User login
Health care providers often need to evaluate allergic disorders such as allergic rhinoconjunctivitis, asthma, and allergies to foods, drugs, latex, and venom, both in the hospital and in the clinic.
Unfortunately, some symptoms, such as chronic nasal symptoms, can occur in both allergic and nonallergic disorders, and this overlap can confound the diagnosis and therapy. Studies suggest that when clinicians use the history and physical examination alone in evaluating possible allergic disease, the accuracy of their diagnoses rarely exceeds 50%.1
Blood tests are now available that measure immunoglobulin E (IgE) directed against specific antigens. These in vitro tests can be important tools in assessing a patient whose history suggests an allergic disease.2 However, neither allergy skin testing nor these blood tests are intended to be used for screening: they may be most useful as confirmatory diagnostic tests in cases in which the pretest clinical impression of allergic disease is high.
ALLERGY IS MEDIATED BY IgE
In susceptible people, IgE is produced by B cells in response to specific antigens such as foods, pollens, latex, and drugs. This antigen-specific (or allergen-specific) IgE circulates in the serum and binds to high-affinity IgE receptors on immune effector cells such as mast cells located throughout the body.
Upon subsequent exposure to the same allergen, IgE receptors cross-link and initiate downstream signaling events that trigger mast cell degranulation and an immediate allergic response—hence the term immediate (or Gell-Coombs type I) hypersensitivity.3
Common manifestations of type I hypersensitivity reactions include signs and symptoms that can be:
- Cutaneous (eg, acute urticaria, angioedema)
- Respiratory (eg, acute bronchospasm, rhinoconjunctivitis)
- Cardiovascular (eg, tachycardia, hypotension)
- Gastrointestinal (eg, vomiting, diarrhea)
- Generalized (eg, anaphylactic shock). By definition, anaphylaxis is a life-threatening reaction that occurs on exposure to an allergen and involves acute respiratory distress, cardiovascular failure, or involvement of two or more organ systems.4
MOST IgE BLOOD TESTS ARE IMMUNOASSAYS
The blood tests for allergic disease are immunoassays that measure the level of IgE specific to a particular allergen. The tests can be used to evaluate sensitivity to various allergens, for example, to common inhalants such as dust mites and pollens and to foods, drugs, venom, and latex.
Types of immunoassays include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), and radioallergosorbent assays (RASTs). At present, most commercial laboratories use one of three autoanalyzer systems to measure specific IgE:
- ImmunoCAP (Phadia AB, Uppsala, Sweden)
- Immulite (Siemens AG, Berlin, Germany)
- HYTEC-288 (Hycor/Agilent, Garden Grove, CA).
These systems use a solid-phase polymer (cellulose or avidin) in which the antigen is embedded. The polymer also facilitates binding of IgE and, therefore, increases the sensitivity of the test.5 Specific IgE from the patient’s serum binds to the allergen embedded in the polymer, and then unbound antibodies are washed off.
Despite the term “RAST,” these systems do not use radiation. A fluorescent antibody is added that binds to the patient’s IgE, and the amount of IgE present is calculated from the amount of fluorescence.6 Results are reported in kilounits of antibody per liter (kU/L) or nanograms per milliliter (ng/mL).5–7
INTERPRETATION IS INDIVIDUALIZED
In general, the sensitivity of these tests ranges from 60% to 95% and their specificity from 30% to 95%, with a concordance among different immunoassays of 75% to 90%.8
Levels of IgE for a particular allergen are also divided into semiquantitative classes, from class I to class V or VI. In general, class I and class II correlate with a low level of allergen sensitization and, often, with a low likelihood of a clinical reaction. On the other hand, classes V and VI reflect higher degrees of sensitization and generally correlate with IgE-mediated clinical reactions upon allergen exposure.
The interpretation of a positive (ie, “nonzero”) test result must be individualized on the basis of clinical presentation and risk factors. A specialist can make an important contribution by helping to interpret any positive test result or a negative test result that does not correlate with the patient’s history.
ADVANTAGES OF ALLERGY BLOOD TESTING
Allergy blood testing is convenient, since it involves only a standard blood draw.
In theory, allergy blood testing may be safer, since it does not expose the patient to any allergens. On the other hand, many patients experience bruising from venipuncture performed for any reason: 16% in one survey.9 In another survey,10 adverse reactions of any type occurred in 0.49% of patients undergoing venipuncture but only in 0.04% of those undergoing allergy skin testing. Therefore, allergy blood testing may be most appropriate in situations in which a patient’s history suggests that he or she may be at risk of a systemic reaction from a traditional skin test or in cases in which skin testing is not possible (eg, extensive eczema).
Another advantage of allergy blood testing is that it is not affected by drugs such as antihistamines or tricyclic antidepressants that suppress the histamine response, which is a problem with skin testing.
Allergy blood testing may also be useful in patients on long-term glucocorticoid therapy, although the data conflict. Prolonged oral glucocorticoid use is associated with a decrease in mast cell density and histamine content in the skin,11,12 although in one study a corticosteroid was found not to affect the results of skin-prick testing for allergy.13 Thus, allergy blood testing can be performed in patients who have severe eczema or dermatographism or who cannot safely suspend taking antihistamines or tricyclic antidepressants.
LIMITATIONS OF THESE TESTS
A limitation of allergy blood tests is that there is no gold-standard test for many allergic conditions. (Double-blind, placebo-controlled oral food challenge testing has been proposed as the gold-standard test for food allergy, and nasal allergen provocation challenge has been proposed for allergic rhinitis.)
Also, allergy blood tests can give false-positive results because of nonspecific binding of antibody in the assay.
Of note: evidence of sensitization to a particular allergen (ie, a positive blood test result) is not synonymous with clinically relevant disease (ie, clinical sensitivity).
Conversely, these tests can give false-negative results in patients who have true IgE-mediated disease as confirmed by skin testing or allergen challenge. The sensitivity of blood allergy testing is approximately 25% to 30% lower than that of skin testing, based on comparative studies.2 The blood tests are usually considered positive if the allergen-specific IgE level is greater than 0.35 kU/L; however, sensitization to certain inhalant allergens can occur at levels as low as 0.12 kU/L.14
Specific IgE levels measured by different commercial assays are not always interchangeable or equivalent, so a clinician should consistently select the same immunoassay if possible when assessing any given patient over time.15
Levels of specific IgE have been shown to depend on age, allergen specificity, total serum IgE, and, with inhalant allergens, the season of the year.15,16
Other limitations of blood testing are its cost and a delay of several days to a week in obtaining the results.17
WHEN TO ORDER ALLERGY BLOOD TESTING
The allergy evaluation should begin with a thorough history to look for possible triggers for the patient’s symptoms.
For example, respiratory conditions such as asthma and rhinitis may be exacerbated during particular times of the year when certain pollens are commonly present. For patients with this pattern, blood testing for allergy to common inhalants, including pollens, may be appropriate. Similarly, peanut allergy evaluation is indicated for a child who has suffered an anaphylactic reaction after consuming peanut butter. Blood testing is also indicated in patients with a history of venom anaphylaxis, especially if venom skin testing was negative.
In cases in which the patient does not have a clear history of sensitization, blood testing for allergy to multiple foods may find evidence of sensitization that does not necessarily correlate with clinical disease.18
Likewise, blood tests are not likely to be clinically relevant in conditions not mediated by IgE, such as food intolerances (eg, lactose intolerance), celiac disease, the DRESS syndrome (drug rash, eosinophilia, and systemic symptoms), Stevens-Johnson syndrome, toxic epidermal necrolysis, or other types of drug hypersensitivity reactions, such as serum sickness.3
INTERPRETING COMMONLY ORDERED BLOOD TESTS FOR ALLERGY
Tests for allergy to hundreds of substances are available.
Foods
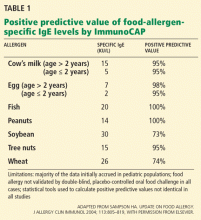
Milk, eggs, soy, wheat, peanuts, tree nuts, fish, and shellfish account for most cases of food allergy in the United States.18
IgE-mediated hypersensitivity to milk, eggs, and peanuts tends to be more common in children, whereas peanuts, tree nuts, fish, and shellfish are more commonly associated with reactions in adults.18 Children are more likely to outgrow allergy to milk, soy, wheat, and eggs than allergy to peanuts, tree nuts, fish, and shellfish—only about 20% of children outgrow peanut allergy.18
Patients with an IgE-mediated reaction to foods should be closely followed by a specialist, who can best help determine the appropriateness of additional testing (such as an oral challenge under observation), avoidance recommendations, and the introduction of foods back into the diet.19
Specific IgE tests for allergy to a variety of foods are available and can be very useful for diagnosis when used in the appropriate setting.
