User login
The most widely used treatment for patients with obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy. Improved quality of life and cardiovascular outcomes for patients with OSA using PAP have been demonstrated. However, for some patients with OSA, PAP therapy is difficult to use or tolerate. Fortunately, there are other available treatment interventions for patients with OSA such as lifestyle interventions, surgical interventions, hypoglossal nerve stimulation (HNS), oral appliance therapy (OAT), and expiratory PAP (EPAP) devices. These alternative treatments can also improve symptoms of OSA though data regarding cardiovascular outcomes are lacking.
LIFESTYLE INTERVENTIONS
Weight loss
Because a higher body mass index (BMI) increases the risk for OSA, weight loss should be recommended for patients with OSA who are overweight. Much of the research evaluating the effect of weight loss on OSA has methodologic limitations such as lack of randomization or controls, potential confounding variables, and limited follow-up. A randomized controlled trial of 72 overweight patients with mild OSA (apnea–hypopnea index [AHI] of 5 to 15) compared a group assigned to a very low calorie diet and lifestyle counseling with a control group.1 At 1 year, weight loss of 15 kg or more resulted in a statistically significant reduction in their AHI to normal, resolving their OSA. A 15 kg weight loss in this study was associated with an overall reduction in the AHI of at least 2 units.
Exercise
Exercise is also recommended for patients with OSA, and it can lessen the severity of symptoms even without weight loss. A meta-analysis of 5 randomized trials of 129 patients reported a reduction in the AHI of as much as 6 events per hour in individuals assigned to a strict exercise regimen.2 The reduction in the AHI occurred despite a slight reduction in BMI (1.37 kg/m2).
Sleep position
For some patients, sleeping in the supine position may worsen their OSA, in which case avoiding the supine sleep position is recommended. A sleep study such as polysomnography should be performed to confirm the resolution of OSA in the nonsupine position.3 Products such as pillows or vibratory feedback devices can help the patient avoid sleeping on the back. The ability to monitor patient adherence to sleep position therapy alone is very limited.
Alcohol avoidance
Alcohol consumption depresses the central nervous system, promotes waking, and increases daytime sleepiness, thus exacerbating OSA. Patients with untreated OSA should avoid alcohol because it worsens the duration and frequency of obstructive respiratory events during sleep, and it can worsen the degree of oxygen desaturation that occurs during abnormal respiratory events.4
Concomitant medications
A review of medications in patients with OSA is warranted. Use of benzodiazepines, benzodiazepine-receptor agonists, barbiturates, and opiates in patients with OSA should be avoided especially if OSA is untreated. If these medications are necessary, careful monitoring is recommended. Medications that can cause weight gain such as some antidepressants should also be avoided.
SURGICAL INTERVENTIONS
Surgical interventions for OSA target the location of the obstruction in the upper airway. The upper airway consists of 3 regions: the palate, oropharynx, and larynx.5 More than 30 surgical soft-tissue and skeletal interventions for OSA are reported in the literature.6
Evaluating the outcomes of various surgical interventions for OSA is hindered by differences in the definition of surgical success or cure. As such, surgical interventions for OSA remain controversial. The practice parameters from 2010 reviewed surgical modifications of the upper airway for adults with OSA.7,8 Success is defined as a greater than 50% reduction in the AHI to fewer than 20 events per hour, whereas surgical cure is defined as a reduction in the AHI to fewer than 5 events per hour.7
Uvulopalatopharyngoplasty
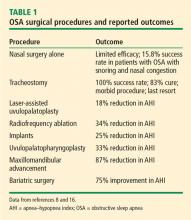
Uvulopalatopharyngoplasty (UPPP) is a surgical procedure that remodels the throat via removal of the tonsils and the posterior surface of the soft palate and uvula and closure of the tonsillar pillars, and thus addresses retropalatal collapse. UPPP rarely achieves a surgical cure (ie, AHI < 5) and has been shown to have a 33% reduction in the AHI, with a postoperative average AHI remaining elevated at 29.8 (ie, moderate to severe OSA).8 In general, 50% of patients have a 50% reduction in AHI.9 The 4-year responder rate for UPPP is 44% to 50%.10 Factors limiting the long-term success of this procedure include weight gain, assessment of surgical candidates,11 and decreased adherence to PAP therapy after the procedure.
The use of UPPP in combination with other surgical procedures has also been evaluated.8 The AHI in patients with OSA improved postoperatively when UPPP was done simultaneously or in a multiphase approach with radiofrequency ablation, midline glossectomy, tongue advancement, hyoid suspension, or maxillomandibular advancement, though greater improvement was noted with the multiphase approach.
Maxillomandibular advancement
Maxillomandibular advancement is a surgical procedure that moves the maxilla and mandible forward and expands the facial skeletal framework via LeFort I maxillary and sagittal split mandibular osteotomies. Maxillomandibular advancement achieves enlargement of the nasopharyngeal, retropalatal, and hypopharyngeal airway. This increases tension on the pharyngeal soft tissue, which enlarges the medial-lateral and anteroposterior dimensions of the upper airway.14
A meta-analysis of 45 studies evaluated the change in the AHI after maxillomandibular advancement in 518 patients.15 Secondary outcomes were surgical success (> 50% reduction in AHI to < 20 events per hour) and surgical cure (AHI < 5). Patients with a higher preoperative AHI achieved the greatest magnitude reduction in AHI but were less likely to achieve surgical success or cure. Patients with a lower preoperative AHI had a greater likelihood of surgical success and cure.
Bariatric surgery
Bariatric surgery is increasingly used for treatment of OSA in individuals with morbid obesity. A systematic review of bariatric surgery including the roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and biliopancreatic diversion evaluated 69 studies with 13,900 patients with OSA.16 OSA was found to be improved or eliminated in 75% of patients for all bariatric surgery procedures.
HYPOGLOSSAL NERVE STIMULATION
Indications and contraindications
The indications and contraindications for HNS are shown in Table 2.
