User login
Since 2008, the FDA has cleared 4 transcranial magnetic stimulation (TMS) devices for treating depression (Related Resources). In that time, the availability of TMS has steadily grown within and outside the United States.
Parallel with increasing clinical utilization of this technology, research continues into the benefit of TMS for treatment-resistant depression; such research includes additional, supportive, acute, sham-controlled trials; comparison trials with electroconvulsive therapy (ECT) for more severe episodes of depression; short- and long-term real-world outcome studies; exploration of alternative treatment parameters to further enhance its efficacy; and the development of other TMS approaches. In this article, we review recent developments in the application of TMS to treat major depressive disorder—in particular, treatment-resistant depression (Box).
Therapeutic neuromodulation
The underlying premise of neuromodulation is that the brain is an electrochemical organ that can be modulated by pharmacotherapy or device-based approaches, or their combination.1 ECT is the prototypic device-based neuromodulation approach, and remains one of the most effective treatments for severe depression.
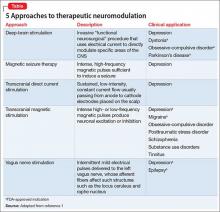
More recently, however, other methods have been, and continue to be, developed to treat patients who do not achieve adequate benefit from psychotherapy or medical therapy, or both, and who might not be an ideal candidate for ECT (Table,1). In addition to the potential therapeutic benefit of these alternative strategies, some could avoid safety and tolerability concerns associated with medication (weight gain, sexual dysfunction) and ECT (eg, cognitive deficits).
TMS, which utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression, represents an important example of this initiative.2
TMS has established efficacy for depression
Sham-controlled trials. Several randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression.
A recent meta-analysis considered 18 studies (N = 1,970) that met the authors’ criteria for inclusion.3 They found that TMS monotherapy was statistically and clinically more effective than a sham procedure based on:
- improvement in depressive symptoms (mean decrease in baseline Hamilton Depression Rating Scale [HDRS] score, −4.53 [95% CI, −6.11 to −2.96])
- response rate; response was 3 times more likely with TMS (relative risk 3.38 [95% CI, 2.24 to 5.10])
- remission rate; remission was 5 times more likely with TMS (relative risk, 5.07 [95% CI, 2.50 to 10.30]).
Another meta-analysis (7 studies, N = 279) considered TMS as an augmentation strategy to standard medication for treatment-resistant depression.4 The authors reported that, based on change in HDRS scores, the pooled standardized mean difference between active and sham TMS augmentation was 0.86 (P < .00001). Furthermore, the pooled response rate with TMS augmentation was 46.6%, compared with 22.1% with the sham procedure (P < .0003).
Acute naturalistic TMS studies. The efficacy of TMS is supported by a large, naturalistic study of 307 patients with treatment-resistant depression who were assessed at baseline and during a standard course of TMS.5 Considering change score in the Clinician Global Impressions-Severity (CGI-S) scale, significant improvement was seen from baseline to end of treatment (−1.9 ± 1.4; P < .0001), with a clinician-assessed response rate of 58.0% and remission rate of 37.1%. Of note: Self-reported quality-of-life measures (on the Medical Outcomes Study 36-Item Short-Form Health Survey and EuroQol 5-Dimensions) also significantly improved during this relatively brief period.6
Maintenance strategies after acute TMS response. Most patients referred for TMS have a depressive illness characterized by a chronic, relapsing course and inadequate response to pharmacotherapy or psychotherapy, or their combination. An effective maintenance strategy after acute response to TMS is paramount. This includes:
- prolonged tapering schedule after an acute TMS course is completed
- maintenance medication or psychotherapy, or both
- scheduled periodic maintenance TMS sessions (usually as an augmentation strategy)
- reintroduction of TMS as needed with early signs of relapse. In this context, several trials have assessed the durability of acute TMS benefit.
A semi-controlled maintenance study followed 99 patients who had at least a 25% decrease in baseline HDRS score after acute TMS treatment.7 They were then tapered from their TMS sessions over 3 weeks while an antidepressant was titrated up. If, at any time during the subsequent 6 months, early signs of depression relapse were noted (ie, change of at least 1 point on the CGI-S for 2 consecutive weeks), TMS was reintroduced. At the end of the trial, 10 patients (13%) had relapsed and 38 (38%) had an exacerbation of symptoms sufficient to warrant reintroduction of TMS. Of those, 32 (84%) re-achieved mood stability.
In another study, 50 patients who had achieved remission during an acute course of TMS were followed for 3 months.8 After TMS taper and continued pharmacotherapy or naturalistic follow-up, 29 (58%) remained in remission; 2 (4%) maintained partial response; and 1 (2%) relapsed.
In a controlled, pilot, maintenance trial, 67 unmedicated patients with treatment-resistant depression received an acute course of TMS.9 Forty-seven of the responders were then randomized to a 1-year follow-up trial with or without a scheduled monthly TMS session. All patients could receive reintroduction TMS if they met criteria for symptom worsening.
Both groups had a similar outcome. The number of patients who did not require TMS reintroduction was 9 of 23 (39%) in the scheduled TMS group vs 9 of 26 (35%) in the no-scheduled TMS group (P < .1). Although no difference was noted between groups, the authors commented that these preliminary results will help inform larger, more definitive trials. They concluded that both acute and maintenance TMS monotherapy might be an option—for some patients.
A long-term, naturalistic outcomes study followed 257 treatment-resistant depressed patients for 1 year after they responded to an acute course of TMS.10 In addition to most patients receiving ongoing maintenance medication, they also could receive reintroduction of TMS if symptoms became worse.
Compared with pre-TMS baseline, there was a statistically significant reduction in the mean total score on the CGI-S scale (primary outcome, P < .0001) at the end of acute treatment that was sustained at follow-up. Ninety-six patients (36.2%) required reintroduction of TMS and 75 of 120 (62.5%) who initially met response or remission criteria after acute treatment continued to meet response criteria after 1 year. The authors concluded that TMS demonstrated both a statistically and clinically meaningful durability of acute benefit during this time frame.
TMS and electroconvulsive therapy
For more than 75 years, ECT has consistently proved to be an effective treatment for major depressive disorder. Although the use of ECT has fluctuated over this period, one practice survey estimated that 100,000 patients receive ECT annually.11
ECT has limitations, however, including cost, the need for general anesthesia, and cognitive deficits that range from short-term confusion to anterograde and retrograde amnesia, which can persist for weeks beyond active treatment.12 Despite increasing awareness of mental illness, stigma also remains a significant barrier to receiving ECT.
TMS vs ECT. Several trials have directly compared ECT and TMS:
- A recent meta-analysis of 9 trials included 384 patients with depression who were considered clinically appropriate for ECT and were randomized to one or the other treatment.13 Both modalities produced a significant reduction in baseline HDRS score, but ECT (15.4 point reduction) was superior to TMS (9.3 point reduction) in the degree of improvement (P < .01).
- Another meta-analysis of 9 trials (N = 425) found ECT superior to TMS in terms of response (P < .03) and remission (P < .006) rates, based on improvement in the HDRS score.14 When psychotic depressed patients were excluded, however, TMS produced effects equivalent to ECT.
In contrast to what was seen with ECT, cognitive testing of patients who received TMS revealed no deterioration in any domain. Furthermore, one of the comparison studies observed a modest, but statistically significant, improvement in patient’s working memory-executive function, objective memory, and fine-motor speed over the course of TMS treatment.15
TMS plus ECT. A 2-week, randomized, single-blind, controlled pilot study (N = 22) examined the combination of TMS and ECT as acute treatment of depression.16 Patients were assigned to receive either unilateral non-dominant (UND) ECT 3 days a week or a combination of 1 UND ECT treatment followed by 4 days of TMS. At the conclusion of treatment, UND ECT plus TMS group produced comparable efficacy and fewer adverse effects compared with the UND ECT-only group.