One caveat about these studies is that many were initially performed in children with a history of food allergy, many of whom had atopic dermatitis, and the findings have not been systematically reexamined in larger studies in more heterogeneous populations.
For example, at least eight studies tried to identify a diagnostic IgE level for cow’s milk allergy. The 95% confidence intervals varied widely, depending on the study design, the age of the study population, the prevalence of food allergy in the population, and the statistical method used for analysis.5 For most other foods for which blood tests are available, few studies have been performed to establish predictive values similar to those in Table 1.
Thus, slight elevations in antigen-specific IgE (> 0.35 kU/L) may correlate only with in vitro sensitization in a patient who has no clinical reactivity upon oral exposure to a particular antigen.
Broad food panels have been shown to have false-positive rates higher than 50%—ie, in more than half of cases, positive results have no clinical relevance. Therefore, these large food panels should not be used for screening.19 Instead, it is recommended that tests be limited to relevant foods based on the patient’s history when evaluating symptoms consistent with an IgE-mediated reaction to a particular food.
Food-specific IgE evaluation is also not helpful in evaluating non-IgE adverse reactions to foods (eg, intolerances).
Therefore, the patient’s history remains the most important tool for evaluation of food allergy. In cases in which the patient’s history suggests a food-associated IgE-mediated reaction and the blood test is negative, the patient should be referred to a specialist for skin testing with commercial extracts or even fresh food extracts, given the higher sensitivity of in vivo testing.20
Inhalants
Common aeroallergens associated with allergic rhinitis, allergic conjunctivitis, and allergic asthma include dust mites, animal dander, cockroach debris, molds, trees, grasses, weeds, and ragweed. Dust mites, animal dander, and mold spores are perennial allergens and may trigger symptoms year-round. Pollen, including pollen from trees, grasses, and weeds, is generally present in a seasonal pattern in many parts of the United States.
A positive blood test for an inhalant allergen can reinforce the physician’s clinical impression in making a diagnosis of allergic rhinoconjunctivitis. Interestingly, studies have suggested a high rate of false-positives based on history alone when in vivo and in vitro allergy testing were negative for IgE-mediated respiratory disease.21
Various studies have aimed to establish threshold values of aeroallergen-specific IgE that predict the likelihood of clinically relevant disease. Unfortunately, other factors also contribute to clinical symptoms of rhinoconjunctivitis; these include concurrent inflammation, infection, physical stress, psychological stress, exposure to irritants, and hormonal changes. These factors introduce variability and make specific IgE cutoffs for inhalant allergens unreliable.22
Prospective studies have suggested that skin testing correlates better with nasal allergen challenge (the gold standard) than blood testing for the diagnosis of inhalant allergy, though more recent studies using modern technologies demonstrate reasonable concordance (67%) between skin testing and blood testing (specifically, ImmunoCAP).23,24 According to current guidelines, skin tests are the preferred method for diagnosing IgE-mediated sensitivity to inhalants.25
Compared with skin prick tests as the gold standard, the sensitivity of specific IgE immunoassays is approximately 70% to 75%.25 Nevertheless, specific IgE values greater than 0.35 kU/L are generally considered positive for aeroallergen sensitization, although lower levels of dog-specific IgE have recently been shown to correlate with clinical disease.14
Drugs, including penicillins
A variety of clinical reactions can occur in response to oral, intravenous, or topical medications.
At present, blood tests are available for the evaluation of IgE-mediated adverse reactions to only a limited number of drugs. Reactions involving other mechanisms, such as those related to the drug’s metabolism, intolerances (eg, nausea), idiosyncratic reactions (eg, Stevens-Johnson syndrome, the DRESS syndrome), or other types of reactions can be diagnosed only by history and physical examination.
The development of specific IgE tests for sensitivity to medications has been limited by incomplete characterization of metabolic products and the possibility that a single medication can have different epitopes or IgE binding sites in different individuals.26
In vitro allergy testing has been most studied for beta-lactam antibiotics (eg, penicillin) and not so much for other drugs.
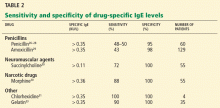
Table 2 summarizes the sensitivity and specificity of blood allergy tests that are commercially available for drugs.
Penicillin, a beta-lactam antibiotic, is degraded into various metabolites known as the major determinant (penicilloyl) and the minor determinants (eg, benzylpenicilloate and benzylpenilloate), which act as haptens. Specific IgE testing is not available for all these determinants.
The sensitivity of blood tests for allergy to penicilloyl (penicillin) and amino-penicillins such as amoxicilloyl (amoxicillin) is reported as between 32% and 50%, and the specificity as 96% to 98%.29
By definition, any nonzero level of IgE specific for penicillin or its derivatives is considered a positive result and may be associated with a higher risk of IgE-mediated reaction to penicillins. However, in a situation analogous to that in people with food allergy who have a food-specific IgE titer lower than the empirically established threshold value (Table 1), low-titer values to penicillin may not predict anaphylactic sensitivity in a penicillin oral challenge.28 Further studies are needed to determine if there is a threshold level of penicillin-specific IgE above which a patient has a higher likelihood of an IgE-mediated systemic reaction.
Other drugs. Specific IgE blood tests are also available for certain neuromuscular agents, insulin, cefaclor (Ceclor), chlorhexidine (contained in various antiseptic products), and gelatin (Table 2). These substances have not been as well studied as penicillins, and the sensitivity and specificity data reported in Table 2 are limited by few studies and small study sizes.
Neuromuscular blocking agents. Tests for IgE against neuromuscular blocking agents are reported to have low sensitivity (30%–60%) using a cutoff value of 0.35 kU/L.30 In small studies, the sensitivity was higher (68% to 92%) when threshold values for rocuronium-specific IgE were lowered from 0.35 to 0.13 kU/L.29
Chlorhexidine, an antiseptic commonly used in surgery, has been linked to IgE-mediated reactions.31 Chlorhexidine-specific IgE levels greater than 0.35 kU/L are considered positive, based on very limited data.
Insulin. Blood tests for allergy to insulin are also commercially available. However, studies have shown a significant overlap in the range of insulin-specific IgE in patients with a clinical history consistent with insulin allergy and in controls. Therefore, this test has a very limited ability to distinguish people who do not have a history of a reaction to insulin.32 More research is needed to determine the clinical utility of insulin-specific IgE testing.
Gelatin. IgE-mediated reactions have occurred after exposure to gelatin (from either cows or pigs) contained in foods and vaccines, including measles-mumps-rubella and yellow fever. One study identified gelatin-specific IgE in 10 of 11 children with a history of systemic reaction to measles or mumps vaccine.33 In the same study, gelatin-specific IgE levels were negative in 24 children who had developed non-IgE-mediated reactions to the vaccine.33
Tests for IgE against bovine gelatin are commercially available; results are considered positive for values higher than 0.35 kU/L. A negative test result does not exclude the possibility of an allergic reaction to porcine gelatin, which can also be found in foods and vaccines, but tests for anti-porcine gelatin IgE are not commercially available.
Latex
Latex, obtained from the rubber tree Hevea brasiliensis, has 13 known polypeptides (allergens Hev b 1–13) that cause IgE-mediated reactions, particularly in health care workers and patients with spina bifida.34 Overall, the incidence of latex allergy has decreased in the United States as most medical institutions have implemented a latex-free environment.
In vitro testing is the only mode of evaluation for allergy to latex approved by the US Food and Drug Administration (FDA).35 Its sensitivity is 80% and its specificity is 95%.36
In a 2007 study, 145 people at risk for latex allergy, including 104 health care workers, 31 patients with spina bifida, and 10 patients requiring multiple surgeries, underwent latex-specific IgE analysis for sensitivity to various recombinant and native latex allergens.34 The three groups differed in their latex allergy profiles, highlighting the diversity of clinical response to latex in high-risk groups and our current inability to establish specific cutoff points for quantitative latex-specific IgE. Thus, at present, any nonzero latex-specific IgE value is considered positive.