Efficacy and outcomes
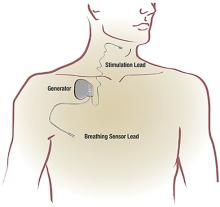
Stimulation of the hypoglossal nerve results in a multilevel mechanism of action: activation and protrusion of the tongue opens the oropharyngeal airway directly but also affects the retropalatal airway by a palatoglossal coupling action.19 Sleep lab testing with polysomnography is used to titrate the voltage of HNS to achieve an open airway that resolves apneic events and normalizes airflow, breathing patterns, and oxygen saturation levels.
Approval of HNS for OSA by the US Food and Drug Administration was based on findings in the Stimulation Therapy for Apnea Reduction (STAR) trial,17 a prospective trial of 126 patients at 22 centers in the United States and Europe with the primary outcomes of AHI and oxygen desaturation index. Secondary outcomes included quality of life as measured by the Functional Outcomes of Sleep Questionnaire and Epworth Sleepiness Scale (ESS). Patient demographics included mean age 54.5, 83% men, mean BMI of 28 kg/m2, and mean baseline AHI of 34 (ie, severe OSA).
Data at 5 years for 97 of the 126 patients on HNS in the STAR trial is available.20 The AHI was reduced an average of 70% to levels in the mild OSA range.20,21 Overall, 85% of the patients had improved quality-of-life measures after HNS implantation, with increased Functional Outcomes of Sleep Questionnaire scores and ESS scores in the normal range over time. Consistent HNS therapy demonstrated sustained benefits at 5 years. The AHI improved by 50% or to less than 20 in 75% of patients, with 44% having resolved OSA and 78% improved to mild OSA (AHI < 15). Device-related adverse events occurred in 6% (9 of 126) of patients requiring replacement or repositioning of the stimulator or leads.20
Moderate to severe snoring was prevalent at baseline in the STAR trial, but over the course of 5 years, 85% of bed partners of patients on HNS reported no or soft snoring.17,21 Nightly use averaged 80% over 60 months based on patient reporting, with 87% reporting use at least 5 nights per week at 36 weeks.20
In terms of predictors of response to HNS therapy, the oxygen desaturation index is the only characteristic that reached a level of statistical significance; patients with higher levels of oxygen desaturation tended to improve and tolerate therapy better long-term.20 A randomized controlled trial of withdrawal of HNS therapy demonstrated increased AHI and oxygen desaturation index when therapy was withdrawn, followed by improvement when therapy resumed.22
A clinical trial of 20 patients implanted with HNS after its approval in 2014 reported that the mean AHI decreased from 33 before implant to 5.1 after implant.23 The ESS also improved from 10.3 before implant to 6 after implant. Mean adherence to device use was 7 (± 2) hours per night. The average stimulation amplitude was 1.89 (± 0.5) volts after the titration sleep study was completed. Similar reductions in AHI were reported by Huntley et al24 for patients receiving HNS implant at 2 academic centers, with no differences between the 2 cohorts in postoperative AHI.
Adverse events
The adverse events reported with HNS are related to the implant procedure or the device.21 Procedure-related adverse events are incision discomfort, temporary tongue weakness, headache, and mild infection of incisions. The most common device-related adverse event is discomfort from the electrical stimulation. Tongue abrasion can also occur if the tongue protrudes and rubs against a sharp tooth. Dry mouth is also commonly reported.
HNS compared with UPPP
Outcomes in patients with moderate to severe OSA matched for BMI, demographics, and preoperative AHI were evaluated comparing patients undergoing HNS (n = 20) with patients receiving UPPP (n = 20).25 The AHI decreased 29% postoperatively in patients with UPPP compared with 88% in patients with HNS, 65% of which had normalization of their AHI. Surgical success was achieved in 40% of patients in the UPPP group compared with 100% in the HNS group. Greater improvement in daytime sleepiness was noted in patients in the HNS group compared with the UPPP group.
ORAL APPLIANCE THERAPY
OAT devices help protrude the mandible forward and stabilize it to maintain a more patent airway during sleep. Oral appliances can be custom-made or prefabricated. Oral appliances can be titratable or nontitratable: titration provides a mechanism to adjust mandibular protrusion analogous to PAP titration, whereas the absence of titration holds the mandible in a single position. The most effective oral appliances are custom-made and titratable.
Types of OAT devices
Custom oral appliances. Custom oral appliances are fabricated using digital or physical impressions of the patient’s oral structures. Custom oral appliances are made of biocompatible materials and engage both the maxillary and mandibular arches.
Custom oral appliances are made by a qualified dentist who takes maxillary and mandibular impressions with a bite registration using the George Gauge with 40% to 50% of maximum protrusion. The appliance is fabricated in a laboratory and then fitted to the patient, who is instructed to titrate the device 0.5 mm to 1 mm per week and follow-up with the dentist at 2-week intervals. Once the patient has titrated the device to the point of comfort or improved sleep quality or snoring, polysomnography should be done with the device in place and titrated to improve the AHI as much as possible. Follow-up is recommended at 6 months and annually thereafter.
Prefabricated oral appliances. Prefabricated oral appliances are of the boil-and-bite type, only partially modified to the patient’s oral structures.
Tongue-retaining devices. Another type of oral appliance is a tongue-retaining device, which is designed to hold the tongue forward and can be custom-made or prefabricated.
Patient considerations for OAT
Practice recommendations
- Prescribed OAT should be done by a qualified dentist, and a custom, titratable appliance is preferred
- OAT is preferred over no therapy for adults with OSA who are intolerant to PAP or prefer alternative therapies
- A qualified dentist should provide oversight for dental-related side effects or occlusal changes
- Follow-up sleep testing should be conducted to confirm efficacy or titrate treatment
- Periodic office visits with the sleep physician and qualified dentist are recommended.26
The quality of evidence for these recommendations is low, with the exception of use of OAT rather than no therapy, which is considered of moderate quality.
Effects of OAT
Anatomic and physiologic effects. With OAT in place in the mouth, the airway caliber in the lateral dimension are increased, and the airway size at the retropalatal level is increased.27–30 With respect to the tongue, increased genioglossus muscle activity has been reported with OAT.