TMS maintenance after acute ECT response. Most patients who are referred for ECT have a depressive illness characterized by repeated episodes and incomplete response to pharmacotherapy or psychotherapy, or both. The need for an effective maintenance strategy after the acute response is therefore critical. Medication or ECT, or both, are commonly used to maintain acute benefit but, regrettably, a recent systematic review of the durability of benefit with such strategies found a substantial percentage (approximately 50%) of patients relapsed within the first year.17
- In this context, a case series report found that 1 or 2 weekly, sequential, bilateral TMS treatments after a successful acute course of ECT maintained response in 5 of 6 patients over 6 to 12 months.18
- Another case series (N = 6) transitioned stable patients from maintenance ECT to maintenance TMS, primarily because of adverse effects with ECT.19 With a mean frequency of 1 TMS treatment every 3.5 weeks, all 6 patients remained stable for as long as 6 months. Subsequently, 2 patients relapsed—1 at 8 months and 1 at 9 months.
Advantages of maintenance TMS over maintenance ECT include lower cost, fewer adverse effects (particularly cognitive deficits), and the ability to remain independent during the period of the treatment sessions.
TMS as an assessment tool for ECT response. TMS can be used to study excitability in cortical circuits. In a study, EEG potentials evoked by TMS before and after a course of ECT in 8 severely depressed patients revealed an increase in frontal cortical excitability, compared with baseline.20 Such findings support the ability of ECT to produce synaptic potentiation in humans. Furthermore, to the extent that depression presents with alterations in frontal cortical excitability, serial EEG-TMS measurements might be an effective tool to guide and monitor treatment progress with ECT, as well as other forms of therapeutic modulation.
Summing up: TMS and ECT. Although a definitive comparative study is needed, available evidence suggests that TMS might be an alternative treatment in a subgroup of patients who are referred for ECT. Factors that might warrant considering TMS over ECT include:
- patient preference
- fear of anesthesia
- concern about cognitive deficits
- stigma.
Although TMS might offer a workable alternative to ECT for acute and maintenance treatment of depression in selected patients, further refinement of the delivery of TMS is also needed to (1) enhance its efficacy and (2) identify clinical and biological markers to better define this select population.
Standard TMS treatment parameters
Superficial TMS. Superficial TMS for depression typically involves a single coil placed over the left dorsolateral prefrontal cortex. The standard, FDA-approved protocol includes stimulating at 110% of motor threshold with 75, 4-second trains at 10 Hz (ie, 40 stimulations) interspersed by 26-second intertrain intervals. Without interruption, a standard treatment session takes 37.5 minutes and delivers a total of 3,000 pulses. Most patients require 20 to 30 sessions, on a Monday-through-Friday schedule, to achieve optimal benefit.
This approach stimulates to a depth of approximately 2 or 3 cm. The coil usually is placed over the left dorsolateral prefrontal cortex because earlier studies indicated that decreased activity in this part of the brain correlates with symptoms of depression. When TMS is administered in a rapid repetitive fashion (at >1 Hz; typically, at 10 Hz), blood flow and metabolism in that area of the brain are increased. In addition, imaging studies indicate that trans-synaptic connections with deeper parts of the brain also allow modulation of other relevant neural circuits.
An alternate approach, less well-studied, involves low-frequency stimulation over the right dorsolateral prefrontal cortex. Parameters differ from what is used in left high-frequency dorsolateral prefrontal cortex TMS: frequency <1 Hz; train durations as long as 15 minutes; an intertrain interval of 25 to 180 seconds; 120 to 900 stimulations per train; and 2,400 to 18,000 total stimulations.
One hypothesis is that this low-frequency approach selectively stimulates inhibitory interneurons, decreases local neuronal activity and diminishes blood flow to deeper structures, such as the amygdala. Although right low-frequency TMS, compared with left high-frequency TMS, has potential advantages of better tolerability and decreased risk for seizures, its relative efficacy is unclear.
Deep TMS. Studies also are pursuing different coil configurations that allow for more direct stimulation of relevant structures (eg, prefrontal neuronal pathways associated with the reward system).
One of these coil designs (ie, the H-coil), coupled to a Magstim TMS stimulator, recently received FDA clearance for treatment-resistant depression. In the pivotal, sham-controlled study, patients received 20 treatment sessions over 4 weeks.21 The treatment protocol consisted of a helmet-like coil placed over the medial and lateral prefrontal cortex. Stimulation parameters included an 18-Hz frequency; stimulation intensity of 120% motor threshold; stimulation train duration of 2 seconds; and an intertrain interval of 20 seconds. The treatment sessions lasted 20.2 minutes and delivered a total of 1,980 stimulations.
Based on the 21-item HDRS, the active treatment coil group achieved a significantly greater decrease in baseline score (6.39 vs 3.28; P < .008); a greater response rate (37% vs 27.8%; P < .03); and a greater remission rate (30.4% vs 15.8%; P < .016) compared with the sham coil group.
Next, in what is the only randomized, controlled maintenance assessment to date, the same patients were followed for an additional 12 weeks, continuing blinded treatments twice weekly. At the end of the second phase, the active treatment group also demonstrated greater benefit than the sham group (P < .03). One seizure did occur, possibly related to excessive alcohol use; but this raises the question of whether treating at a higher frequency (18 Hz) with greater depth and less focality might increase the risk of seizure.
To assess the potential advantages, as well as the relative safety, of this approach over standard TMS delivery, an adequately designed and powered trial comparing the H-coil and a single-coil device is needed.
Alternate TMS approaches
Efforts to improve the clinical effectiveness of TMS for treating depression include several approaches.
Theta burst stimulation (TBS) is a patterned form of TMS pulse delivery that utilizes high and low frequencies in the same stimulus train (eg, three 50-Hz bursts delivered 5 times a second). Such a pulse sequence can modulate long-term depression and long-term potentiation mechanisms that induce plasticity in areas such as the hippocampus.22
Intermittent TBS (iTBS) administers stimulations over a relatively brief duration (eg, 2 seconds) or intermittently (eg, every 10 seconds) for a specific period (eg, 190 seconds [600 pulses in total]) over the left dorsolateral prefrontal cortex. This technique induces long-term potentiation and produces effects similar to those of high-frequency TMS.
In contrast, continuous TBS (cTBS) administers a continuous train (eg, 40 seconds [600 total pulses]) over the right dorsolateral prefrontal cortex. This induces long-term depression and produces effects similar to low-frequency TMS.
Recent studies using different delivery paradigms have generated mixed results:
Study 1: Fifty-six patients with depression received active treatment; 17 others, a sham procedure.23 This study used 3 different conditions:
- a combination of low-frequency and high-frequency TMS stimulation, administered over the right and left dorsolateral prefrontal cortices, respectively
- a combination of iTBS over the left dorsolateral prefrontal cortex and cTBS over the right dorsolateral prefrontal cortex
- a sham procedure, in which no magnetic field was created.
Neither active treatment arm separated from the sham procedure based on change scores in the 21-item HDRS (P = not significant).
Study 2: Sixty treatment-resistant depression patients were assigned to cTBS, iTBS, a combination of the 2 procedures, or a sham procedure.24 After 2 weeks, the active treatment arms produced the greatest benefit, based on change in scores on the 17-item HDRS, which differed significantly among the 4 groups (F value = 6.166; P < .001); the iTBS and combination arms demonstrated the most robust effect.