A formal evaluation for allergy is recommended for patients who have a strong history of an IgE-mediated reaction to latex and a latex-specific IgE value of zero. Blood tests for allergy to some native or recombinant latex allergens are available; these allergens may be underrepresented in the native total latex extract.33 Skin testing for allergy to latex, although not FDA-approved or standardized, can also be useful in this setting.37
Insect venom
Type I hypersensitivity reactions can occur from the stings of Vespidae (vespids), Apidae (bees), and Formicidae (fire ants). Large localized reactions after an insect sting are not infrequent and typically do not predict anaphylactic sensitivity with future stings, even though they are considered mild IgE-mediated reactions. However, systemic reactions are considered life-threatening and warrant allergy testing.38
The level of venom-specific IgE usually increases weeks to months after a sting.39 Therefore, blood tests can be falsely negative if performed within a short time of the sting.
Patients who have suffered a systemic reaction to venom and have evidence of sensitization by either in vitro or in vivo allergy testing are candidates for venom immunotherapy.40
At present, any nonzero venom-specific IgE test is considered positive, as there is no specific value for venom-specific IgE that predicts clinical risk.
A negative blood test does not exclude the possibility of an IgE-mediated reaction.41 In cases in which a patient has a clinical history compatible with venom allergy but the blood test is negative, the patient should be referred to an allergist for further evaluation, including venom skin testing and possibly repeat blood testing at a later time.
Conversely, specific IgE testing to venom is recommended when a patient has a history consistent with venom allergy and negative skin test results.38
As mentioned previously, in vitro test performance can vary with the laboratory and testing method used, and sending samples directly to a reference laboratory could be considered.41
TESTING FOR IgG AGAINST FOODS IS UNVALIDATED AND INAPPROPRIATE
In recent years, some practitioners of alternative medicine have started testing for allergen-specific IgG or IgG4 as part of evaluations for hypersensitivity, especially in cases in which patients describe atypical gastrointestinal, neurologic, or other symptoms after eating specific foods.19
However, this testing often finds IgG or IgG4 against foods that are well tolerated. At present, allergen-specific IgG testing lacks scientific evidence to support its clinical use in the evaluation of allergic disease.5,19
- Williams PB, Ahlstedt S, Barnes JH, Söderström L, Portnoy J. Are our impressions of allergy test performances correct? Ann Allergy Asthma Immunol 2003; 91:26–33.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Pichler WJ. Immune mechanism of drug hypersensitivity. Immunol Allergy Clin North Am 2004; 24:373–397.
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–480.
- Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010; 125(suppl 2):S284–S296.
- Cox L, Williams B, Sicherer S, et al; American College of Allergy, Asthma and Immunology Test Task Force; American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 2008; 101:580–592.
- Hamilton RG, Franklin Adkinson N. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol 2004; 114:213–225.
- Williams PB, Dolen WK, Koepke JW, Selner JC. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy 1992; 68:35–45.
- Howanitz PJ, Cembrowski GS, Bachner P. Laboratory phlebotomy. College of American Pathologists Q-Probe study of patient satisfaction and complications in 23,783 patients. Arch Pathol Lab Med 1991; 115:867–872.
- Turkeltaub PC, Gergen PJ. The risk of adverse reactions from percutaneous prick-puncture allergen skin testing, venipuncture, and body measurements: data from the second National Health and Nutrition Examination Survey 1976–80 (NHANES II). J Allergy Clin Immunol 1989; 84:886–890.
- Pipkorn U, Hammarlund A, Enerbäck L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 1989; 19:19–25.
- Cole ZA, Clough GF, Church MK. Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br J Pharmacol 2001; 132:286–292.
- Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol 1996; 98:522–527.
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011; 106:153–158.
- Hamilton RG, Williams PB; Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology; American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol 2010; 126:33–38.
- Somville MA, Machiels J, Gilles JG, Saint-Remy JM. Seasonal variation in specific IgE antibodies of grass-pollen hypersensitive patients depends on the steady state IgE concentration and is not related to clinical symptoms. J Allergy Clin Immunol 1989; 83( 2 Pt 1):486–494.
- Poon AW, Goodman CS, Rubin RJ. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Manag Care 1998; 4:969–985.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol 1994; 93:1068–1070.
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001; 86:196–202.
- Söderström L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy 2003; 58:921–928.
- Bousquet J, Lebel B, Dhivert H, Bataille Y, Martinot B, Michel FB. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin Allergy 1987; 17:529–536.
- Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc 2003; 24:253–258.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Mayorga C, Sanz ML, Gamboa PM, et al; Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010; 20:103–109.
- Garcia JJ, Blanca M, Moreno F, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal 1997; 11:251–257.
- Macy E, Goldberg B, Poon KY. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol 2010; 105:136–141.
- Blanca M, Mayorga C, Torres MJ, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001; 56:862–870.
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007; 107:253–259.
- Ebo DG, Bridts CH, Stevens WJ. IgE-mediated anaphylaxis from chlorhexidine: diagnostic possibilities. Contact Dermatitis 2006; 55:301–302.
- deShazo RD, Mather P, Grant W, et al. Evaluation of patients with local reactions to insulin with skin tests and in vitro techniques. Diabetes Care 1987; 10:330–336.
- Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol 1995; 96:563–565.
- Raulf-Heimsoth M, Rihs HP, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy 2007; 37:1657–1667.
- Biagini RE, MacKenzie BA, Sammons DL, et al. Latex specific IgE: performance characteristics of the IMMULITE 2000 3gAllergy assay compared with skin testing. Ann Allergy Asthma Immunol 2006; 97:196–202.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S47–S56.
- Safadi GS, Corey EC, Taylor JS, Wagner WO, Pien LC, Melton AL. Latex hypersensitivity in emergency medical service providers. Ann Allergy Asthma Immunol 1996; 77:39–42.
- Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004; 114:869–886.
- Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339–1349.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001; 107:897–901.
Health care providers often need to evaluate allergic disorders such as allergic rhinoconjunctivitis, asthma, and allergies to foods, drugs, latex, and venom, both in the hospital and in the clinic.
Unfortunately, some symptoms, such as chronic nasal symptoms, can occur in both allergic and nonallergic disorders, and this overlap can confound the diagnosis and therapy. Studies suggest that when clinicians use the history and physical examination alone in evaluating possible allergic disease, the accuracy of their diagnoses rarely exceeds 50%.1
Blood tests are now available that measure immunoglobulin E (IgE) directed against specific antigens. These in vitro tests can be important tools in assessing a patient whose history suggests an allergic disease.2 However, neither allergy skin testing nor these blood tests are intended to be used for screening: they may be most useful as confirmatory diagnostic tests in cases in which the pretest clinical impression of allergic disease is high.
ALLERGY IS MEDIATED BY IgE
In susceptible people, IgE is produced by B cells in response to specific antigens such as foods, pollens, latex, and drugs. This antigen-specific (or allergen-specific) IgE circulates in the serum and binds to high-affinity IgE receptors on immune effector cells such as mast cells located throughout the body.
Upon subsequent exposure to the same allergen, IgE receptors cross-link and initiate downstream signaling events that trigger mast cell degranulation and an immediate allergic response—hence the term immediate (or Gell-Coombs type I) hypersensitivity.3
Common manifestations of type I hypersensitivity reactions include signs and symptoms that can be:
- Cutaneous (eg, acute urticaria, angioedema)
- Respiratory (eg, acute bronchospasm, rhinoconjunctivitis)
- Cardiovascular (eg, tachycardia, hypotension)
- Gastrointestinal (eg, vomiting, diarrhea)
- Generalized (eg, anaphylactic shock). By definition, anaphylaxis is a life-threatening reaction that occurs on exposure to an allergen and involves acute respiratory distress, cardiovascular failure, or involvement of two or more organ systems.4
MOST IgE BLOOD TESTS ARE IMMUNOASSAYS
The blood tests for allergic disease are immunoassays that measure the level of IgE specific to a particular allergen. The tests can be used to evaluate sensitivity to various allergens, for example, to common inhalants such as dust mites and pollens and to foods, drugs, venom, and latex.
Types of immunoassays include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), and radioallergosorbent assays (RASTs). At present, most commercial laboratories use one of three autoanalyzer systems to measure specific IgE:
- ImmunoCAP (Phadia AB, Uppsala, Sweden)
- Immulite (Siemens AG, Berlin, Germany)
- HYTEC-288 (Hycor/Agilent, Garden Grove, CA).
These systems use a solid-phase polymer (cellulose or avidin) in which the antigen is embedded. The polymer also facilitates binding of IgE and, therefore, increases the sensitivity of the test.5 Specific IgE from the patient’s serum binds to the allergen embedded in the polymer, and then unbound antibodies are washed off.