Side effects. Side effects of OAT include excess salivation, dry mouth, tooth tenderness, soft-tissue changes, jaw discomfort, tooth movement, and occlusal changes such as difficulty chewing in the morning. Feelings of suffocation, vivid dreams, and anxiety have also been reported with OAT.31–33
Efficacy and outcomes
Review of the data on the efficacy of OAT did not illuminate factors that predict treatment success.26 Data indicate that in patients with mild OSA using OAT or PAP therapy, there was no significant difference in the percentage achieving their target AHI; however, patients with moderate to severe OSA had a statistically significant greater odds of achieving their target AHI using PAP therapy compared with OAT. Therefore, OAT should be reserved for patients with severe OSA who cannot use or are intolerant to PAP.
Moderate-grade quality of evidence was reviewed for the established OAT practice recommendations for OSA outcomes before and after use of custom, titratable OAT devices.26 Use of a custom OAT device reduced the mean AHI, increased mean oxygen saturation, decreased the mean oxygen desaturation, decreased the arousal index, decreased the ESS, and increased quality of life compared with values prior to use of OAT.
With respect to adherence and discontinuation, patients using OAT had higher mean adherence and lower discontinuation because of side effects compared with patients using continuous PAP.26
NASAL EPAP THERAPY
Nasal EPAP is a new treatment for OSA that consists of a mechanical valve worn in each naris at night. The valves have a low inspiratory resistance and a high expiratory resistance thus increased pressure occurs at exhalation.
Pressure at exhalation may counter the airway collapse in OSA. With the mouth closed and use of the nasal valves, the positive pressure during the normal respiratory cycle is utilized to maintain an open airway.34 At the onset and throughout inspiration, the activity of the airway dilator muscles increases. At maximum expiration, right before the end of the expiratory pause, the dilator muscle stops abruptly and the airway is of its smallest caliber. The presence of the nasal valve at this point is thought to act as a pneumatic splint to the airway, and the nasal EPAP helps keep the airway patent during the next inspiratory phase.
Nasal EPAP valves are available in a 30-day starter kit. Intended for single-night use, the kit includes valves of increasing levels of expiration resistance: low (nights 1 and 2), medium (nights 3 and 4), and normal (nights 5–30).
Outcomes of nasal EPAP therapy
A multicenter 30-day in-home trial evaluated efficacy and compliance of nasal EPAP therapy.35 The AHI was reduced by 50% or more in 14 of 34 (41%) patients using nasal EPAP compared with the control group at the 30-day follow-up. The patient-reported compliance with nasal EPAP was 94%. Patients in this study had mild to moderate OSA and did not have obesity or other comorbidities such as pulmonary hypertension or cardiovascular disease.
A randomized controlled trial compared nasal EPAP with a sham device in patients with newly diagnosed or untreated OSA (N = 250) for 3 months.36 A median reduction of 52% in the AHI was noted in the intention-to-treat group (N = 229) during rapid eye movement (REM) and non-REM sleep, though it was statistically significant only during REM sleep and supine sleep. At 3 months, improved OSA was maintained in 42% of the patients using nasal EPAP compared with 10% of patients using a sham device. Improvements in daytime sleepiness and adherence with 88% using EPAP the entire night were also noted.
In a 12-month study of nasal EPAP, 67% of patients (34 of 51) used nasal EPAP for the full trial duration.37 Of patients using nasal EPAP for 12 months, the median AHI was reduced by 71%, the ESS improved, and adherence to full-night use was 89%.
Patient considerations for nasal EPAP
In clinical practice, nasal EPAP therapy requires nasal patency and use of a chin strap in patients with mouth leakage. Nasal EPAP may be recommended for patients who travel frequently and can go without continuous PAP or bilevel PAP for short periods of time, and for patients who do not have significant medical comorbidities.
Side effects and limitations of nasal EPAP
Reported side effects of nasal EPAP include difficulty with exhalation, nasal discomfort, dry mouth, and headache. Nasal EPAP therapy is of limited use in patients with severe OSA and severe oxygen desaturation. The efficacy of nasal EPAP beyond 12 months is unknown. Use of nasal EPAP in patients with prior upper-airway surgery and in combination with other therapies is yet to be evaluated.
- Tuomilehto HPI, Seppä JM, Partinen MM, et al; Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179(4):320–327.
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192(1):175–184.
- de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015; 11(2):131–137.
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45(4):353–359.
- Rowley JA, Badr MS. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th edition. Philadelphia, PA: Elsevier; 2017:1076–1087.
- Camacho M, Certal V, Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2013; 79(6):780–788.
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33(10):1408–1413.
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33(10):1396–1407.
- Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc 2009; 84(9):795–800.
- Larson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994; 104(11 Pt 1):1362–1368.
- Aboussouan LS, Golish JA, Wood BG, Mehta AC, Wood DE, Dinner DS. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest 1995; 107(4):946–951.
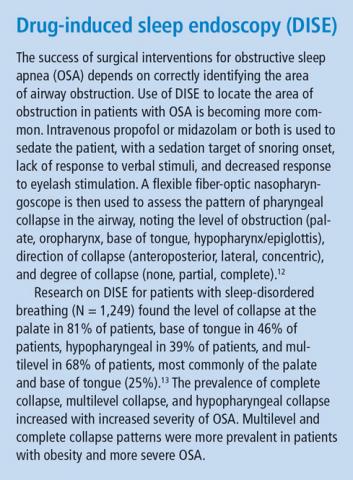
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9(5):433–438.
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124(3):797–802.
- Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop 2014; 146(4):477–492.
- Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016; 142(1):58–66.
- Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013; 23(3):414–423.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):139–149.
- Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg 2016; 154(5):970–977.
- Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015; 45(1):129–138.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018; 159(1):194–202.
- Woodson BT, Soose RJ, Gillespie MB; STAR Trial Investigators. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 2016; 154(1):181–188.
- Woodson BT, Gillespie MB, Soose RJ, et al; STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short-and long-term effect. Otolaryngol Head Neck Surg 2014; 151(5):880–887.
- Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016; 155(1):188–193.
- Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017; 13(9):1075–1079.
- Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018; 39(3):266–270.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015—an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015; 11(7):773–827.
- Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011; 34(4):469–477.
- Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake caliber of the velopharynx. Thorax 1999; 54(11):972–977.
- Tsuiki S, Ono T, Kuroda T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000; 4(2):53–58.
- Lowe AA. Oral appliances for sleep breathing disorders. Principles and Practice of Sleep Medicine. 3rd edition. In: Kryger MH, Roth T, Dement WE, eds. Philadelphia: Saunders; 2000:929–939.
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129(2):214–221.
- Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132(6):806–814.
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep 1999; 22(2):237–240.
- Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med 2008; 4(5):426–433.
- Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med 2009; 5(6):532–537.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34(4):479–485.
- Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011; 7(5):449–453.
The most widely used treatment for patients with obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy. Improved quality of life and cardiovascular outcomes for patients with OSA using PAP have been demonstrated. However, for some patients with OSA, PAP therapy is difficult to use or tolerate. Fortunately, there are other available treatment interventions for patients with OSA such as lifestyle interventions, surgical interventions, hypoglossal nerve stimulation (HNS), oral appliance therapy (OAT), and expiratory PAP (EPAP) devices. These alternative treatments can also improve symptoms of OSA though data regarding cardiovascular outcomes are lacking.
LIFESTYLE INTERVENTIONS
Weight loss
Because a higher body mass index (BMI) increases the risk for OSA, weight loss should be recommended for patients with OSA who are overweight. Much of the research evaluating the effect of weight loss on OSA has methodologic limitations such as lack of randomization or controls, potential confounding variables, and limited follow-up. A randomized controlled trial of 72 overweight patients with mild OSA (apnea–hypopnea index [AHI] of 5 to 15) compared a group assigned to a very low calorie diet and lifestyle counseling with a control group.1 At 1 year, weight loss of 15 kg or more resulted in a statistically significant reduction in their AHI to normal, resolving their OSA. A 15 kg weight loss in this study was associated with an overall reduction in the AHI of at least 2 units.
Exercise
Exercise is also recommended for patients with OSA, and it can lessen the severity of symptoms even without weight loss. A meta-analysis of 5 randomized trials of 129 patients reported a reduction in the AHI of as much as 6 events per hour in individuals assigned to a strict exercise regimen.2 The reduction in the AHI occurred despite a slight reduction in BMI (1.37 kg/m2).
Sleep position
For some patients, sleeping in the supine position may worsen their OSA, in which case avoiding the supine sleep position is recommended. A sleep study such as polysomnography should be performed to confirm the resolution of OSA in the nonsupine position.3 Products such as pillows or vibratory feedback devices can help the patient avoid sleeping on the back. The ability to monitor patient adherence to sleep position therapy alone is very limited.
Alcohol avoidance
Alcohol consumption depresses the central nervous system, promotes waking, and increases daytime sleepiness, thus exacerbating OSA. Patients with untreated OSA should avoid alcohol because it worsens the duration and frequency of obstructive respiratory events during sleep, and it can worsen the degree of oxygen desaturation that occurs during abnormal respiratory events.4
Concomitant medications
A review of medications in patients with OSA is warranted. Use of benzodiazepines, benzodiazepine-receptor agonists, barbiturates, and opiates in patients with OSA should be avoided especially if OSA is untreated. If these medications are necessary, careful monitoring is recommended. Medications that can cause weight gain such as some antidepressants should also be avoided.
SURGICAL INTERVENTIONS
Surgical interventions for OSA target the location of the obstruction in the upper airway. The upper airway consists of 3 regions: the palate, oropharynx, and larynx.5 More than 30 surgical soft-tissue and skeletal interventions for OSA are reported in the literature.6
Evaluating the outcomes of various surgical interventions for OSA is hindered by differences in the definition of surgical success or cure. As such, surgical interventions for OSA remain controversial. The practice parameters from 2010 reviewed surgical modifications of the upper airway for adults with OSA.7,8 Success is defined as a greater than 50% reduction in the AHI to fewer than 20 events per hour, whereas surgical cure is defined as a reduction in the AHI to fewer than 5 events per hour.7
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) is a surgical procedure that remodels the throat via removal of the tonsils and the posterior surface of the soft palate and uvula and closure of the tonsillar pillars, and thus addresses retropalatal collapse. UPPP rarely achieves a surgical cure (ie, AHI < 5) and has been shown to have a 33% reduction in the AHI, with a postoperative average AHI remaining elevated at 29.8 (ie, moderate to severe OSA).8 In general, 50% of patients have a 50% reduction in AHI.9 The 4-year responder rate for UPPP is 44% to 50%.10 Factors limiting the long-term success of this procedure include weight gain, assessment of surgical candidates,11 and decreased adherence to PAP therapy after the procedure.
The use of UPPP in combination with other surgical procedures has also been evaluated.8 The AHI in patients with OSA improved postoperatively when UPPP was done simultaneously or in a multiphase approach with radiofrequency ablation, midline glossectomy, tongue advancement, hyoid suspension, or maxillomandibular advancement, though greater improvement was noted with the multiphase approach.
Maxillomandibular advancement
Maxillomandibular advancement is a surgical procedure that moves the maxilla and mandible forward and expands the facial skeletal framework via LeFort I maxillary and sagittal split mandibular osteotomies. Maxillomandibular advancement achieves enlargement of the nasopharyngeal, retropalatal, and hypopharyngeal airway. This increases tension on the pharyngeal soft tissue, which enlarges the medial-lateral and anteroposterior dimensions of the upper airway.14
A meta-analysis of 45 studies evaluated the change in the AHI after maxillomandibular advancement in 518 patients.15 Secondary outcomes were surgical success (> 50% reduction in AHI to < 20 events per hour) and surgical cure (AHI < 5). Patients with a higher preoperative AHI achieved the greatest magnitude reduction in AHI but were less likely to achieve surgical success or cure. Patients with a lower preoperative AHI had a greater likelihood of surgical success and cure.