There were also significantly more responders in the iTBS (40.0%) and combination groups (66.7%) than in the cTBS (25.0%) and sham groups (13.3%) (P < .010). A lower level of treatment refractoriness predicted a better outcome.
Study 3: Twenty-nine depressed patients were randomized to cTBS over the right dorsolateral prefrontal cortex or a sham procedure.25 Overall, there was no difference between groups; however, actively treated patients who were unmedicated (n = 3) or remained on a stable dosage of medication during treatment (n = 8) did experience a significantly greater reduction in the HDRS score.
Study 4: In a pilot trial, 32 depressed patients were randomized to 30 sessions of adjunctive combined iTBS plus cTBS or bilateral sham TBS.26 Based on reduction from the baseline Montgomery-Åsberg Depression Rating Scale score, 9 patients in the active treatment group and 4 in the sham group achieved response (odds ratio, 3.86; P < .048).
If at least comparable efficacy can be clearly demonstrated, advantages of TBS over standard TMS include a significantly reduced administration time, which might allow for more patients to be treated and reduce associated costs of treatment.27
Magnetic low-field synchronized stimulation is produced by rotating spherical rare-earth magnets that are synchronized to an individual’s alpha frequency. A recent 6-week, double-blind, sham-controlled trial (N = 202) reported that, in the intention-to-treat population, there was no difference in outcome between treatment arms. In patients who completed the study according to protocol (120 of 202), however, active treatment was significantly better in decreasing baseline HDRS score (P < .033).28
Magnetic seizure therapy (MST) is an experimental approach to treating patients with more severe depression that is resistant to medical therapy. The primary aim is to use TMS to induce a seizure, thus achieving the same efficacy as provided by ECT but without the adverse cognitive effects of ECT. With MST, the TMS device uses much higher stimulation settings to produce a seizure—the goal being to avoid direct electrical current to the brain’s memory centers.29
A pilot study considered the clinical and cognitive effects of MST in a group of 26 treatment-resistant depression patients (10 randomized; 16 open-label).30 Based on reduction in baseline HDRS scores at the end of the trial, 69% of patients achieved response and 46% met remission criteria; however, one-half of patients relapsed within 6 months.
Importantly, no cognitive adverse effects were observed. Furthermore, the antidepressant and anti-anxiety effects of MST were associated with localized metabolic changes in brain areas implicated in the pathophysiology of depression.
The investigators concluded that MST might constitute an effective, well-tolerated, and safe treatment for patients unable to benefit from available medical therapies for depression. In addition to confirmation of acute benefit in more definitive trials, the issue of durability of effect needs further clarification.
TMS is a key component of neuropsychiatric practice
It has been 3 decades since Barker et al31 developed the technology to deliver intense, localized magnetic pulses to specific areas of the nervous system. During this period, the role of TMS as a probe of the central and peripheral nervous systems has expanded to include various therapeutic applications, primarily focusing on treatment-resistant major depressive disorder.
Now, increasing sophistication in the choice of stimulation parameters and other ongoing efforts to optimize the benefits of TMS are yielding improved clinical outcomes. Research is still needed to better define the place of TMS in the management of subtypes of depression that are particularly difficult to treat and that do not benefit adequately from medications or psychotherapy or their combination.
Growing support from controlled trials, systematic reviews, meta-analyses, naturalistic outcome studies, and professional guidelines indicate that TMS has an increasingly important role in clinical practice.
1. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):66-70,72-74.
2. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
3. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
4. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
5. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
6. Janicak PG, Dunner DL, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice. CNS Spectr. 2013;18(6):322-332.
7. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
8. Mantovani A, Pavlicova M, Avery D, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883-890.
9. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
10. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
11. Hermann RC, Dorwart RA, Hoover CW. Variation in ECT use in the United States. Am J Psychiatry. 1995;152(6):869-875.
12. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16(2):87-96.
13. Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049. doi: 10.1155/2014/135049.
14. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
15. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
16. Pridmore S, Rybak M, Turnier-Shea Y, et al. Comparison of transcranial magnetic stimulation and electroconvulsive therapy in depression. In: Miyoshi K, Shapiro CM, Gaviria M, et al, eds. Contemporary neuropsychiatry. Tokyo, Japan: Springer; 2001:237-241.
17. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
18. Noda Y, Daskalakis Z, Ramos C, et al. Repetitive transcranial magnetic stimulation to maintain treatment response to electroconvulsive therapy in depression: a case series. Front Psychiatry. 2013;4:73.
19. Cristancho MA, Helmer A, Connolly R, et al. Transcranial magnetic stimulation maintenance as a substitute for maintenance electroconvulsive therapy: a case series. J ECT. 2013;29(2):106-108.
20. Casarotto S, Canali P, Rosanova M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326-337.
21. Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64-73.
22. Daskalakis ZJ. Theta-burst transcranial magnetic stimulation in depression: when less may be more. Brain. 2014;137(pt 7):1860-1862.
23. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57-65.
24. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(pt 7):2088-2098.
25. Chistyakov A, Kreinin B, Marmor S, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225-229.
26. Plewnia C, Pasqualetti P, Große S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219-223.
27. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
28. Leuchter AF, Cook IA, Feifel D, et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;8(4):787-794.
29. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
30. Kayser S, Bewernick BH, Matusch A, et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073-1092.
31. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107.
Since 2008, the FDA has cleared 4 transcranial magnetic stimulation (TMS) devices for treating depression (Related Resources). In that time, the availability of TMS has steadily grown within and outside the United States.
Parallel with increasing clinical utilization of this technology, research continues into the benefit of TMS for treatment-resistant depression; such research includes additional, supportive, acute, sham-controlled trials; comparison trials with electroconvulsive therapy (ECT) for more severe episodes of depression; short- and long-term real-world outcome studies; exploration of alternative treatment parameters to further enhance its efficacy; and the development of other TMS approaches. In this article, we review recent developments in the application of TMS to treat major depressive disorder—in particular, treatment-resistant depression (Box).
Therapeutic neuromodulation
The underlying premise of neuromodulation is that the brain is an electrochemical organ that can be modulated by pharmacotherapy or device-based approaches, or their combination.1 ECT is the prototypic device-based neuromodulation approach, and remains one of the most effective treatments for severe depression.
More recently, however, other methods have been, and continue to be, developed to treat patients who do not achieve adequate benefit from psychotherapy or medical therapy, or both, and who might not be an ideal candidate for ECT (Table,1). In addition to the potential therapeutic benefit of these alternative strategies, some could avoid safety and tolerability concerns associated with medication (weight gain, sexual dysfunction) and ECT (eg, cognitive deficits).
TMS, which utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression, represents an important example of this initiative.2
TMS has established efficacy for depression
Sham-controlled trials. Several randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression.
A recent meta-analysis considered 18 studies (N = 1,970) that met the authors’ criteria for inclusion.3 They found that TMS monotherapy was statistically and clinically more effective than a sham procedure based on:
- improvement in depressive symptoms (mean decrease in baseline Hamilton Depression Rating Scale [HDRS] score, −4.53 [95% CI, −6.11 to −2.96])
- response rate; response was 3 times more likely with TMS (relative risk 3.38 [95% CI, 2.24 to 5.10])
- remission rate; remission was 5 times more likely with TMS (relative risk, 5.07 [95% CI, 2.50 to 10.30]).
Another meta-analysis (7 studies, N = 279) considered TMS as an augmentation strategy to standard medication for treatment-resistant depression.4 The authors reported that, based on change in HDRS scores, the pooled standardized mean difference between active and sham TMS augmentation was 0.86 (P < .00001). Furthermore, the pooled response rate with TMS augmentation was 46.6%, compared with 22.1% with the sham procedure (P < .0003).