Despite the term “RAST,” these systems do not use radiation. A fluorescent antibody is added that binds to the patient’s IgE, and the amount of IgE present is calculated from the amount of fluorescence.6 Results are reported in kilounits of antibody per liter (kU/L) or nanograms per milliliter (ng/mL).5–7
INTERPRETATION IS INDIVIDUALIZED
In general, the sensitivity of these tests ranges from 60% to 95% and their specificity from 30% to 95%, with a concordance among different immunoassays of 75% to 90%.8
Levels of IgE for a particular allergen are also divided into semiquantitative classes, from class I to class V or VI. In general, class I and class II correlate with a low level of allergen sensitization and, often, with a low likelihood of a clinical reaction. On the other hand, classes V and VI reflect higher degrees of sensitization and generally correlate with IgE-mediated clinical reactions upon allergen exposure.
The interpretation of a positive (ie, “nonzero”) test result must be individualized on the basis of clinical presentation and risk factors. A specialist can make an important contribution by helping to interpret any positive test result or a negative test result that does not correlate with the patient’s history.
ADVANTAGES OF ALLERGY BLOOD TESTING
Allergy blood testing is convenient, since it involves only a standard blood draw.
In theory, allergy blood testing may be safer, since it does not expose the patient to any allergens. On the other hand, many patients experience bruising from venipuncture performed for any reason: 16% in one survey.9 In another survey,10 adverse reactions of any type occurred in 0.49% of patients undergoing venipuncture but only in 0.04% of those undergoing allergy skin testing. Therefore, allergy blood testing may be most appropriate in situations in which a patient’s history suggests that he or she may be at risk of a systemic reaction from a traditional skin test or in cases in which skin testing is not possible (eg, extensive eczema).
Another advantage of allergy blood testing is that it is not affected by drugs such as antihistamines or tricyclic antidepressants that suppress the histamine response, which is a problem with skin testing.
Allergy blood testing may also be useful in patients on long-term glucocorticoid therapy, although the data conflict. Prolonged oral glucocorticoid use is associated with a decrease in mast cell density and histamine content in the skin,11,12 although in one study a corticosteroid was found not to affect the results of skin-prick testing for allergy.13 Thus, allergy blood testing can be performed in patients who have severe eczema or dermatographism or who cannot safely suspend taking antihistamines or tricyclic antidepressants.
LIMITATIONS OF THESE TESTS
A limitation of allergy blood tests is that there is no gold-standard test for many allergic conditions. (Double-blind, placebo-controlled oral food challenge testing has been proposed as the gold-standard test for food allergy, and nasal allergen provocation challenge has been proposed for allergic rhinitis.)
Also, allergy blood tests can give false-positive results because of nonspecific binding of antibody in the assay.
Of note: evidence of sensitization to a particular allergen (ie, a positive blood test result) is not synonymous with clinically relevant disease (ie, clinical sensitivity).
Conversely, these tests can give false-negative results in patients who have true IgE-mediated disease as confirmed by skin testing or allergen challenge. The sensitivity of blood allergy testing is approximately 25% to 30% lower than that of skin testing, based on comparative studies.2 The blood tests are usually considered positive if the allergen-specific IgE level is greater than 0.35 kU/L; however, sensitization to certain inhalant allergens can occur at levels as low as 0.12 kU/L.14
Specific IgE levels measured by different commercial assays are not always interchangeable or equivalent, so a clinician should consistently select the same immunoassay if possible when assessing any given patient over time.15
Levels of specific IgE have been shown to depend on age, allergen specificity, total serum IgE, and, with inhalant allergens, the season of the year.15,16
Other limitations of blood testing are its cost and a delay of several days to a week in obtaining the results.17
WHEN TO ORDER ALLERGY BLOOD TESTING
The allergy evaluation should begin with a thorough history to look for possible triggers for the patient’s symptoms.
For example, respiratory conditions such as asthma and rhinitis may be exacerbated during particular times of the year when certain pollens are commonly present. For patients with this pattern, blood testing for allergy to common inhalants, including pollens, may be appropriate. Similarly, peanut allergy evaluation is indicated for a child who has suffered an anaphylactic reaction after consuming peanut butter. Blood testing is also indicated in patients with a history of venom anaphylaxis, especially if venom skin testing was negative.
In cases in which the patient does not have a clear history of sensitization, blood testing for allergy to multiple foods may find evidence of sensitization that does not necessarily correlate with clinical disease.18
Likewise, blood tests are not likely to be clinically relevant in conditions not mediated by IgE, such as food intolerances (eg, lactose intolerance), celiac disease, the DRESS syndrome (drug rash, eosinophilia, and systemic symptoms), Stevens-Johnson syndrome, toxic epidermal necrolysis, or other types of drug hypersensitivity reactions, such as serum sickness.3
INTERPRETING COMMONLY ORDERED BLOOD TESTS FOR ALLERGY
Tests for allergy to hundreds of substances are available.
Foods
Milk, eggs, soy, wheat, peanuts, tree nuts, fish, and shellfish account for most cases of food allergy in the United States.18
IgE-mediated hypersensitivity to milk, eggs, and peanuts tends to be more common in children, whereas peanuts, tree nuts, fish, and shellfish are more commonly associated with reactions in adults.18 Children are more likely to outgrow allergy to milk, soy, wheat, and eggs than allergy to peanuts, tree nuts, fish, and shellfish—only about 20% of children outgrow peanut allergy.18
Patients with an IgE-mediated reaction to foods should be closely followed by a specialist, who can best help determine the appropriateness of additional testing (such as an oral challenge under observation), avoidance recommendations, and the introduction of foods back into the diet.19
Specific IgE tests for allergy to a variety of foods are available and can be very useful for diagnosis when used in the appropriate setting.
One caveat about these studies is that many were initially performed in children with a history of food allergy, many of whom had atopic dermatitis, and the findings have not been systematically reexamined in larger studies in more heterogeneous populations.
For example, at least eight studies tried to identify a diagnostic IgE level for cow’s milk allergy. The 95% confidence intervals varied widely, depending on the study design, the age of the study population, the prevalence of food allergy in the population, and the statistical method used for analysis.5 For most other foods for which blood tests are available, few studies have been performed to establish predictive values similar to those in Table 1.
Thus, slight elevations in antigen-specific IgE (> 0.35 kU/L) may correlate only with in vitro sensitization in a patient who has no clinical reactivity upon oral exposure to a particular antigen.
Broad food panels have been shown to have false-positive rates higher than 50%—ie, in more than half of cases, positive results have no clinical relevance. Therefore, these large food panels should not be used for screening.19 Instead, it is recommended that tests be limited to relevant foods based on the patient’s history when evaluating symptoms consistent with an IgE-mediated reaction to a particular food.
Food-specific IgE evaluation is also not helpful in evaluating non-IgE adverse reactions to foods (eg, intolerances).
Therefore, the patient’s history remains the most important tool for evaluation of food allergy. In cases in which the patient’s history suggests a food-associated IgE-mediated reaction and the blood test is negative, the patient should be referred to a specialist for skin testing with commercial extracts or even fresh food extracts, given the higher sensitivity of in vivo testing.20
Inhalants
Common aeroallergens associated with allergic rhinitis, allergic conjunctivitis, and allergic asthma include dust mites, animal dander, cockroach debris, molds, trees, grasses, weeds, and ragweed. Dust mites, animal dander, and mold spores are perennial allergens and may trigger symptoms year-round. Pollen, including pollen from trees, grasses, and weeds, is generally present in a seasonal pattern in many parts of the United States.
A positive blood test for an inhalant allergen can reinforce the physician’s clinical impression in making a diagnosis of allergic rhinoconjunctivitis. Interestingly, studies have suggested a high rate of false-positives based on history alone when in vivo and in vitro allergy testing were negative for IgE-mediated respiratory disease.21
Various studies have aimed to establish threshold values of aeroallergen-specific IgE that predict the likelihood of clinically relevant disease. Unfortunately, other factors also contribute to clinical symptoms of rhinoconjunctivitis; these include concurrent inflammation, infection, physical stress, psychological stress, exposure to irritants, and hormonal changes. These factors introduce variability and make specific IgE cutoffs for inhalant allergens unreliable.22
Prospective studies have suggested that skin testing correlates better with nasal allergen challenge (the gold standard) than blood testing for the diagnosis of inhalant allergy, though more recent studies using modern technologies demonstrate reasonable concordance (67%) between skin testing and blood testing (specifically, ImmunoCAP).23,24 According to current guidelines, skin tests are the preferred method for diagnosing IgE-mediated sensitivity to inhalants.25
Compared with skin prick tests as the gold standard, the sensitivity of specific IgE immunoassays is approximately 70% to 75%.25 Nevertheless, specific IgE values greater than 0.35 kU/L are generally considered positive for aeroallergen sensitization, although lower levels of dog-specific IgE have recently been shown to correlate with clinical disease.14
Drugs, including penicillins
A variety of clinical reactions can occur in response to oral, intravenous, or topical medications.