Bariatric surgery
Bariatric surgery is increasingly used for treatment of OSA in individuals with morbid obesity. A systematic review of bariatric surgery including the roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and biliopancreatic diversion evaluated 69 studies with 13,900 patients with OSA.16 OSA was found to be improved or eliminated in 75% of patients for all bariatric surgery procedures.
HYPOGLOSSAL NERVE STIMULATION
Indications and contraindications
The indications and contraindications for HNS are shown in Table 2.
Efficacy and outcomes
Stimulation of the hypoglossal nerve results in a multilevel mechanism of action: activation and protrusion of the tongue opens the oropharyngeal airway directly but also affects the retropalatal airway by a palatoglossal coupling action.19 Sleep lab testing with polysomnography is used to titrate the voltage of HNS to achieve an open airway that resolves apneic events and normalizes airflow, breathing patterns, and oxygen saturation levels.
Approval of HNS for OSA by the US Food and Drug Administration was based on findings in the Stimulation Therapy for Apnea Reduction (STAR) trial,17 a prospective trial of 126 patients at 22 centers in the United States and Europe with the primary outcomes of AHI and oxygen desaturation index. Secondary outcomes included quality of life as measured by the Functional Outcomes of Sleep Questionnaire and Epworth Sleepiness Scale (ESS). Patient demographics included mean age 54.5, 83% men, mean BMI of 28 kg/m2, and mean baseline AHI of 34 (ie, severe OSA).
Data at 5 years for 97 of the 126 patients on HNS in the STAR trial is available.20 The AHI was reduced an average of 70% to levels in the mild OSA range.20,21 Overall, 85% of the patients had improved quality-of-life measures after HNS implantation, with increased Functional Outcomes of Sleep Questionnaire scores and ESS scores in the normal range over time. Consistent HNS therapy demonstrated sustained benefits at 5 years. The AHI improved by 50% or to less than 20 in 75% of patients, with 44% having resolved OSA and 78% improved to mild OSA (AHI < 15). Device-related adverse events occurred in 6% (9 of 126) of patients requiring replacement or repositioning of the stimulator or leads.20
Moderate to severe snoring was prevalent at baseline in the STAR trial, but over the course of 5 years, 85% of bed partners of patients on HNS reported no or soft snoring.17,21 Nightly use averaged 80% over 60 months based on patient reporting, with 87% reporting use at least 5 nights per week at 36 weeks.20
In terms of predictors of response to HNS therapy, the oxygen desaturation index is the only characteristic that reached a level of statistical significance; patients with higher levels of oxygen desaturation tended to improve and tolerate therapy better long-term.20 A randomized controlled trial of withdrawal of HNS therapy demonstrated increased AHI and oxygen desaturation index when therapy was withdrawn, followed by improvement when therapy resumed.22
A clinical trial of 20 patients implanted with HNS after its approval in 2014 reported that the mean AHI decreased from 33 before implant to 5.1 after implant.23 The ESS also improved from 10.3 before implant to 6 after implant. Mean adherence to device use was 7 (± 2) hours per night. The average stimulation amplitude was 1.89 (± 0.5) volts after the titration sleep study was completed. Similar reductions in AHI were reported by Huntley et al24 for patients receiving HNS implant at 2 academic centers, with no differences between the 2 cohorts in postoperative AHI.
Adverse events
The adverse events reported with HNS are related to the implant procedure or the device.21 Procedure-related adverse events are incision discomfort, temporary tongue weakness, headache, and mild infection of incisions. The most common device-related adverse event is discomfort from the electrical stimulation. Tongue abrasion can also occur if the tongue protrudes and rubs against a sharp tooth. Dry mouth is also commonly reported.
HNS compared with UPPP
Outcomes in patients with moderate to severe OSA matched for BMI, demographics, and preoperative AHI were evaluated comparing patients undergoing HNS (n = 20) with patients receiving UPPP (n = 20).25 The AHI decreased 29% postoperatively in patients with UPPP compared with 88% in patients with HNS, 65% of which had normalization of their AHI. Surgical success was achieved in 40% of patients in the UPPP group compared with 100% in the HNS group. Greater improvement in daytime sleepiness was noted in patients in the HNS group compared with the UPPP group.
ORAL APPLIANCE THERAPY
OAT devices help protrude the mandible forward and stabilize it to maintain a more patent airway during sleep. Oral appliances can be custom-made or prefabricated. Oral appliances can be titratable or nontitratable: titration provides a mechanism to adjust mandibular protrusion analogous to PAP titration, whereas the absence of titration holds the mandible in a single position. The most effective oral appliances are custom-made and titratable.
Types of OAT devices
Custom oral appliances. Custom oral appliances are fabricated using digital or physical impressions of the patient’s oral structures. Custom oral appliances are made of biocompatible materials and engage both the maxillary and mandibular arches.
Custom oral appliances are made by a qualified dentist who takes maxillary and mandibular impressions with a bite registration using the George Gauge with 40% to 50% of maximum protrusion. The appliance is fabricated in a laboratory and then fitted to the patient, who is instructed to titrate the device 0.5 mm to 1 mm per week and follow-up with the dentist at 2-week intervals. Once the patient has titrated the device to the point of comfort or improved sleep quality or snoring, polysomnography should be done with the device in place and titrated to improve the AHI as much as possible. Follow-up is recommended at 6 months and annually thereafter.
Prefabricated oral appliances. Prefabricated oral appliances are of the boil-and-bite type, only partially modified to the patient’s oral structures.
Tongue-retaining devices. Another type of oral appliance is a tongue-retaining device, which is designed to hold the tongue forward and can be custom-made or prefabricated.
Patient considerations for OAT
Practice recommendations
- Prescribed OAT should be done by a qualified dentist, and a custom, titratable appliance is preferred
- OAT is preferred over no therapy for adults with OSA who are intolerant to PAP or prefer alternative therapies
- A qualified dentist should provide oversight for dental-related side effects or occlusal changes
- Follow-up sleep testing should be conducted to confirm efficacy or titrate treatment
- Periodic office visits with the sleep physician and qualified dentist are recommended.26
The quality of evidence for these recommendations is low, with the exception of use of OAT rather than no therapy, which is considered of moderate quality.