Acute naturalistic TMS studies. The efficacy of TMS is supported by a large, naturalistic study of 307 patients with treatment-resistant depression who were assessed at baseline and during a standard course of TMS.5 Considering change score in the Clinician Global Impressions-Severity (CGI-S) scale, significant improvement was seen from baseline to end of treatment (−1.9 ± 1.4; P < .0001), with a clinician-assessed response rate of 58.0% and remission rate of 37.1%. Of note: Self-reported quality-of-life measures (on the Medical Outcomes Study 36-Item Short-Form Health Survey and EuroQol 5-Dimensions) also significantly improved during this relatively brief period.6
Maintenance strategies after acute TMS response. Most patients referred for TMS have a depressive illness characterized by a chronic, relapsing course and inadequate response to pharmacotherapy or psychotherapy, or their combination. An effective maintenance strategy after acute response to TMS is paramount. This includes:
- prolonged tapering schedule after an acute TMS course is completed
- maintenance medication or psychotherapy, or both
- scheduled periodic maintenance TMS sessions (usually as an augmentation strategy)
- reintroduction of TMS as needed with early signs of relapse. In this context, several trials have assessed the durability of acute TMS benefit.
A semi-controlled maintenance study followed 99 patients who had at least a 25% decrease in baseline HDRS score after acute TMS treatment.7 They were then tapered from their TMS sessions over 3 weeks while an antidepressant was titrated up. If, at any time during the subsequent 6 months, early signs of depression relapse were noted (ie, change of at least 1 point on the CGI-S for 2 consecutive weeks), TMS was reintroduced. At the end of the trial, 10 patients (13%) had relapsed and 38 (38%) had an exacerbation of symptoms sufficient to warrant reintroduction of TMS. Of those, 32 (84%) re-achieved mood stability.
In another study, 50 patients who had achieved remission during an acute course of TMS were followed for 3 months.8 After TMS taper and continued pharmacotherapy or naturalistic follow-up, 29 (58%) remained in remission; 2 (4%) maintained partial response; and 1 (2%) relapsed.
In a controlled, pilot, maintenance trial, 67 unmedicated patients with treatment-resistant depression received an acute course of TMS.9 Forty-seven of the responders were then randomized to a 1-year follow-up trial with or without a scheduled monthly TMS session. All patients could receive reintroduction TMS if they met criteria for symptom worsening.
Both groups had a similar outcome. The number of patients who did not require TMS reintroduction was 9 of 23 (39%) in the scheduled TMS group vs 9 of 26 (35%) in the no-scheduled TMS group (P < .1). Although no difference was noted between groups, the authors commented that these preliminary results will help inform larger, more definitive trials. They concluded that both acute and maintenance TMS monotherapy might be an option—for some patients.
A long-term, naturalistic outcomes study followed 257 treatment-resistant depressed patients for 1 year after they responded to an acute course of TMS.10 In addition to most patients receiving ongoing maintenance medication, they also could receive reintroduction of TMS if symptoms became worse.
Compared with pre-TMS baseline, there was a statistically significant reduction in the mean total score on the CGI-S scale (primary outcome, P < .0001) at the end of acute treatment that was sustained at follow-up. Ninety-six patients (36.2%) required reintroduction of TMS and 75 of 120 (62.5%) who initially met response or remission criteria after acute treatment continued to meet response criteria after 1 year. The authors concluded that TMS demonstrated both a statistically and clinically meaningful durability of acute benefit during this time frame.
TMS and electroconvulsive therapy
For more than 75 years, ECT has consistently proved to be an effective treatment for major depressive disorder. Although the use of ECT has fluctuated over this period, one practice survey estimated that 100,000 patients receive ECT annually.11
ECT has limitations, however, including cost, the need for general anesthesia, and cognitive deficits that range from short-term confusion to anterograde and retrograde amnesia, which can persist for weeks beyond active treatment.12 Despite increasing awareness of mental illness, stigma also remains a significant barrier to receiving ECT.
TMS vs ECT. Several trials have directly compared ECT and TMS:
- A recent meta-analysis of 9 trials included 384 patients with depression who were considered clinically appropriate for ECT and were randomized to one or the other treatment.13 Both modalities produced a significant reduction in baseline HDRS score, but ECT (15.4 point reduction) was superior to TMS (9.3 point reduction) in the degree of improvement (P < .01).
- Another meta-analysis of 9 trials (N = 425) found ECT superior to TMS in terms of response (P < .03) and remission (P < .006) rates, based on improvement in the HDRS score.14 When psychotic depressed patients were excluded, however, TMS produced effects equivalent to ECT.
In contrast to what was seen with ECT, cognitive testing of patients who received TMS revealed no deterioration in any domain. Furthermore, one of the comparison studies observed a modest, but statistically significant, improvement in patient’s working memory-executive function, objective memory, and fine-motor speed over the course of TMS treatment.15
TMS plus ECT. A 2-week, randomized, single-blind, controlled pilot study (N = 22) examined the combination of TMS and ECT as acute treatment of depression.16 Patients were assigned to receive either unilateral non-dominant (UND) ECT 3 days a week or a combination of 1 UND ECT treatment followed by 4 days of TMS. At the conclusion of treatment, UND ECT plus TMS group produced comparable efficacy and fewer adverse effects compared with the UND ECT-only group.
TMS maintenance after acute ECT response. Most patients who are referred for ECT have a depressive illness characterized by repeated episodes and incomplete response to pharmacotherapy or psychotherapy, or both. The need for an effective maintenance strategy after the acute response is therefore critical. Medication or ECT, or both, are commonly used to maintain acute benefit but, regrettably, a recent systematic review of the durability of benefit with such strategies found a substantial percentage (approximately 50%) of patients relapsed within the first year.17
- In this context, a case series report found that 1 or 2 weekly, sequential, bilateral TMS treatments after a successful acute course of ECT maintained response in 5 of 6 patients over 6 to 12 months.18
- Another case series (N = 6) transitioned stable patients from maintenance ECT to maintenance TMS, primarily because of adverse effects with ECT.19 With a mean frequency of 1 TMS treatment every 3.5 weeks, all 6 patients remained stable for as long as 6 months. Subsequently, 2 patients relapsed—1 at 8 months and 1 at 9 months.
Advantages of maintenance TMS over maintenance ECT include lower cost, fewer adverse effects (particularly cognitive deficits), and the ability to remain independent during the period of the treatment sessions.
TMS as an assessment tool for ECT response. TMS can be used to study excitability in cortical circuits. In a study, EEG potentials evoked by TMS before and after a course of ECT in 8 severely depressed patients revealed an increase in frontal cortical excitability, compared with baseline.20 Such findings support the ability of ECT to produce synaptic potentiation in humans. Furthermore, to the extent that depression presents with alterations in frontal cortical excitability, serial EEG-TMS measurements might be an effective tool to guide and monitor treatment progress with ECT, as well as other forms of therapeutic modulation.
Summing up: TMS and ECT. Although a definitive comparative study is needed, available evidence suggests that TMS might be an alternative treatment in a subgroup of patients who are referred for ECT. Factors that might warrant considering TMS over ECT include:
- patient preference
- fear of anesthesia
- concern about cognitive deficits
- stigma.
Although TMS might offer a workable alternative to ECT for acute and maintenance treatment of depression in selected patients, further refinement of the delivery of TMS is also needed to (1) enhance its efficacy and (2) identify clinical and biological markers to better define this select population.