At present, blood tests are available for the evaluation of IgE-mediated adverse reactions to only a limited number of drugs. Reactions involving other mechanisms, such as those related to the drug’s metabolism, intolerances (eg, nausea), idiosyncratic reactions (eg, Stevens-Johnson syndrome, the DRESS syndrome), or other types of reactions can be diagnosed only by history and physical examination.
The development of specific IgE tests for sensitivity to medications has been limited by incomplete characterization of metabolic products and the possibility that a single medication can have different epitopes or IgE binding sites in different individuals.26
In vitro allergy testing has been most studied for beta-lactam antibiotics (eg, penicillin) and not so much for other drugs.
Table 2 summarizes the sensitivity and specificity of blood allergy tests that are commercially available for drugs.
Penicillin, a beta-lactam antibiotic, is degraded into various metabolites known as the major determinant (penicilloyl) and the minor determinants (eg, benzylpenicilloate and benzylpenilloate), which act as haptens. Specific IgE testing is not available for all these determinants.
The sensitivity of blood tests for allergy to penicilloyl (penicillin) and amino-penicillins such as amoxicilloyl (amoxicillin) is reported as between 32% and 50%, and the specificity as 96% to 98%.29
By definition, any nonzero level of IgE specific for penicillin or its derivatives is considered a positive result and may be associated with a higher risk of IgE-mediated reaction to penicillins. However, in a situation analogous to that in people with food allergy who have a food-specific IgE titer lower than the empirically established threshold value (Table 1), low-titer values to penicillin may not predict anaphylactic sensitivity in a penicillin oral challenge.28 Further studies are needed to determine if there is a threshold level of penicillin-specific IgE above which a patient has a higher likelihood of an IgE-mediated systemic reaction.
Other drugs. Specific IgE blood tests are also available for certain neuromuscular agents, insulin, cefaclor (Ceclor), chlorhexidine (contained in various antiseptic products), and gelatin (Table 2). These substances have not been as well studied as penicillins, and the sensitivity and specificity data reported in Table 2 are limited by few studies and small study sizes.
Neuromuscular blocking agents. Tests for IgE against neuromuscular blocking agents are reported to have low sensitivity (30%–60%) using a cutoff value of 0.35 kU/L.30 In small studies, the sensitivity was higher (68% to 92%) when threshold values for rocuronium-specific IgE were lowered from 0.35 to 0.13 kU/L.29
Chlorhexidine, an antiseptic commonly used in surgery, has been linked to IgE-mediated reactions.31 Chlorhexidine-specific IgE levels greater than 0.35 kU/L are considered positive, based on very limited data.
Insulin. Blood tests for allergy to insulin are also commercially available. However, studies have shown a significant overlap in the range of insulin-specific IgE in patients with a clinical history consistent with insulin allergy and in controls. Therefore, this test has a very limited ability to distinguish people who do not have a history of a reaction to insulin.32 More research is needed to determine the clinical utility of insulin-specific IgE testing.
Gelatin. IgE-mediated reactions have occurred after exposure to gelatin (from either cows or pigs) contained in foods and vaccines, including measles-mumps-rubella and yellow fever. One study identified gelatin-specific IgE in 10 of 11 children with a history of systemic reaction to measles or mumps vaccine.33 In the same study, gelatin-specific IgE levels were negative in 24 children who had developed non-IgE-mediated reactions to the vaccine.33
Tests for IgE against bovine gelatin are commercially available; results are considered positive for values higher than 0.35 kU/L. A negative test result does not exclude the possibility of an allergic reaction to porcine gelatin, which can also be found in foods and vaccines, but tests for anti-porcine gelatin IgE are not commercially available.
Latex
Latex, obtained from the rubber tree Hevea brasiliensis, has 13 known polypeptides (allergens Hev b 1–13) that cause IgE-mediated reactions, particularly in health care workers and patients with spina bifida.34 Overall, the incidence of latex allergy has decreased in the United States as most medical institutions have implemented a latex-free environment.
In vitro testing is the only mode of evaluation for allergy to latex approved by the US Food and Drug Administration (FDA).35 Its sensitivity is 80% and its specificity is 95%.36
In a 2007 study, 145 people at risk for latex allergy, including 104 health care workers, 31 patients with spina bifida, and 10 patients requiring multiple surgeries, underwent latex-specific IgE analysis for sensitivity to various recombinant and native latex allergens.34 The three groups differed in their latex allergy profiles, highlighting the diversity of clinical response to latex in high-risk groups and our current inability to establish specific cutoff points for quantitative latex-specific IgE. Thus, at present, any nonzero latex-specific IgE value is considered positive.
A formal evaluation for allergy is recommended for patients who have a strong history of an IgE-mediated reaction to latex and a latex-specific IgE value of zero. Blood tests for allergy to some native or recombinant latex allergens are available; these allergens may be underrepresented in the native total latex extract.33 Skin testing for allergy to latex, although not FDA-approved or standardized, can also be useful in this setting.37
Insect venom
Type I hypersensitivity reactions can occur from the stings of Vespidae (vespids), Apidae (bees), and Formicidae (fire ants). Large localized reactions after an insect sting are not infrequent and typically do not predict anaphylactic sensitivity with future stings, even though they are considered mild IgE-mediated reactions. However, systemic reactions are considered life-threatening and warrant allergy testing.38
The level of venom-specific IgE usually increases weeks to months after a sting.39 Therefore, blood tests can be falsely negative if performed within a short time of the sting.
Patients who have suffered a systemic reaction to venom and have evidence of sensitization by either in vitro or in vivo allergy testing are candidates for venom immunotherapy.40
At present, any nonzero venom-specific IgE test is considered positive, as there is no specific value for venom-specific IgE that predicts clinical risk.
A negative blood test does not exclude the possibility of an IgE-mediated reaction.41 In cases in which a patient has a clinical history compatible with venom allergy but the blood test is negative, the patient should be referred to an allergist for further evaluation, including venom skin testing and possibly repeat blood testing at a later time.
Conversely, specific IgE testing to venom is recommended when a patient has a history consistent with venom allergy and negative skin test results.38
As mentioned previously, in vitro test performance can vary with the laboratory and testing method used, and sending samples directly to a reference laboratory could be considered.41
TESTING FOR IgG AGAINST FOODS IS UNVALIDATED AND INAPPROPRIATE
In recent years, some practitioners of alternative medicine have started testing for allergen-specific IgG or IgG4 as part of evaluations for hypersensitivity, especially in cases in which patients describe atypical gastrointestinal, neurologic, or other symptoms after eating specific foods.19
However, this testing often finds IgG or IgG4 against foods that are well tolerated. At present, allergen-specific IgG testing lacks scientific evidence to support its clinical use in the evaluation of allergic disease.5,19
Health care providers often need to evaluate allergic disorders such as allergic rhinoconjunctivitis, asthma, and allergies to foods, drugs, latex, and venom, both in the hospital and in the clinic.
Unfortunately, some symptoms, such as chronic nasal symptoms, can occur in both allergic and nonallergic disorders, and this overlap can confound the diagnosis and therapy. Studies suggest that when clinicians use the history and physical examination alone in evaluating possible allergic disease, the accuracy of their diagnoses rarely exceeds 50%.1
Blood tests are now available that measure immunoglobulin E (IgE) directed against specific antigens. These in vitro tests can be important tools in assessing a patient whose history suggests an allergic disease.2 However, neither allergy skin testing nor these blood tests are intended to be used for screening: they may be most useful as confirmatory diagnostic tests in cases in which the pretest clinical impression of allergic disease is high.
ALLERGY IS MEDIATED BY IgE
In susceptible people, IgE is produced by B cells in response to specific antigens such as foods, pollens, latex, and drugs. This antigen-specific (or allergen-specific) IgE circulates in the serum and binds to high-affinity IgE receptors on immune effector cells such as mast cells located throughout the body.