Effects of OAT
Anatomic and physiologic effects. With OAT in place in the mouth, the airway caliber in the lateral dimension are increased, and the airway size at the retropalatal level is increased.27–30 With respect to the tongue, increased genioglossus muscle activity has been reported with OAT.
Side effects. Side effects of OAT include excess salivation, dry mouth, tooth tenderness, soft-tissue changes, jaw discomfort, tooth movement, and occlusal changes such as difficulty chewing in the morning. Feelings of suffocation, vivid dreams, and anxiety have also been reported with OAT.31–33
Efficacy and outcomes
Review of the data on the efficacy of OAT did not illuminate factors that predict treatment success.26 Data indicate that in patients with mild OSA using OAT or PAP therapy, there was no significant difference in the percentage achieving their target AHI; however, patients with moderate to severe OSA had a statistically significant greater odds of achieving their target AHI using PAP therapy compared with OAT. Therefore, OAT should be reserved for patients with severe OSA who cannot use or are intolerant to PAP.
Moderate-grade quality of evidence was reviewed for the established OAT practice recommendations for OSA outcomes before and after use of custom, titratable OAT devices.26 Use of a custom OAT device reduced the mean AHI, increased mean oxygen saturation, decreased the mean oxygen desaturation, decreased the arousal index, decreased the ESS, and increased quality of life compared with values prior to use of OAT.
With respect to adherence and discontinuation, patients using OAT had higher mean adherence and lower discontinuation because of side effects compared with patients using continuous PAP.26
NASAL EPAP THERAPY
Nasal EPAP is a new treatment for OSA that consists of a mechanical valve worn in each naris at night. The valves have a low inspiratory resistance and a high expiratory resistance thus increased pressure occurs at exhalation.
Pressure at exhalation may counter the airway collapse in OSA. With the mouth closed and use of the nasal valves, the positive pressure during the normal respiratory cycle is utilized to maintain an open airway.34 At the onset and throughout inspiration, the activity of the airway dilator muscles increases. At maximum expiration, right before the end of the expiratory pause, the dilator muscle stops abruptly and the airway is of its smallest caliber. The presence of the nasal valve at this point is thought to act as a pneumatic splint to the airway, and the nasal EPAP helps keep the airway patent during the next inspiratory phase.
Nasal EPAP valves are available in a 30-day starter kit. Intended for single-night use, the kit includes valves of increasing levels of expiration resistance: low (nights 1 and 2), medium (nights 3 and 4), and normal (nights 5–30).
Outcomes of nasal EPAP therapy
A multicenter 30-day in-home trial evaluated efficacy and compliance of nasal EPAP therapy.35 The AHI was reduced by 50% or more in 14 of 34 (41%) patients using nasal EPAP compared with the control group at the 30-day follow-up. The patient-reported compliance with nasal EPAP was 94%. Patients in this study had mild to moderate OSA and did not have obesity or other comorbidities such as pulmonary hypertension or cardiovascular disease.
A randomized controlled trial compared nasal EPAP with a sham device in patients with newly diagnosed or untreated OSA (N = 250) for 3 months.36 A median reduction of 52% in the AHI was noted in the intention-to-treat group (N = 229) during rapid eye movement (REM) and non-REM sleep, though it was statistically significant only during REM sleep and supine sleep. At 3 months, improved OSA was maintained in 42% of the patients using nasal EPAP compared with 10% of patients using a sham device. Improvements in daytime sleepiness and adherence with 88% using EPAP the entire night were also noted.
In a 12-month study of nasal EPAP, 67% of patients (34 of 51) used nasal EPAP for the full trial duration.37 Of patients using nasal EPAP for 12 months, the median AHI was reduced by 71%, the ESS improved, and adherence to full-night use was 89%.
Patient considerations for nasal EPAP
In clinical practice, nasal EPAP therapy requires nasal patency and use of a chin strap in patients with mouth leakage. Nasal EPAP may be recommended for patients who travel frequently and can go without continuous PAP or bilevel PAP for short periods of time, and for patients who do not have significant medical comorbidities.
Side effects and limitations of nasal EPAP
Reported side effects of nasal EPAP include difficulty with exhalation, nasal discomfort, dry mouth, and headache. Nasal EPAP therapy is of limited use in patients with severe OSA and severe oxygen desaturation. The efficacy of nasal EPAP beyond 12 months is unknown. Use of nasal EPAP in patients with prior upper-airway surgery and in combination with other therapies is yet to be evaluated.
The most widely used treatment for patients with obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy. Improved quality of life and cardiovascular outcomes for patients with OSA using PAP have been demonstrated. However, for some patients with OSA, PAP therapy is difficult to use or tolerate. Fortunately, there are other available treatment interventions for patients with OSA such as lifestyle interventions, surgical interventions, hypoglossal nerve stimulation (HNS), oral appliance therapy (OAT), and expiratory PAP (EPAP) devices. These alternative treatments can also improve symptoms of OSA though data regarding cardiovascular outcomes are lacking.
LIFESTYLE INTERVENTIONS
Weight loss
Because a higher body mass index (BMI) increases the risk for OSA, weight loss should be recommended for patients with OSA who are overweight. Much of the research evaluating the effect of weight loss on OSA has methodologic limitations such as lack of randomization or controls, potential confounding variables, and limited follow-up. A randomized controlled trial of 72 overweight patients with mild OSA (apnea–hypopnea index [AHI] of 5 to 15) compared a group assigned to a very low calorie diet and lifestyle counseling with a control group.1 At 1 year, weight loss of 15 kg or more resulted in a statistically significant reduction in their AHI to normal, resolving their OSA. A 15 kg weight loss in this study was associated with an overall reduction in the AHI of at least 2 units.
Exercise
Exercise is also recommended for patients with OSA, and it can lessen the severity of symptoms even without weight loss. A meta-analysis of 5 randomized trials of 129 patients reported a reduction in the AHI of as much as 6 events per hour in individuals assigned to a strict exercise regimen.2 The reduction in the AHI occurred despite a slight reduction in BMI (1.37 kg/m2).