Standard TMS treatment parameters
Superficial TMS. Superficial TMS for depression typically involves a single coil placed over the left dorsolateral prefrontal cortex. The standard, FDA-approved protocol includes stimulating at 110% of motor threshold with 75, 4-second trains at 10 Hz (ie, 40 stimulations) interspersed by 26-second intertrain intervals. Without interruption, a standard treatment session takes 37.5 minutes and delivers a total of 3,000 pulses. Most patients require 20 to 30 sessions, on a Monday-through-Friday schedule, to achieve optimal benefit.
This approach stimulates to a depth of approximately 2 or 3 cm. The coil usually is placed over the left dorsolateral prefrontal cortex because earlier studies indicated that decreased activity in this part of the brain correlates with symptoms of depression. When TMS is administered in a rapid repetitive fashion (at >1 Hz; typically, at 10 Hz), blood flow and metabolism in that area of the brain are increased. In addition, imaging studies indicate that trans-synaptic connections with deeper parts of the brain also allow modulation of other relevant neural circuits.
An alternate approach, less well-studied, involves low-frequency stimulation over the right dorsolateral prefrontal cortex. Parameters differ from what is used in left high-frequency dorsolateral prefrontal cortex TMS: frequency <1 Hz; train durations as long as 15 minutes; an intertrain interval of 25 to 180 seconds; 120 to 900 stimulations per train; and 2,400 to 18,000 total stimulations.
One hypothesis is that this low-frequency approach selectively stimulates inhibitory interneurons, decreases local neuronal activity and diminishes blood flow to deeper structures, such as the amygdala. Although right low-frequency TMS, compared with left high-frequency TMS, has potential advantages of better tolerability and decreased risk for seizures, its relative efficacy is unclear.
Deep TMS. Studies also are pursuing different coil configurations that allow for more direct stimulation of relevant structures (eg, prefrontal neuronal pathways associated with the reward system).
One of these coil designs (ie, the H-coil), coupled to a Magstim TMS stimulator, recently received FDA clearance for treatment-resistant depression. In the pivotal, sham-controlled study, patients received 20 treatment sessions over 4 weeks.21 The treatment protocol consisted of a helmet-like coil placed over the medial and lateral prefrontal cortex. Stimulation parameters included an 18-Hz frequency; stimulation intensity of 120% motor threshold; stimulation train duration of 2 seconds; and an intertrain interval of 20 seconds. The treatment sessions lasted 20.2 minutes and delivered a total of 1,980 stimulations.
Based on the 21-item HDRS, the active treatment coil group achieved a significantly greater decrease in baseline score (6.39 vs 3.28; P < .008); a greater response rate (37% vs 27.8%; P < .03); and a greater remission rate (30.4% vs 15.8%; P < .016) compared with the sham coil group.
Next, in what is the only randomized, controlled maintenance assessment to date, the same patients were followed for an additional 12 weeks, continuing blinded treatments twice weekly. At the end of the second phase, the active treatment group also demonstrated greater benefit than the sham group (P < .03). One seizure did occur, possibly related to excessive alcohol use; but this raises the question of whether treating at a higher frequency (18 Hz) with greater depth and less focality might increase the risk of seizure.
To assess the potential advantages, as well as the relative safety, of this approach over standard TMS delivery, an adequately designed and powered trial comparing the H-coil and a single-coil device is needed.
Alternate TMS approaches
Efforts to improve the clinical effectiveness of TMS for treating depression include several approaches.
Theta burst stimulation (TBS) is a patterned form of TMS pulse delivery that utilizes high and low frequencies in the same stimulus train (eg, three 50-Hz bursts delivered 5 times a second). Such a pulse sequence can modulate long-term depression and long-term potentiation mechanisms that induce plasticity in areas such as the hippocampus.22
Intermittent TBS (iTBS) administers stimulations over a relatively brief duration (eg, 2 seconds) or intermittently (eg, every 10 seconds) for a specific period (eg, 190 seconds [600 pulses in total]) over the left dorsolateral prefrontal cortex. This technique induces long-term potentiation and produces effects similar to those of high-frequency TMS.
In contrast, continuous TBS (cTBS) administers a continuous train (eg, 40 seconds [600 total pulses]) over the right dorsolateral prefrontal cortex. This induces long-term depression and produces effects similar to low-frequency TMS.
Recent studies using different delivery paradigms have generated mixed results:
Study 1: Fifty-six patients with depression received active treatment; 17 others, a sham procedure.23 This study used 3 different conditions:
- a combination of low-frequency and high-frequency TMS stimulation, administered over the right and left dorsolateral prefrontal cortices, respectively
- a combination of iTBS over the left dorsolateral prefrontal cortex and cTBS over the right dorsolateral prefrontal cortex
- a sham procedure, in which no magnetic field was created.
Neither active treatment arm separated from the sham procedure based on change scores in the 21-item HDRS (P = not significant).
Study 2: Sixty treatment-resistant depression patients were assigned to cTBS, iTBS, a combination of the 2 procedures, or a sham procedure.24 After 2 weeks, the active treatment arms produced the greatest benefit, based on change in scores on the 17-item HDRS, which differed significantly among the 4 groups (F value = 6.166; P < .001); the iTBS and combination arms demonstrated the most robust effect.
There were also significantly more responders in the iTBS (40.0%) and combination groups (66.7%) than in the cTBS (25.0%) and sham groups (13.3%) (P < .010). A lower level of treatment refractoriness predicted a better outcome.
Study 3: Twenty-nine depressed patients were randomized to cTBS over the right dorsolateral prefrontal cortex or a sham procedure.25 Overall, there was no difference between groups; however, actively treated patients who were unmedicated (n = 3) or remained on a stable dosage of medication during treatment (n = 8) did experience a significantly greater reduction in the HDRS score.
Study 4: In a pilot trial, 32 depressed patients were randomized to 30 sessions of adjunctive combined iTBS plus cTBS or bilateral sham TBS.26 Based on reduction from the baseline Montgomery-Åsberg Depression Rating Scale score, 9 patients in the active treatment group and 4 in the sham group achieved response (odds ratio, 3.86; P < .048).
If at least comparable efficacy can be clearly demonstrated, advantages of TBS over standard TMS include a significantly reduced administration time, which might allow for more patients to be treated and reduce associated costs of treatment.27
Magnetic low-field synchronized stimulation is produced by rotating spherical rare-earth magnets that are synchronized to an individual’s alpha frequency. A recent 6-week, double-blind, sham-controlled trial (N = 202) reported that, in the intention-to-treat population, there was no difference in outcome between treatment arms. In patients who completed the study according to protocol (120 of 202), however, active treatment was significantly better in decreasing baseline HDRS score (P < .033).28
Magnetic seizure therapy (MST) is an experimental approach to treating patients with more severe depression that is resistant to medical therapy. The primary aim is to use TMS to induce a seizure, thus achieving the same efficacy as provided by ECT but without the adverse cognitive effects of ECT. With MST, the TMS device uses much higher stimulation settings to produce a seizure—the goal being to avoid direct electrical current to the brain’s memory centers.29
A pilot study considered the clinical and cognitive effects of MST in a group of 26 treatment-resistant depression patients (10 randomized; 16 open-label).30 Based on reduction in baseline HDRS scores at the end of the trial, 69% of patients achieved response and 46% met remission criteria; however, one-half of patients relapsed within 6 months.
Importantly, no cognitive adverse effects were observed. Furthermore, the antidepressant and anti-anxiety effects of MST were associated with localized metabolic changes in brain areas implicated in the pathophysiology of depression.
The investigators concluded that MST might constitute an effective, well-tolerated, and safe treatment for patients unable to benefit from available medical therapies for depression. In addition to confirmation of acute benefit in more definitive trials, the issue of durability of effect needs further clarification.