Upon subsequent exposure to the same allergen, IgE receptors cross-link and initiate downstream signaling events that trigger mast cell degranulation and an immediate allergic response—hence the term immediate (or Gell-Coombs type I) hypersensitivity.3
Common manifestations of type I hypersensitivity reactions include signs and symptoms that can be:
- Cutaneous (eg, acute urticaria, angioedema)
- Respiratory (eg, acute bronchospasm, rhinoconjunctivitis)
- Cardiovascular (eg, tachycardia, hypotension)
- Gastrointestinal (eg, vomiting, diarrhea)
- Generalized (eg, anaphylactic shock). By definition, anaphylaxis is a life-threatening reaction that occurs on exposure to an allergen and involves acute respiratory distress, cardiovascular failure, or involvement of two or more organ systems.4
MOST IgE BLOOD TESTS ARE IMMUNOASSAYS
The blood tests for allergic disease are immunoassays that measure the level of IgE specific to a particular allergen. The tests can be used to evaluate sensitivity to various allergens, for example, to common inhalants such as dust mites and pollens and to foods, drugs, venom, and latex.
Types of immunoassays include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), and radioallergosorbent assays (RASTs). At present, most commercial laboratories use one of three autoanalyzer systems to measure specific IgE:
- ImmunoCAP (Phadia AB, Uppsala, Sweden)
- Immulite (Siemens AG, Berlin, Germany)
- HYTEC-288 (Hycor/Agilent, Garden Grove, CA).
These systems use a solid-phase polymer (cellulose or avidin) in which the antigen is embedded. The polymer also facilitates binding of IgE and, therefore, increases the sensitivity of the test.5 Specific IgE from the patient’s serum binds to the allergen embedded in the polymer, and then unbound antibodies are washed off.
Despite the term “RAST,” these systems do not use radiation. A fluorescent antibody is added that binds to the patient’s IgE, and the amount of IgE present is calculated from the amount of fluorescence.6 Results are reported in kilounits of antibody per liter (kU/L) or nanograms per milliliter (ng/mL).5–7
INTERPRETATION IS INDIVIDUALIZED
In general, the sensitivity of these tests ranges from 60% to 95% and their specificity from 30% to 95%, with a concordance among different immunoassays of 75% to 90%.8
Levels of IgE for a particular allergen are also divided into semiquantitative classes, from class I to class V or VI. In general, class I and class II correlate with a low level of allergen sensitization and, often, with a low likelihood of a clinical reaction. On the other hand, classes V and VI reflect higher degrees of sensitization and generally correlate with IgE-mediated clinical reactions upon allergen exposure.
The interpretation of a positive (ie, “nonzero”) test result must be individualized on the basis of clinical presentation and risk factors. A specialist can make an important contribution by helping to interpret any positive test result or a negative test result that does not correlate with the patient’s history.
ADVANTAGES OF ALLERGY BLOOD TESTING
Allergy blood testing is convenient, since it involves only a standard blood draw.
In theory, allergy blood testing may be safer, since it does not expose the patient to any allergens. On the other hand, many patients experience bruising from venipuncture performed for any reason: 16% in one survey.9 In another survey,10 adverse reactions of any type occurred in 0.49% of patients undergoing venipuncture but only in 0.04% of those undergoing allergy skin testing. Therefore, allergy blood testing may be most appropriate in situations in which a patient’s history suggests that he or she may be at risk of a systemic reaction from a traditional skin test or in cases in which skin testing is not possible (eg, extensive eczema).
Another advantage of allergy blood testing is that it is not affected by drugs such as antihistamines or tricyclic antidepressants that suppress the histamine response, which is a problem with skin testing.
Allergy blood testing may also be useful in patients on long-term glucocorticoid therapy, although the data conflict. Prolonged oral glucocorticoid use is associated with a decrease in mast cell density and histamine content in the skin,11,12 although in one study a corticosteroid was found not to affect the results of skin-prick testing for allergy.13 Thus, allergy blood testing can be performed in patients who have severe eczema or dermatographism or who cannot safely suspend taking antihistamines or tricyclic antidepressants.
LIMITATIONS OF THESE TESTS
A limitation of allergy blood tests is that there is no gold-standard test for many allergic conditions. (Double-blind, placebo-controlled oral food challenge testing has been proposed as the gold-standard test for food allergy, and nasal allergen provocation challenge has been proposed for allergic rhinitis.)
Also, allergy blood tests can give false-positive results because of nonspecific binding of antibody in the assay.
Of note: evidence of sensitization to a particular allergen (ie, a positive blood test result) is not synonymous with clinically relevant disease (ie, clinical sensitivity).
Conversely, these tests can give false-negative results in patients who have true IgE-mediated disease as confirmed by skin testing or allergen challenge. The sensitivity of blood allergy testing is approximately 25% to 30% lower than that of skin testing, based on comparative studies.2 The blood tests are usually considered positive if the allergen-specific IgE level is greater than 0.35 kU/L; however, sensitization to certain inhalant allergens can occur at levels as low as 0.12 kU/L.14
Specific IgE levels measured by different commercial assays are not always interchangeable or equivalent, so a clinician should consistently select the same immunoassay if possible when assessing any given patient over time.15
Levels of specific IgE have been shown to depend on age, allergen specificity, total serum IgE, and, with inhalant allergens, the season of the year.15,16
Other limitations of blood testing are its cost and a delay of several days to a week in obtaining the results.17
WHEN TO ORDER ALLERGY BLOOD TESTING
The allergy evaluation should begin with a thorough history to look for possible triggers for the patient’s symptoms.
For example, respiratory conditions such as asthma and rhinitis may be exacerbated during particular times of the year when certain pollens are commonly present. For patients with this pattern, blood testing for allergy to common inhalants, including pollens, may be appropriate. Similarly, peanut allergy evaluation is indicated for a child who has suffered an anaphylactic reaction after consuming peanut butter. Blood testing is also indicated in patients with a history of venom anaphylaxis, especially if venom skin testing was negative.
In cases in which the patient does not have a clear history of sensitization, blood testing for allergy to multiple foods may find evidence of sensitization that does not necessarily correlate with clinical disease.18
Likewise, blood tests are not likely to be clinically relevant in conditions not mediated by IgE, such as food intolerances (eg, lactose intolerance), celiac disease, the DRESS syndrome (drug rash, eosinophilia, and systemic symptoms), Stevens-Johnson syndrome, toxic epidermal necrolysis, or other types of drug hypersensitivity reactions, such as serum sickness.3
INTERPRETING COMMONLY ORDERED BLOOD TESTS FOR ALLERGY
Tests for allergy to hundreds of substances are available.
Foods
Milk, eggs, soy, wheat, peanuts, tree nuts, fish, and shellfish account for most cases of food allergy in the United States.18
IgE-mediated hypersensitivity to milk, eggs, and peanuts tends to be more common in children, whereas peanuts, tree nuts, fish, and shellfish are more commonly associated with reactions in adults.18 Children are more likely to outgrow allergy to milk, soy, wheat, and eggs than allergy to peanuts, tree nuts, fish, and shellfish—only about 20% of children outgrow peanut allergy.18
Patients with an IgE-mediated reaction to foods should be closely followed by a specialist, who can best help determine the appropriateness of additional testing (such as an oral challenge under observation), avoidance recommendations, and the introduction of foods back into the diet.19
Specific IgE tests for allergy to a variety of foods are available and can be very useful for diagnosis when used in the appropriate setting.
One caveat about these studies is that many were initially performed in children with a history of food allergy, many of whom had atopic dermatitis, and the findings have not been systematically reexamined in larger studies in more heterogeneous populations.
For example, at least eight studies tried to identify a diagnostic IgE level for cow’s milk allergy. The 95% confidence intervals varied widely, depending on the study design, the age of the study population, the prevalence of food allergy in the population, and the statistical method used for analysis.5 For most other foods for which blood tests are available, few studies have been performed to establish predictive values similar to those in Table 1.
Thus, slight elevations in antigen-specific IgE (> 0.35 kU/L) may correlate only with in vitro sensitization in a patient who has no clinical reactivity upon oral exposure to a particular antigen.
Broad food panels have been shown to have false-positive rates higher than 50%—ie, in more than half of cases, positive results have no clinical relevance. Therefore, these large food panels should not be used for screening.19 Instead, it is recommended that tests be limited to relevant foods based on the patient’s history when evaluating symptoms consistent with an IgE-mediated reaction to a particular food.
Food-specific IgE evaluation is also not helpful in evaluating non-IgE adverse reactions to foods (eg, intolerances).
