User login
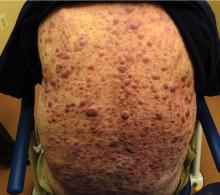
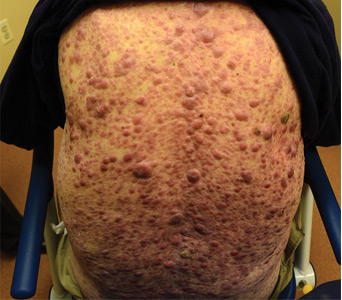
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
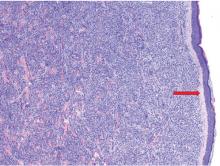
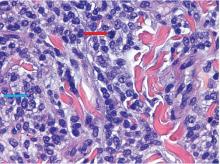
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164