrane analysis of five of these trials (294 patients) suggested that patients who got antibiotics had a lower risk of death (odds ratio 0.37, 95% confidence interval [CI] 0.17–0.83) but no difference in the rates of pancreatic infection or surgery.30 These paradoxical results suggest that antibiotics may prevent death by preventing nonpancreatic infections (eg, pneumonia, line infections) rather than by preventing infection of necrotic pancreatic tissue. The five trials in the meta-analysis are limited by significant methodologic heterogeneity and by lack of double-blinding.
In spite of the overall lower death rate observed in the meta-analysis, the prophylactic use of antibiotics in sterile necrosis remains controversial. One concern is that patients given long prophylactic courses of antibiotics may develop resistant bacterial or fungal infections. However, the Cochrane and other meta-analyses have not shown a higher rate of fungal infections in those given antibiotics.31
Recommendation: No routine antibiotics for mild cases
The AGA guidelines recommend against routinely giving antibiotics in mild acute pancreatitis and do not provide strict recommendations for prophylactic antibiotic use in necrotizing acute pancreatitis.2 The guidelines state that antibiotics can be used “on demand” based on clinical signs of infection (eg, high fevers, rising leukocytosis, hypotension) or worsening organ failure.
If a purely prophylactic strategy is used, only patients at high risk of developing infection (eg, those with necrosis in more than 30% of the pancreas) should receive antibiotics. Antibiotics with high tissue-penetration should be used, such as imipenem-cilastin (Primaxin IV) or ciprofloxacin (Cipro) plus metronidazole (Flagyl).
Adherence to these guidelines is not optimal. For example, in an Italian multicenter study, 9% of patients with mild acute pancreatitis were treated with antibiotics.19 Moreover, many patients with proven infected necrosis received antibiotics that do not penetrate the pancreatic tissue very well.
ERCP IN SEVERE BILIARY ACUTE PANCREATITIS
Problem: Endoscopic retrograde cholangiopancreatography (ERCP) often is performed inappropriately in mild biliary acute pancreatitis or is not performed urgently in severe cases.
In most cases of mild biliary pancreatitis, the stones pass spontaneously, as verified by cholangiography done during laparoscopic cholecystectomy. Ongoing ampullary obstruction by impacted biliary stones can perpetuate pancreatic inflammation and delay recovery.
Two early randomized trials showed a benefit from early ERCP (within 72 hours) with sphincterotomy and stone extraction, primarily in those with severe biliary acute pancreatitis or ascending cholangitis,32,33 but a third trial failed to reveal a benefit.34 A Cochrane metaanalysis of these three trials failed to show a lower death rate with ERCP in mild or severe biliary pancreatitis.35 However, early ERCP did prevent complications in severe biliary pancreatitis (odds ratio 0.27, 95% CI 0.14–0.53).
Later, a fourth randomized trial was restricted to patients with suspected biliary pancreatitis, evidence of biliary obstruction, and no signs of cholangitis36: 103 patients were randomized to undergo either ERCP within 72 hours or conservative management. No difference was observed in rates of death or organ failure or in the CT severity index.
Recommendation: ER CP for suspected retained stones
ERCP has a limited role in patients with biliary pancreatitis, being used to clear retained bile duct stones or to relieve ongoing biliary obstruction.
The decision to perform ERCP before surgery should be based on how strongly one suspects retained stones. ERCP is most appropriate if the suspicion of retained stones and the likelihood of therapeutic intervention are high (eg, if the serum bilirubin and alkaline phosphatase levels are rising and ultrasonography shows a dilated bile duct). If there is moderate suspicion, a safer and less-invasive imaging study such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography can be done to screen for bile duct stones before proceeding to ERCP.
The ACG guidelines suggest urgent ERCP (preferably within 24 hours) for those with severe biliary pancreatitis complicated by organ failure or those with suspicion of cholangitis. The level of evidence is I, ie, “strong evidence from at least one published systematic review of multiple well-designed randomized controlled trials.”1
Elective ERCP is recommended for those who are poor surgical candidates. ERCP is also recommended for those with rising liver enzyme values or imaging findings suggesting a retained common bile duct stone (including intraoperative cholangiography). Endoscopic ultrasonography or MRCP is recommended for those with slow clinical resolution, who are pregnant, or in whom uncertainty exists regarding the biliary etiology of pancreatitis.