Sleep position
For some patients, sleeping in the supine position may worsen their OSA, in which case avoiding the supine sleep position is recommended. A sleep study such as polysomnography should be performed to confirm the resolution of OSA in the nonsupine position.3 Products such as pillows or vibratory feedback devices can help the patient avoid sleeping on the back. The ability to monitor patient adherence to sleep position therapy alone is very limited.
Alcohol avoidance
Alcohol consumption depresses the central nervous system, promotes waking, and increases daytime sleepiness, thus exacerbating OSA. Patients with untreated OSA should avoid alcohol because it worsens the duration and frequency of obstructive respiratory events during sleep, and it can worsen the degree of oxygen desaturation that occurs during abnormal respiratory events.4
Concomitant medications
A review of medications in patients with OSA is warranted. Use of benzodiazepines, benzodiazepine-receptor agonists, barbiturates, and opiates in patients with OSA should be avoided especially if OSA is untreated. If these medications are necessary, careful monitoring is recommended. Medications that can cause weight gain such as some antidepressants should also be avoided.
SURGICAL INTERVENTIONS
Surgical interventions for OSA target the location of the obstruction in the upper airway. The upper airway consists of 3 regions: the palate, oropharynx, and larynx.5 More than 30 surgical soft-tissue and skeletal interventions for OSA are reported in the literature.6
Evaluating the outcomes of various surgical interventions for OSA is hindered by differences in the definition of surgical success or cure. As such, surgical interventions for OSA remain controversial. The practice parameters from 2010 reviewed surgical modifications of the upper airway for adults with OSA.7,8 Success is defined as a greater than 50% reduction in the AHI to fewer than 20 events per hour, whereas surgical cure is defined as a reduction in the AHI to fewer than 5 events per hour.7
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) is a surgical procedure that remodels the throat via removal of the tonsils and the posterior surface of the soft palate and uvula and closure of the tonsillar pillars, and thus addresses retropalatal collapse. UPPP rarely achieves a surgical cure (ie, AHI < 5) and has been shown to have a 33% reduction in the AHI, with a postoperative average AHI remaining elevated at 29.8 (ie, moderate to severe OSA).8 In general, 50% of patients have a 50% reduction in AHI.9 The 4-year responder rate for UPPP is 44% to 50%.10 Factors limiting the long-term success of this procedure include weight gain, assessment of surgical candidates,11 and decreased adherence to PAP therapy after the procedure.
The use of UPPP in combination with other surgical procedures has also been evaluated.8 The AHI in patients with OSA improved postoperatively when UPPP was done simultaneously or in a multiphase approach with radiofrequency ablation, midline glossectomy, tongue advancement, hyoid suspension, or maxillomandibular advancement, though greater improvement was noted with the multiphase approach.
Maxillomandibular advancement
Maxillomandibular advancement is a surgical procedure that moves the maxilla and mandible forward and expands the facial skeletal framework via LeFort I maxillary and sagittal split mandibular osteotomies. Maxillomandibular advancement achieves enlargement of the nasopharyngeal, retropalatal, and hypopharyngeal airway. This increases tension on the pharyngeal soft tissue, which enlarges the medial-lateral and anteroposterior dimensions of the upper airway.14
A meta-analysis of 45 studies evaluated the change in the AHI after maxillomandibular advancement in 518 patients.15 Secondary outcomes were surgical success (> 50% reduction in AHI to < 20 events per hour) and surgical cure (AHI < 5). Patients with a higher preoperative AHI achieved the greatest magnitude reduction in AHI but were less likely to achieve surgical success or cure. Patients with a lower preoperative AHI had a greater likelihood of surgical success and cure.
Bariatric surgery
Bariatric surgery is increasingly used for treatment of OSA in individuals with morbid obesity. A systematic review of bariatric surgery including the roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and biliopancreatic diversion evaluated 69 studies with 13,900 patients with OSA.16 OSA was found to be improved or eliminated in 75% of patients for all bariatric surgery procedures.
HYPOGLOSSAL NERVE STIMULATION
Indications and contraindications
The indications and contraindications for HNS are shown in Table 2.
Efficacy and outcomes
Stimulation of the hypoglossal nerve results in a multilevel mechanism of action: activation and protrusion of the tongue opens the oropharyngeal airway directly but also affects the retropalatal airway by a palatoglossal coupling action.19 Sleep lab testing with polysomnography is used to titrate the voltage of HNS to achieve an open airway that resolves apneic events and normalizes airflow, breathing patterns, and oxygen saturation levels.
Approval of HNS for OSA by the US Food and Drug Administration was based on findings in the Stimulation Therapy for Apnea Reduction (STAR) trial,17 a prospective trial of 126 patients at 22 centers in the United States and Europe with the primary outcomes of AHI and oxygen desaturation index. Secondary outcomes included quality of life as measured by the Functional Outcomes of Sleep Questionnaire and Epworth Sleepiness Scale (ESS). Patient demographics included mean age 54.5, 83% men, mean BMI of 28 kg/m2, and mean baseline AHI of 34 (ie, severe OSA).
Data at 5 years for 97 of the 126 patients on HNS in the STAR trial is available.20 The AHI was reduced an average of 70% to levels in the mild OSA range.20,21 Overall, 85% of the patients had improved quality-of-life measures after HNS implantation, with increased Functional Outcomes of Sleep Questionnaire scores and ESS scores in the normal range over time. Consistent HNS therapy demonstrated sustained benefits at 5 years. The AHI improved by 50% or to less than 20 in 75% of patients, with 44% having resolved OSA and 78% improved to mild OSA (AHI < 15). Device-related adverse events occurred in 6% (9 of 126) of patients requiring replacement or repositioning of the stimulator or leads.20
Moderate to severe snoring was prevalent at baseline in the STAR trial, but over the course of 5 years, 85% of bed partners of patients on HNS reported no or soft snoring.17,21 Nightly use averaged 80% over 60 months based on patient reporting, with 87% reporting use at least 5 nights per week at 36 weeks.20
In terms of predictors of response to HNS therapy, the oxygen desaturation index is the only characteristic that reached a level of statistical significance; patients with higher levels of oxygen desaturation tended to improve and tolerate therapy better long-term.20 A randomized controlled trial of withdrawal of HNS therapy demonstrated increased AHI and oxygen desaturation index when therapy was withdrawn, followed by improvement when therapy resumed.22
A clinical trial of 20 patients implanted with HNS after its approval in 2014 reported that the mean AHI decreased from 33 before implant to 5.1 after implant.23 The ESS also improved from 10.3 before implant to 6 after implant. Mean adherence to device use was 7 (± 2) hours per night. The average stimulation amplitude was 1.89 (± 0.5) volts after the titration sleep study was completed. Similar reductions in AHI were reported by Huntley et al24 for patients receiving HNS implant at 2 academic centers, with no differences between the 2 cohorts in postoperative AHI.