Therefore, the patient’s history remains the most important tool for evaluation of food allergy. In cases in which the patient’s history suggests a food-associated IgE-mediated reaction and the blood test is negative, the patient should be referred to a specialist for skin testing with commercial extracts or even fresh food extracts, given the higher sensitivity of in vivo testing.20
Inhalants
Common aeroallergens associated with allergic rhinitis, allergic conjunctivitis, and allergic asthma include dust mites, animal dander, cockroach debris, molds, trees, grasses, weeds, and ragweed. Dust mites, animal dander, and mold spores are perennial allergens and may trigger symptoms year-round. Pollen, including pollen from trees, grasses, and weeds, is generally present in a seasonal pattern in many parts of the United States.
A positive blood test for an inhalant allergen can reinforce the physician’s clinical impression in making a diagnosis of allergic rhinoconjunctivitis. Interestingly, studies have suggested a high rate of false-positives based on history alone when in vivo and in vitro allergy testing were negative for IgE-mediated respiratory disease.21
Various studies have aimed to establish threshold values of aeroallergen-specific IgE that predict the likelihood of clinically relevant disease. Unfortunately, other factors also contribute to clinical symptoms of rhinoconjunctivitis; these include concurrent inflammation, infection, physical stress, psychological stress, exposure to irritants, and hormonal changes. These factors introduce variability and make specific IgE cutoffs for inhalant allergens unreliable.22
Prospective studies have suggested that skin testing correlates better with nasal allergen challenge (the gold standard) than blood testing for the diagnosis of inhalant allergy, though more recent studies using modern technologies demonstrate reasonable concordance (67%) between skin testing and blood testing (specifically, ImmunoCAP).23,24 According to current guidelines, skin tests are the preferred method for diagnosing IgE-mediated sensitivity to inhalants.25
Compared with skin prick tests as the gold standard, the sensitivity of specific IgE immunoassays is approximately 70% to 75%.25 Nevertheless, specific IgE values greater than 0.35 kU/L are generally considered positive for aeroallergen sensitization, although lower levels of dog-specific IgE have recently been shown to correlate with clinical disease.14
Drugs, including penicillins
A variety of clinical reactions can occur in response to oral, intravenous, or topical medications.
At present, blood tests are available for the evaluation of IgE-mediated adverse reactions to only a limited number of drugs. Reactions involving other mechanisms, such as those related to the drug’s metabolism, intolerances (eg, nausea), idiosyncratic reactions (eg, Stevens-Johnson syndrome, the DRESS syndrome), or other types of reactions can be diagnosed only by history and physical examination.
The development of specific IgE tests for sensitivity to medications has been limited by incomplete characterization of metabolic products and the possibility that a single medication can have different epitopes or IgE binding sites in different individuals.26
In vitro allergy testing has been most studied for beta-lactam antibiotics (eg, penicillin) and not so much for other drugs.
Table 2 summarizes the sensitivity and specificity of blood allergy tests that are commercially available for drugs.
Penicillin, a beta-lactam antibiotic, is degraded into various metabolites known as the major determinant (penicilloyl) and the minor determinants (eg, benzylpenicilloate and benzylpenilloate), which act as haptens. Specific IgE testing is not available for all these determinants.
The sensitivity of blood tests for allergy to penicilloyl (penicillin) and amino-penicillins such as amoxicilloyl (amoxicillin) is reported as between 32% and 50%, and the specificity as 96% to 98%.29
By definition, any nonzero level of IgE specific for penicillin or its derivatives is considered a positive result and may be associated with a higher risk of IgE-mediated reaction to penicillins. However, in a situation analogous to that in people with food allergy who have a food-specific IgE titer lower than the empirically established threshold value (Table 1), low-titer values to penicillin may not predict anaphylactic sensitivity in a penicillin oral challenge.28 Further studies are needed to determine if there is a threshold level of penicillin-specific IgE above which a patient has a higher likelihood of an IgE-mediated systemic reaction.
Other drugs. Specific IgE blood tests are also available for certain neuromuscular agents, insulin, cefaclor (Ceclor), chlorhexidine (contained in various antiseptic products), and gelatin (Table 2). These substances have not been as well studied as penicillins, and the sensitivity and specificity data reported in Table 2 are limited by few studies and small study sizes.
Neuromuscular blocking agents. Tests for IgE against neuromuscular blocking agents are reported to have low sensitivity (30%–60%) using a cutoff value of 0.35 kU/L.30 In small studies, the sensitivity was higher (68% to 92%) when threshold values for rocuronium-specific IgE were lowered from 0.35 to 0.13 kU/L.29
Chlorhexidine, an antiseptic commonly used in surgery, has been linked to IgE-mediated reactions.31 Chlorhexidine-specific IgE levels greater than 0.35 kU/L are considered positive, based on very limited data.
Insulin. Blood tests for allergy to insulin are also commercially available. However, studies have shown a significant overlap in the range of insulin-specific IgE in patients with a clinical history consistent with insulin allergy and in controls. Therefore, this test has a very limited ability to distinguish people who do not have a history of a reaction to insulin.32 More research is needed to determine the clinical utility of insulin-specific IgE testing.
Gelatin. IgE-mediated reactions have occurred after exposure to gelatin (from either cows or pigs) contained in foods and vaccines, including measles-mumps-rubella and yellow fever. One study identified gelatin-specific IgE in 10 of 11 children with a history of systemic reaction to measles or mumps vaccine.33 In the same study, gelatin-specific IgE levels were negative in 24 children who had developed non-IgE-mediated reactions to the vaccine.33
Tests for IgE against bovine gelatin are commercially available; results are considered positive for values higher than 0.35 kU/L. A negative test result does not exclude the possibility of an allergic reaction to porcine gelatin, which can also be found in foods and vaccines, but tests for anti-porcine gelatin IgE are not commercially available.
Latex
Latex, obtained from the rubber tree Hevea brasiliensis, has 13 known polypeptides (allergens Hev b 1–13) that cause IgE-mediated reactions, particularly in health care workers and patients with spina bifida.34 Overall, the incidence of latex allergy has decreased in the United States as most medical institutions have implemented a latex-free environment.
In vitro testing is the only mode of evaluation for allergy to latex approved by the US Food and Drug Administration (FDA).35 Its sensitivity is 80% and its specificity is 95%.36
In a 2007 study, 145 people at risk for latex allergy, including 104 health care workers, 31 patients with spina bifida, and 10 patients requiring multiple surgeries, underwent latex-specific IgE analysis for sensitivity to various recombinant and native latex allergens.34 The three groups differed in their latex allergy profiles, highlighting the diversity of clinical response to latex in high-risk groups and our current inability to establish specific cutoff points for quantitative latex-specific IgE. Thus, at present, any nonzero latex-specific IgE value is considered positive.
A formal evaluation for allergy is recommended for patients who have a strong history of an IgE-mediated reaction to latex and a latex-specific IgE value of zero. Blood tests for allergy to some native or recombinant latex allergens are available; these allergens may be underrepresented in the native total latex extract.33 Skin testing for allergy to latex, although not FDA-approved or standardized, can also be useful in this setting.37
Insect venom
Type I hypersensitivity reactions can occur from the stings of Vespidae (vespids), Apidae (bees), and Formicidae (fire ants). Large localized reactions after an insect sting are not infrequent and typically do not predict anaphylactic sensitivity with future stings, even though they are considered mild IgE-mediated reactions. However, systemic reactions are considered life-threatening and warrant allergy testing.38
The level of venom-specific IgE usually increases weeks to months after a sting.39 Therefore, blood tests can be falsely negative if performed within a short time of the sting.
Patients who have suffered a systemic reaction to venom and have evidence of sensitization by either in vitro or in vivo allergy testing are candidates for venom immunotherapy.40
At present, any nonzero venom-specific IgE test is considered positive, as there is no specific value for venom-specific IgE that predicts clinical risk.
A negative blood test does not exclude the possibility of an IgE-mediated reaction.41 In cases in which a patient has a clinical history compatible with venom allergy but the blood test is negative, the patient should be referred to an allergist for further evaluation, including venom skin testing and possibly repeat blood testing at a later time.
Conversely, specific IgE testing to venom is recommended when a patient has a history consistent with venom allergy and negative skin test results.38
As mentioned previously, in vitro test performance can vary with the laboratory and testing method used, and sending samples directly to a reference laboratory could be considered.41
TESTING FOR IgG AGAINST FOODS IS UNVALIDATED AND INAPPROPRIATE
In recent years, some practitioners of alternative medicine have started testing for allergen-specific IgG or IgG4 as part of evaluations for hypersensitivity, especially in cases in which patients describe atypical gastrointestinal, neurologic, or other symptoms after eating specific foods.19
However, this testing often finds IgG or IgG4 against foods that are well tolerated. At present, allergen-specific IgG testing lacks scientific evidence to support its clinical use in the evaluation of allergic disease.5,19
- Williams PB, Ahlstedt S, Barnes JH, Söderström L, Portnoy J. Are our impressions of allergy test performances correct? Ann Allergy Asthma Immunol 2003; 91:26–33.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Pichler WJ. Immune mechanism of drug hypersensitivity. Immunol Allergy Clin North Am 2004; 24:373–397.