TMS is a key component of neuropsychiatric practice
It has been 3 decades since Barker et al31 developed the technology to deliver intense, localized magnetic pulses to specific areas of the nervous system. During this period, the role of TMS as a probe of the central and peripheral nervous systems has expanded to include various therapeutic applications, primarily focusing on treatment-resistant major depressive disorder.
Now, increasing sophistication in the choice of stimulation parameters and other ongoing efforts to optimize the benefits of TMS are yielding improved clinical outcomes. Research is still needed to better define the place of TMS in the management of subtypes of depression that are particularly difficult to treat and that do not benefit adequately from medications or psychotherapy or their combination.
Growing support from controlled trials, systematic reviews, meta-analyses, naturalistic outcome studies, and professional guidelines indicate that TMS has an increasingly important role in clinical practice.
Since 2008, the FDA has cleared 4 transcranial magnetic stimulation (TMS) devices for treating depression (Related Resources). In that time, the availability of TMS has steadily grown within and outside the United States.
Parallel with increasing clinical utilization of this technology, research continues into the benefit of TMS for treatment-resistant depression; such research includes additional, supportive, acute, sham-controlled trials; comparison trials with electroconvulsive therapy (ECT) for more severe episodes of depression; short- and long-term real-world outcome studies; exploration of alternative treatment parameters to further enhance its efficacy; and the development of other TMS approaches. In this article, we review recent developments in the application of TMS to treat major depressive disorder—in particular, treatment-resistant depression (Box).
Therapeutic neuromodulation
The underlying premise of neuromodulation is that the brain is an electrochemical organ that can be modulated by pharmacotherapy or device-based approaches, or their combination.1 ECT is the prototypic device-based neuromodulation approach, and remains one of the most effective treatments for severe depression.
More recently, however, other methods have been, and continue to be, developed to treat patients who do not achieve adequate benefit from psychotherapy or medical therapy, or both, and who might not be an ideal candidate for ECT (Table,1). In addition to the potential therapeutic benefit of these alternative strategies, some could avoid safety and tolerability concerns associated with medication (weight gain, sexual dysfunction) and ECT (eg, cognitive deficits).
TMS, which utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression, represents an important example of this initiative.2
TMS has established efficacy for depression
Sham-controlled trials. Several randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression.
A recent meta-analysis considered 18 studies (N = 1,970) that met the authors’ criteria for inclusion.3 They found that TMS monotherapy was statistically and clinically more effective than a sham procedure based on:
- improvement in depressive symptoms (mean decrease in baseline Hamilton Depression Rating Scale [HDRS] score, −4.53 [95% CI, −6.11 to −2.96])
- response rate; response was 3 times more likely with TMS (relative risk 3.38 [95% CI, 2.24 to 5.10])
- remission rate; remission was 5 times more likely with TMS (relative risk, 5.07 [95% CI, 2.50 to 10.30]).
Another meta-analysis (7 studies, N = 279) considered TMS as an augmentation strategy to standard medication for treatment-resistant depression.4 The authors reported that, based on change in HDRS scores, the pooled standardized mean difference between active and sham TMS augmentation was 0.86 (P < .00001). Furthermore, the pooled response rate with TMS augmentation was 46.6%, compared with 22.1% with the sham procedure (P < .0003).
Acute naturalistic TMS studies. The efficacy of TMS is supported by a large, naturalistic study of 307 patients with treatment-resistant depression who were assessed at baseline and during a standard course of TMS.5 Considering change score in the Clinician Global Impressions-Severity (CGI-S) scale, significant improvement was seen from baseline to end of treatment (−1.9 ± 1.4; P < .0001), with a clinician-assessed response rate of 58.0% and remission rate of 37.1%. Of note: Self-reported quality-of-life measures (on the Medical Outcomes Study 36-Item Short-Form Health Survey and EuroQol 5-Dimensions) also significantly improved during this relatively brief period.6
Maintenance strategies after acute TMS response. Most patients referred for TMS have a depressive illness characterized by a chronic, relapsing course and inadequate response to pharmacotherapy or psychotherapy, or their combination. An effective maintenance strategy after acute response to TMS is paramount. This includes:
- prolonged tapering schedule after an acute TMS course is completed
- maintenance medication or psychotherapy, or both
- scheduled periodic maintenance TMS sessions (usually as an augmentation strategy)
- reintroduction of TMS as needed with early signs of relapse. In this context, several trials have assessed the durability of acute TMS benefit.
A semi-controlled maintenance study followed 99 patients who had at least a 25% decrease in baseline HDRS score after acute TMS treatment.7 They were then tapered from their TMS sessions over 3 weeks while an antidepressant was titrated up. If, at any time during the subsequent 6 months, early signs of depression relapse were noted (ie, change of at least 1 point on the CGI-S for 2 consecutive weeks), TMS was reintroduced. At the end of the trial, 10 patients (13%) had relapsed and 38 (38%) had an exacerbation of symptoms sufficient to warrant reintroduction of TMS. Of those, 32 (84%) re-achieved mood stability.
In another study, 50 patients who had achieved remission during an acute course of TMS were followed for 3 months.8 After TMS taper and continued pharmacotherapy or naturalistic follow-up, 29 (58%) remained in remission; 2 (4%) maintained partial response; and 1 (2%) relapsed.
In a controlled, pilot, maintenance trial, 67 unmedicated patients with treatment-resistant depression received an acute course of TMS.9 Forty-seven of the responders were then randomized to a 1-year follow-up trial with or without a scheduled monthly TMS session. All patients could receive reintroduction TMS if they met criteria for symptom worsening.
Both groups had a similar outcome. The number of patients who did not require TMS reintroduction was 9 of 23 (39%) in the scheduled TMS group vs 9 of 26 (35%) in the no-scheduled TMS group (P < .1). Although no difference was noted between groups, the authors commented that these preliminary results will help inform larger, more definitive trials. They concluded that both acute and maintenance TMS monotherapy might be an option—for some patients.
A long-term, naturalistic outcomes study followed 257 treatment-resistant depressed patients for 1 year after they responded to an acute course of TMS.10 In addition to most patients receiving ongoing maintenance medication, they also could receive reintroduction of TMS if symptoms became worse.
Compared with pre-TMS baseline, there was a statistically significant reduction in the mean total score on the CGI-S scale (primary outcome, P < .0001) at the end of acute treatment that was sustained at follow-up. Ninety-six patients (36.2%) required reintroduction of TMS and 75 of 120 (62.5%) who initially met response or remission criteria after acute treatment continued to meet response criteria after 1 year. The authors concluded that TMS demonstrated both a statistically and clinically meaningful durability of acute benefit during this time frame.
TMS and electroconvulsive therapy
For more than 75 years, ECT has consistently proved to be an effective treatment for major depressive disorder. Although the use of ECT has fluctuated over this period, one practice survey estimated that 100,000 patients receive ECT annually.11
ECT has limitations, however, including cost, the need for general anesthesia, and cognitive deficits that range from short-term confusion to anterograde and retrograde amnesia, which can persist for weeks beyond active treatment.12 Despite increasing awareness of mental illness, stigma also remains a significant barrier to receiving ECT.
TMS vs ECT. Several trials have directly compared ECT and TMS:
- A recent meta-analysis of 9 trials included 384 patients with depression who were considered clinically appropriate for ECT and were randomized to one or the other treatment.13 Both modalities produced a significant reduction in baseline HDRS score, but ECT (15.4 point reduction) was superior to TMS (9.3 point reduction) in the degree of improvement (P < .01).
- Another meta-analysis of 9 trials (N = 425) found ECT superior to TMS in terms of response (P < .03) and remission (P < .006) rates, based on improvement in the HDRS score.14 When psychotic depressed patients were excluded, however, TMS produced effects equivalent to ECT.