Compliance rates with these and similar guidelines are not adequate. In an audit of adherence to the British Society of Gastroenterology guidelines, early ERCP was performed in only 25% of patients with severe biliary acute pancreatitis.6
LAPAROSCOPIC CHOLECYSTECTOMY FOR MILD BILIARY PANCREATITIS
Problem: Laparoscopic cholecystectomy is not done at admission or within 2 weeks in many patients with mild biliary pancreatitis.
If the gallbladder is not removed, biliary pancreatitis may recur in up to 61% of patients within 6 weeks of hospital discharge.37 This is the basis for guideline recommendations for surgery (or a confirmation of a surgery date) prior to hospital discharge.
The International Association of Pancreatology recommends early cholecystectomy (preferably during the same hospitalization) for patients with mild gallstone-associated acute pancreatitis.38 In severe gallstone-associated acute pancreatitis, cholecystectomy should be delayed until there is sufficient resolution of the inflammatory response and clinical recovery. The AGA guidelines advocate cholecystectomy as soon as possible and in no case later than 4 weeks after discharge to prevent relapse. ERCP with biliary sphinc-terotomy may also protect against relapse in those who are not fit to undergo surgery.
Recommendations for definitive management of gallstones (laparoscopic cholecystectomy or ERCP, or both) are not always followed. For example, a British study showed 70% compliance with this recommendation.4 A similar compliance audit in Germany revealed that cholecystectomy was performed during the initial hospital stay in only 23% of cases.7 In a New Zealand study, a regular compliance audit with feedback to surgeons resulted in an increase in the early cholecystectomy rate from 54% to 80%.8
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007; 132:2022–2044.
- United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42(suppl 2):S1–S13.
- Norton SA, Cheruvu CV, Collins J, Dix FP, Eyre-Brook IA. An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 2001; 83:399–405.
- Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg 2004; 74:218–221.
- Barnard J, Siriwardena AK. Variations in implementation of current national guidelines for the treatment of acute pancreatitis: implications for acute surgical service provision. Ann R Coll Surg Engl 2002; 84:79–81.
- Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis in Germany [letter]. Pancreatology 2005; 5:591–593.
- Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J 2008; 121:19–25.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction, and death in acute pancreatitis. Br J Surg 2006; 93:738–744.
- Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000; 20:367–372.
- Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96:2081–2085.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Larvin M. Assessment of clinical severity and prognosis. In:Beger HG, Warshaw AL, Buchler MW, et al, editors. The Pancreas. Blackwell Science: New York, 1998:489–502.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993, 128:586–590.
- Forgacs B, Eible G, Faulhaber J, Kahrau S, Buhr H, Foitzik T. Effect of fluid resuscitation with and without endothelin A receptor blockade on hemoconcentration and organ function in experimental pancreatitis. Eur Surg Res 2000; 32:162–168.
- Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2:104–107.
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007; 132:1127–1151.
- Pezzilli R, Uomo G, Gabbrielli A, et al; ProInf-AISP Study Group. A prospective multicenter survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis 2007; 39:838–846.
- Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008; 6:1070–1076.
- Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther 2008; 28:704–712.
- Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004; 328:1407.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Foitzik T, Bassi DG, Schmidt J, et al. Intravenous contrast medium accentuates the severity of acute necrotizing pancreatitis in the rat. Gastroenterology 1994; 106:207–214.
- Carmona-Sanchez R, Uscanga L, Bezaury-Rivas P, Robles-Díaz G, Suazo-Barahona J, Vargas-Vorácková F. Potential harmful effect of iodinated intravenous contrast medium on the clinical course of mild acute pancreatitis. Arch Surg 2000; 135:1280–1284.
- Uhl W, Roggo A, Kirschstein T, et al. Influence of contrast-enhanced computed tomography on couse and outcome in patients with acute pancreatitis. Pancreas 2002; 24:191–197.
- Foitzik T, Klar E. Non-compliance with guidelines for the management of severe acute pancreatitis among German surgeons. Pancreatology 2007; 7:80–85.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006;CD002941.
- Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg 2006; 243:154–168.
- Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988; 2:979–983.
- Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993; 328:228–232.
- Folsch UR, Nitsche R, Ludtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997; 336:237–242.
- Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone associated pancreatitis. Cochrane Database Syst Rev 2004;CD003630
- Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. A randomized clinical trial. Ann Surg 2007; 245:10–17.
- Frei GJ, Frei VT, Thirlby RC, McClelland RN. Biliary pancreatitis: clinical presentation and surgical management. Am J Surg 1986; 151:170–175.
- Uhl W, Warshaw A, Imrie C, et al; International Association of Pancreatology. IAP guidelines on the surgical management of acute pancreatitis. Pancreatology 2002; 2:565–573.
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007; 132:2022–2044.
- United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42(suppl 2):S1–S13.
- Norton SA, Cheruvu CV, Collins J, Dix FP, Eyre-Brook IA. An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 2001; 83:399–405.
- Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg 2004; 74:218–221.
- Barnard J, Siriwardena AK. Variations in implementation of current national guidelines for the treatment of acute pancreatitis: implications for acute surgical service provision. Ann R Coll Surg Engl 2002; 84:79–81.
- Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis in Germany [letter]. Pancreatology 2005; 5:591–593.
- Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J 2008; 121:19–25.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction, and death in acute pancreatitis. Br J Surg 2006; 93:738–744.
- Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000; 20:367–372.
- Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96:2081–2085.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Larvin M. Assessment of clinical severity and prognosis. In:Beger HG, Warshaw AL, Buchler MW, et al, editors. The Pancreas. Blackwell Science: New York, 1998:489–502.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993, 128:586–590.
- Forgacs B, Eible G, Faulhaber J, Kahrau S, Buhr H, Foitzik T. Effect of fluid resuscitation with and without endothelin A receptor blockade on hemoconcentration and organ function in experimental pancreatitis. Eur Surg Res 2000; 32:162–168.
- Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2:104–107.
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007; 132:1127–1151.
- Pezzilli R, Uomo G, Gabbrielli A, et al; ProInf-AISP Study Group. A prospective multicenter survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis 2007; 39:838–846.
- Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008; 6:1070–1076.
- Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther 2008; 28:704–712.
- Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004; 328:1407.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Foitzik T, Bassi DG, Schmidt J, et al. Intravenous contrast medium accentuates the severity of acute necrotizing pancreatitis in the rat. Gastroenterology 1994; 106:207–214.
- Carmona-Sanchez R, Uscanga L, Bezaury-Rivas P, Robles-Díaz G, Suazo-Barahona J, Vargas-Vorácková F. Potential harmful effect of iodinated intravenous contrast medium on the clinical course of mild acute pancreatitis. Arch Surg 2000; 135:1280–1284.
- Uhl W, Roggo A, Kirschstein T, et al. Influence of contrast-enhanced computed tomography on couse and outcome in patients with acute pancreatitis. Pancreas 2002; 24:191–197.
- Foitzik T, Klar E. Non-compliance with guidelines for the management of severe acute pancreatitis among German surgeons. Pancreatology 2007; 7:80–85.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006;CD002941.
- Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg 2006; 243:154–168.
- Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988; 2:979–983.
- Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993; 328:228–232.
- Folsch UR, Nitsche R, Ludtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997; 336:237–242.
- Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone associated pancreatitis. Cochrane Database Syst Rev 2004;CD003630
- Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. A randomized clinical trial. Ann Surg 2007; 245:10–17.
- Frei GJ, Frei VT, Thirlby RC, McClelland RN. Biliary pancreatitis: clinical presentation and surgical management. Am J Surg 1986; 151:170–175.
- Uhl W, Warshaw A, Imrie C, et al; International Association of Pancreatology. IAP guidelines on the surgical management of acute pancreatitis. Pancreatology 2002; 2:565–573.
KEY POINTS
- Serum amylase and lipase levels are often needlessly measured every day.
- Often, severity assessments are not performed regularly or acted on.
- Often, not enough fluid is replaced, or fluid status is not adequately monitored.
- In many severe cases, enteral or parenteral feeding is not started soon enough.
- Computed tomography is not done in many patients with severe acute pancreatitis, or it is performed too soon.
- In many cases of suspected infected necrosis, fine-needle aspiration is not done.
- Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.