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–480.
- Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010; 125(suppl 2):S284–S296.
- Cox L, Williams B, Sicherer S, et al; American College of Allergy, Asthma and Immunology Test Task Force; American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 2008; 101:580–592.
- Hamilton RG, Franklin Adkinson N. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol 2004; 114:213–225.
- Williams PB, Dolen WK, Koepke JW, Selner JC. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy 1992; 68:35–45.
- Howanitz PJ, Cembrowski GS, Bachner P. Laboratory phlebotomy. College of American Pathologists Q-Probe study of patient satisfaction and complications in 23,783 patients. Arch Pathol Lab Med 1991; 115:867–872.
- Turkeltaub PC, Gergen PJ. The risk of adverse reactions from percutaneous prick-puncture allergen skin testing, venipuncture, and body measurements: data from the second National Health and Nutrition Examination Survey 1976–80 (NHANES II). J Allergy Clin Immunol 1989; 84:886–890.
- Pipkorn U, Hammarlund A, Enerbäck L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 1989; 19:19–25.
- Cole ZA, Clough GF, Church MK. Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br J Pharmacol 2001; 132:286–292.
- Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol 1996; 98:522–527.
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011; 106:153–158.
- Hamilton RG, Williams PB; Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology; American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol 2010; 126:33–38.
- Somville MA, Machiels J, Gilles JG, Saint-Remy JM. Seasonal variation in specific IgE antibodies of grass-pollen hypersensitive patients depends on the steady state IgE concentration and is not related to clinical symptoms. J Allergy Clin Immunol 1989; 83( 2 Pt 1):486–494.
- Poon AW, Goodman CS, Rubin RJ. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Manag Care 1998; 4:969–985.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol 1994; 93:1068–1070.
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001; 86:196–202.
- Söderström L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy 2003; 58:921–928.
- Bousquet J, Lebel B, Dhivert H, Bataille Y, Martinot B, Michel FB. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin Allergy 1987; 17:529–536.
- Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc 2003; 24:253–258.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Mayorga C, Sanz ML, Gamboa PM, et al; Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010; 20:103–109.
- Garcia JJ, Blanca M, Moreno F, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal 1997; 11:251–257.
- Macy E, Goldberg B, Poon KY. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol 2010; 105:136–141.
- Blanca M, Mayorga C, Torres MJ, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001; 56:862–870.
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007; 107:253–259.
- Ebo DG, Bridts CH, Stevens WJ. IgE-mediated anaphylaxis from chlorhexidine: diagnostic possibilities. Contact Dermatitis 2006; 55:301–302.
- deShazo RD, Mather P, Grant W, et al. Evaluation of patients with local reactions to insulin with skin tests and in vitro techniques. Diabetes Care 1987; 10:330–336.
- Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol 1995; 96:563–565.
- Raulf-Heimsoth M, Rihs HP, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy 2007; 37:1657–1667.
- Biagini RE, MacKenzie BA, Sammons DL, et al. Latex specific IgE: performance characteristics of the IMMULITE 2000 3gAllergy assay compared with skin testing. Ann Allergy Asthma Immunol 2006; 97:196–202.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S47–S56.
- Safadi GS, Corey EC, Taylor JS, Wagner WO, Pien LC, Melton AL. Latex hypersensitivity in emergency medical service providers. Ann Allergy Asthma Immunol 1996; 77:39–42.
- Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004; 114:869–886.
- Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339–1349.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001; 107:897–901.
- Williams PB, Ahlstedt S, Barnes JH, Söderström L, Portnoy J. Are our impressions of allergy test performances correct? Ann Allergy Asthma Immunol 2003; 91:26–33.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Pichler WJ. Immune mechanism of drug hypersensitivity. Immunol Allergy Clin North Am 2004; 24:373–397.
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–480.
- Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010; 125(suppl 2):S284–S296.
- Cox L, Williams B, Sicherer S, et al; American College of Allergy, Asthma and Immunology Test Task Force; American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 2008; 101:580–592.
- Hamilton RG, Franklin Adkinson N. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol 2004; 114:213–225.
- Williams PB, Dolen WK, Koepke JW, Selner JC. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy 1992; 68:35–45.
- Howanitz PJ, Cembrowski GS, Bachner P. Laboratory phlebotomy. College of American Pathologists Q-Probe study of patient satisfaction and complications in 23,783 patients. Arch Pathol Lab Med 1991; 115:867–872.
- Turkeltaub PC, Gergen PJ. The risk of adverse reactions from percutaneous prick-puncture allergen skin testing, venipuncture, and body measurements: data from the second National Health and Nutrition Examination Survey 1976–80 (NHANES II). J Allergy Clin Immunol 1989; 84:886–890.
- Pipkorn U, Hammarlund A, Enerbäck L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 1989; 19:19–25.
- Cole ZA, Clough GF, Church MK. Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br J Pharmacol 2001; 132:286–292.
- Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol 1996; 98:522–527.
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011; 106:153–158.
- Hamilton RG, Williams PB; Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology; American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol 2010; 126:33–38.
- Somville MA, Machiels J, Gilles JG, Saint-Remy JM. Seasonal variation in specific IgE antibodies of grass-pollen hypersensitive patients depends on the steady state IgE concentration and is not related to clinical symptoms. J Allergy Clin Immunol 1989; 83( 2 Pt 1):486–494.
- Poon AW, Goodman CS, Rubin RJ. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Manag Care 1998; 4:969–985.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol 1994; 93:1068–1070.
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001; 86:196–202.
- Söderström L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy 2003; 58:921–928.
- Bousquet J, Lebel B, Dhivert H, Bataille Y, Martinot B, Michel FB. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin Allergy 1987; 17:529–536.
- Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc 2003; 24:253–258.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Mayorga C, Sanz ML, Gamboa PM, et al; Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010; 20:103–109.
- Garcia JJ, Blanca M, Moreno F, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal 1997; 11:251–257.
- Macy E, Goldberg B, Poon KY. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Ann Allergy Asthma Immunol 2010; 105:136–141.
- Blanca M, Mayorga C, Torres MJ, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001; 56:862–870.
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007; 107:253–259.
- Ebo DG, Bridts CH, Stevens WJ. IgE-mediated anaphylaxis from chlorhexidine: diagnostic possibilities. Contact Dermatitis 2006; 55:301–302.
- deShazo RD, Mather P, Grant W, et al. Evaluation of patients with local reactions to insulin with skin tests and in vitro techniques. Diabetes Care 1987; 10:330–336.
- Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol 1995; 96:563–565.
- Raulf-Heimsoth M, Rihs HP, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy 2007; 37:1657–1667.
- Biagini RE, MacKenzie BA, Sammons DL, et al. Latex specific IgE: performance characteristics of the IMMULITE 2000 3gAllergy assay compared with skin testing. Ann Allergy Asthma Immunol 2006; 97:196–202.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol 2002; 110(suppl 2):S47–S56.
- Safadi GS, Corey EC, Taylor JS, Wagner WO, Pien LC, Melton AL. Latex hypersensitivity in emergency medical service providers. Ann Allergy Asthma Immunol 1996; 77:39–42.
- Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004; 114:869–886.
- Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339–1349.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001; 107:897–901.
KEY POINTS
- Specific IgE levels higher than 0.35 kU/L suggest sensitization, but that is not synonymous with clinical disease.
- Prospective studies have identified IgE levels that can predict clinical reactivity with greater than 95% certainty for certain foods, but similar studies have not been performed for most other foods, drugs, latex, or venom.
- The likelihood of an IgE-mediated clinical reaction often increases with the level of specific IgE, but these levels do not predict severity or guarantee a reaction will occur.
- The sensitivity of allergy blood tests ranges from 60% to 95%, and the specificity ranges from 30% to 95%.
- In the appropriate setting, these tests can help in identifying specific allergens and assessing allergic disease.
- Neither allergy blood testing nor skin testing should be used for screening: they may be most useful as confirmatory tests when the patient’s history is compatible with an IgE-mediated reaction.