In contrast to what was seen with ECT, cognitive testing of patients who received TMS revealed no deterioration in any domain. Furthermore, one of the comparison studies observed a modest, but statistically significant, improvement in patient’s working memory-executive function, objective memory, and fine-motor speed over the course of TMS treatment.15
TMS plus ECT. A 2-week, randomized, single-blind, controlled pilot study (N = 22) examined the combination of TMS and ECT as acute treatment of depression.16 Patients were assigned to receive either unilateral non-dominant (UND) ECT 3 days a week or a combination of 1 UND ECT treatment followed by 4 days of TMS. At the conclusion of treatment, UND ECT plus TMS group produced comparable efficacy and fewer adverse effects compared with the UND ECT-only group.
TMS maintenance after acute ECT response. Most patients who are referred for ECT have a depressive illness characterized by repeated episodes and incomplete response to pharmacotherapy or psychotherapy, or both. The need for an effective maintenance strategy after the acute response is therefore critical. Medication or ECT, or both, are commonly used to maintain acute benefit but, regrettably, a recent systematic review of the durability of benefit with such strategies found a substantial percentage (approximately 50%) of patients relapsed within the first year.17
- In this context, a case series report found that 1 or 2 weekly, sequential, bilateral TMS treatments after a successful acute course of ECT maintained response in 5 of 6 patients over 6 to 12 months.18
- Another case series (N = 6) transitioned stable patients from maintenance ECT to maintenance TMS, primarily because of adverse effects with ECT.19 With a mean frequency of 1 TMS treatment every 3.5 weeks, all 6 patients remained stable for as long as 6 months. Subsequently, 2 patients relapsed—1 at 8 months and 1 at 9 months.
Advantages of maintenance TMS over maintenance ECT include lower cost, fewer adverse effects (particularly cognitive deficits), and the ability to remain independent during the period of the treatment sessions.
TMS as an assessment tool for ECT response. TMS can be used to study excitability in cortical circuits. In a study, EEG potentials evoked by TMS before and after a course of ECT in 8 severely depressed patients revealed an increase in frontal cortical excitability, compared with baseline.20 Such findings support the ability of ECT to produce synaptic potentiation in humans. Furthermore, to the extent that depression presents with alterations in frontal cortical excitability, serial EEG-TMS measurements might be an effective tool to guide and monitor treatment progress with ECT, as well as other forms of therapeutic modulation.
Summing up: TMS and ECT. Although a definitive comparative study is needed, available evidence suggests that TMS might be an alternative treatment in a subgroup of patients who are referred for ECT. Factors that might warrant considering TMS over ECT include:
- patient preference
- fear of anesthesia
- concern about cognitive deficits
- stigma.
Although TMS might offer a workable alternative to ECT for acute and maintenance treatment of depression in selected patients, further refinement of the delivery of TMS is also needed to (1) enhance its efficacy and (2) identify clinical and biological markers to better define this select population.
Standard TMS treatment parameters
Superficial TMS. Superficial TMS for depression typically involves a single coil placed over the left dorsolateral prefrontal cortex. The standard, FDA-approved protocol includes stimulating at 110% of motor threshold with 75, 4-second trains at 10 Hz (ie, 40 stimulations) interspersed by 26-second intertrain intervals. Without interruption, a standard treatment session takes 37.5 minutes and delivers a total of 3,000 pulses. Most patients require 20 to 30 sessions, on a Monday-through-Friday schedule, to achieve optimal benefit.
This approach stimulates to a depth of approximately 2 or 3 cm. The coil usually is placed over the left dorsolateral prefrontal cortex because earlier studies indicated that decreased activity in this part of the brain correlates with symptoms of depression. When TMS is administered in a rapid repetitive fashion (at >1 Hz; typically, at 10 Hz), blood flow and metabolism in that area of the brain are increased. In addition, imaging studies indicate that trans-synaptic connections with deeper parts of the brain also allow modulation of other relevant neural circuits.
An alternate approach, less well-studied, involves low-frequency stimulation over the right dorsolateral prefrontal cortex. Parameters differ from what is used in left high-frequency dorsolateral prefrontal cortex TMS: frequency <1 Hz; train durations as long as 15 minutes; an intertrain interval of 25 to 180 seconds; 120 to 900 stimulations per train; and 2,400 to 18,000 total stimulations.
One hypothesis is that this low-frequency approach selectively stimulates inhibitory interneurons, decreases local neuronal activity and diminishes blood flow to deeper structures, such as the amygdala. Although right low-frequency TMS, compared with left high-frequency TMS, has potential advantages of better tolerability and decreased risk for seizures, its relative efficacy is unclear.
Deep TMS. Studies also are pursuing different coil configurations that allow for more direct stimulation of relevant structures (eg, prefrontal neuronal pathways associated with the reward system).
One of these coil designs (ie, the H-coil), coupled to a Magstim TMS stimulator, recently received FDA clearance for treatment-resistant depression. In the pivotal, sham-controlled study, patients received 20 treatment sessions over 4 weeks.21 The treatment protocol consisted of a helmet-like coil placed over the medial and lateral prefrontal cortex. Stimulation parameters included an 18-Hz frequency; stimulation intensity of 120% motor threshold; stimulation train duration of 2 seconds; and an intertrain interval of 20 seconds. The treatment sessions lasted 20.2 minutes and delivered a total of 1,980 stimulations.
Based on the 21-item HDRS, the active treatment coil group achieved a significantly greater decrease in baseline score (6.39 vs 3.28; P < .008); a greater response rate (37% vs 27.8%; P < .03); and a greater remission rate (30.4% vs 15.8%; P < .016) compared with the sham coil group.
Next, in what is the only randomized, controlled maintenance assessment to date, the same patients were followed for an additional 12 weeks, continuing blinded treatments twice weekly. At the end of the second phase, the active treatment group also demonstrated greater benefit than the sham group (P < .03). One seizure did occur, possibly related to excessive alcohol use; but this raises the question of whether treating at a higher frequency (18 Hz) with greater depth and less focality might increase the risk of seizure.
To assess the potential advantages, as well as the relative safety, of this approach over standard TMS delivery, an adequately designed and powered trial comparing the H-coil and a single-coil device is needed.
Alternate TMS approaches
Efforts to improve the clinical effectiveness of TMS for treating depression include several approaches.
Theta burst stimulation (TBS) is a patterned form of TMS pulse delivery that utilizes high and low frequencies in the same stimulus train (eg, three 50-Hz bursts delivered 5 times a second). Such a pulse sequence can modulate long-term depression and long-term potentiation mechanisms that induce plasticity in areas such as the hippocampus.22
Intermittent TBS (iTBS) administers stimulations over a relatively brief duration (eg, 2 seconds) or intermittently (eg, every 10 seconds) for a specific period (eg, 190 seconds [600 pulses in total]) over the left dorsolateral prefrontal cortex. This technique induces long-term potentiation and produces effects similar to those of high-frequency TMS.
In contrast, continuous TBS (cTBS) administers a continuous train (eg, 40 seconds [600 total pulses]) over the right dorsolateral prefrontal cortex. This induces long-term depression and produces effects similar to low-frequency TMS.
Recent studies using different delivery paradigms have generated mixed results:
Study 1: Fifty-six patients with depression received active treatment; 17 others, a sham procedure.23 This study used 3 different conditions:
- a combination of low-frequency and high-frequency TMS stimulation, administered over the right and left dorsolateral prefrontal cortices, respectively
- a combination of iTBS over the left dorsolateral prefrontal cortex and cTBS over the right dorsolateral prefrontal cortex
- a sham procedure, in which no magnetic field was created.
Neither active treatment arm separated from the sham procedure based on change scores in the 21-item HDRS (P = not significant).
Study 2: Sixty treatment-resistant depression patients were assigned to cTBS, iTBS, a combination of the 2 procedures, or a sham procedure.24 After 2 weeks, the active treatment arms produced the greatest benefit, based on change in scores on the 17-item HDRS, which differed significantly among the 4 groups (F value = 6.166; P < .001); the iTBS and combination arms demonstrated the most robust effect.
There were also significantly more responders in the iTBS (40.0%) and combination groups (66.7%) than in the cTBS (25.0%) and sham groups (13.3%) (P < .010). A lower level of treatment refractoriness predicted a better outcome.
Study 3: Twenty-nine depressed patients were randomized to cTBS over the right dorsolateral prefrontal cortex or a sham procedure.25 Overall, there was no difference between groups; however, actively treated patients who were unmedicated (n = 3) or remained on a stable dosage of medication during treatment (n = 8) did experience a significantly greater reduction in the HDRS score.
Study 4: In a pilot trial, 32 depressed patients were randomized to 30 sessions of adjunctive combined iTBS plus cTBS or bilateral sham TBS.26 Based on reduction from the baseline Montgomery-Åsberg Depression Rating Scale score, 9 patients in the active treatment group and 4 in the sham group achieved response (odds ratio, 3.86; P < .048).
If at least comparable efficacy can be clearly demonstrated, advantages of TBS over standard TMS include a significantly reduced administration time, which might allow for more patients to be treated and reduce associated costs of treatment.27
Magnetic low-field synchronized stimulation is produced by rotating spherical rare-earth magnets that are synchronized to an individual’s alpha frequency. A recent 6-week, double-blind, sham-controlled trial (N = 202) reported that, in the intention-to-treat population, there was no difference in outcome between treatment arms. In patients who completed the study according to protocol (120 of 202), however, active treatment was significantly better in decreasing baseline HDRS score (P < .033).28
Magnetic seizure therapy (MST) is an experimental approach to treating patients with more severe depression that is resistant to medical therapy. The primary aim is to use TMS to induce a seizure, thus achieving the same efficacy as provided by ECT but without the adverse cognitive effects of ECT. With MST, the TMS device uses much higher stimulation settings to produce a seizure—the goal being to avoid direct electrical current to the brain’s memory centers.29
A pilot study considered the clinical and cognitive effects of MST in a group of 26 treatment-resistant depression patients (10 randomized; 16 open-label).30 Based on reduction in baseline HDRS scores at the end of the trial, 69% of patients achieved response and 46% met remission criteria; however, one-half of patients relapsed within 6 months.
Importantly, no cognitive adverse effects were observed. Furthermore, the antidepressant and anti-anxiety effects of MST were associated with localized metabolic changes in brain areas implicated in the pathophysiology of depression.
The investigators concluded that MST might constitute an effective, well-tolerated, and safe treatment for patients unable to benefit from available medical therapies for depression. In addition to confirmation of acute benefit in more definitive trials, the issue of durability of effect needs further clarification.
TMS is a key component of neuropsychiatric practice
It has been 3 decades since Barker et al31 developed the technology to deliver intense, localized magnetic pulses to specific areas of the nervous system. During this period, the role of TMS as a probe of the central and peripheral nervous systems has expanded to include various therapeutic applications, primarily focusing on treatment-resistant major depressive disorder.
Now, increasing sophistication in the choice of stimulation parameters and other ongoing efforts to optimize the benefits of TMS are yielding improved clinical outcomes. Research is still needed to better define the place of TMS in the management of subtypes of depression that are particularly difficult to treat and that do not benefit adequately from medications or psychotherapy or their combination.
Growing support from controlled trials, systematic reviews, meta-analyses, naturalistic outcome studies, and professional guidelines indicate that TMS has an increasingly important role in clinical practice.
1. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):66-70,72-74.
2. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
3. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
4. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
5. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
6. Janicak PG, Dunner DL, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice. CNS Spectr. 2013;18(6):322-332.
7. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
8. Mantovani A, Pavlicova M, Avery D, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883-890.
9. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
10. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
11. Hermann RC, Dorwart RA, Hoover CW. Variation in ECT use in the United States. Am J Psychiatry. 1995;152(6):869-875.
12. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16(2):87-96.
13. Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049. doi: 10.1155/2014/135049.
14. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
15. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
16. Pridmore S, Rybak M, Turnier-Shea Y, et al. Comparison of transcranial magnetic stimulation and electroconvulsive therapy in depression. In: Miyoshi K, Shapiro CM, Gaviria M, et al, eds. Contemporary neuropsychiatry. Tokyo, Japan: Springer; 2001:237-241.
17. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
18. Noda Y, Daskalakis Z, Ramos C, et al. Repetitive transcranial magnetic stimulation to maintain treatment response to electroconvulsive therapy in depression: a case series. Front Psychiatry. 2013;4:73.
19. Cristancho MA, Helmer A, Connolly R, et al. Transcranial magnetic stimulation maintenance as a substitute for maintenance electroconvulsive therapy: a case series. J ECT. 2013;29(2):106-108.
20. Casarotto S, Canali P, Rosanova M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326-337.
21. Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64-73.
22. Daskalakis ZJ. Theta-burst transcranial magnetic stimulation in depression: when less may be more. Brain. 2014;137(pt 7):1860-1862.
23. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57-65.
24. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(pt 7):2088-2098.
25. Chistyakov A, Kreinin B, Marmor S, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225-229.
26. Plewnia C, Pasqualetti P, Große S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219-223.
27. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
28. Leuchter AF, Cook IA, Feifel D, et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;8(4):787-794.
29. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
30. Kayser S, Bewernick BH, Matusch A, et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073-1092.
31. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107.
1. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):66-70,72-74.
2. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
3. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
4. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
5. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
6. Janicak PG, Dunner DL, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice. CNS Spectr. 2013;18(6):322-332.
7. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
8. Mantovani A, Pavlicova M, Avery D, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883-890.
9. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
10. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
11. Hermann RC, Dorwart RA, Hoover CW. Variation in ECT use in the United States. Am J Psychiatry. 1995;152(6):869-875.
12. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16(2):87-96.
13. Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049. doi: 10.1155/2014/135049.
14. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
15. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
16. Pridmore S, Rybak M, Turnier-Shea Y, et al. Comparison of transcranial magnetic stimulation and electroconvulsive therapy in depression. In: Miyoshi K, Shapiro CM, Gaviria M, et al, eds. Contemporary neuropsychiatry. Tokyo, Japan: Springer; 2001:237-241.
17. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
18. Noda Y, Daskalakis Z, Ramos C, et al. Repetitive transcranial magnetic stimulation to maintain treatment response to electroconvulsive therapy in depression: a case series. Front Psychiatry. 2013;4:73.
19. Cristancho MA, Helmer A, Connolly R, et al. Transcranial magnetic stimulation maintenance as a substitute for maintenance electroconvulsive therapy: a case series. J ECT. 2013;29(2):106-108.
20. Casarotto S, Canali P, Rosanova M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326-337.
21. Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64-73.
22. Daskalakis ZJ. Theta-burst transcranial magnetic stimulation in depression: when less may be more. Brain. 2014;137(pt 7):1860-1862.
23. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57-65.
24. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(pt 7):2088-2098.
25. Chistyakov A, Kreinin B, Marmor S, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225-229.
26. Plewnia C, Pasqualetti P, Große S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219-223.
27. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
28. Leuchter AF, Cook IA, Feifel D, et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;8(4):787-794.
29. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
30. Kayser S, Bewernick BH, Matusch A, et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073-1092.
31. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107.