Adverse events
The adverse events reported with HNS are related to the implant procedure or the device.21 Procedure-related adverse events are incision discomfort, temporary tongue weakness, headache, and mild infection of incisions. The most common device-related adverse event is discomfort from the electrical stimulation. Tongue abrasion can also occur if the tongue protrudes and rubs against a sharp tooth. Dry mouth is also commonly reported.
HNS compared with UPPP
Outcomes in patients with moderate to severe OSA matched for BMI, demographics, and preoperative AHI were evaluated comparing patients undergoing HNS (n = 20) with patients receiving UPPP (n = 20).25 The AHI decreased 29% postoperatively in patients with UPPP compared with 88% in patients with HNS, 65% of which had normalization of their AHI. Surgical success was achieved in 40% of patients in the UPPP group compared with 100% in the HNS group. Greater improvement in daytime sleepiness was noted in patients in the HNS group compared with the UPPP group.
ORAL APPLIANCE THERAPY
OAT devices help protrude the mandible forward and stabilize it to maintain a more patent airway during sleep. Oral appliances can be custom-made or prefabricated. Oral appliances can be titratable or nontitratable: titration provides a mechanism to adjust mandibular protrusion analogous to PAP titration, whereas the absence of titration holds the mandible in a single position. The most effective oral appliances are custom-made and titratable.
Types of OAT devices
Custom oral appliances. Custom oral appliances are fabricated using digital or physical impressions of the patient’s oral structures. Custom oral appliances are made of biocompatible materials and engage both the maxillary and mandibular arches.
Custom oral appliances are made by a qualified dentist who takes maxillary and mandibular impressions with a bite registration using the George Gauge with 40% to 50% of maximum protrusion. The appliance is fabricated in a laboratory and then fitted to the patient, who is instructed to titrate the device 0.5 mm to 1 mm per week and follow-up with the dentist at 2-week intervals. Once the patient has titrated the device to the point of comfort or improved sleep quality or snoring, polysomnography should be done with the device in place and titrated to improve the AHI as much as possible. Follow-up is recommended at 6 months and annually thereafter.
Prefabricated oral appliances. Prefabricated oral appliances are of the boil-and-bite type, only partially modified to the patient’s oral structures.
Tongue-retaining devices. Another type of oral appliance is a tongue-retaining device, which is designed to hold the tongue forward and can be custom-made or prefabricated.
Patient considerations for OAT
Practice recommendations
- Prescribed OAT should be done by a qualified dentist, and a custom, titratable appliance is preferred
- OAT is preferred over no therapy for adults with OSA who are intolerant to PAP or prefer alternative therapies
- A qualified dentist should provide oversight for dental-related side effects or occlusal changes
- Follow-up sleep testing should be conducted to confirm efficacy or titrate treatment
- Periodic office visits with the sleep physician and qualified dentist are recommended.26
The quality of evidence for these recommendations is low, with the exception of use of OAT rather than no therapy, which is considered of moderate quality.
Effects of OAT
Anatomic and physiologic effects. With OAT in place in the mouth, the airway caliber in the lateral dimension are increased, and the airway size at the retropalatal level is increased.27–30 With respect to the tongue, increased genioglossus muscle activity has been reported with OAT.
Side effects. Side effects of OAT include excess salivation, dry mouth, tooth tenderness, soft-tissue changes, jaw discomfort, tooth movement, and occlusal changes such as difficulty chewing in the morning. Feelings of suffocation, vivid dreams, and anxiety have also been reported with OAT.31–33
Efficacy and outcomes
Review of the data on the efficacy of OAT did not illuminate factors that predict treatment success.26 Data indicate that in patients with mild OSA using OAT or PAP therapy, there was no significant difference in the percentage achieving their target AHI; however, patients with moderate to severe OSA had a statistically significant greater odds of achieving their target AHI using PAP therapy compared with OAT. Therefore, OAT should be reserved for patients with severe OSA who cannot use or are intolerant to PAP.
Moderate-grade quality of evidence was reviewed for the established OAT practice recommendations for OSA outcomes before and after use of custom, titratable OAT devices.26 Use of a custom OAT device reduced the mean AHI, increased mean oxygen saturation, decreased the mean oxygen desaturation, decreased the arousal index, decreased the ESS, and increased quality of life compared with values prior to use of OAT.
With respect to adherence and discontinuation, patients using OAT had higher mean adherence and lower discontinuation because of side effects compared with patients using continuous PAP.26
NASAL EPAP THERAPY
Nasal EPAP is a new treatment for OSA that consists of a mechanical valve worn in each naris at night. The valves have a low inspiratory resistance and a high expiratory resistance thus increased pressure occurs at exhalation.
Pressure at exhalation may counter the airway collapse in OSA. With the mouth closed and use of the nasal valves, the positive pressure during the normal respiratory cycle is utilized to maintain an open airway.34 At the onset and throughout inspiration, the activity of the airway dilator muscles increases. At maximum expiration, right before the end of the expiratory pause, the dilator muscle stops abruptly and the airway is of its smallest caliber. The presence of the nasal valve at this point is thought to act as a pneumatic splint to the airway, and the nasal EPAP helps keep the airway patent during the next inspiratory phase.
Nasal EPAP valves are available in a 30-day starter kit. Intended for single-night use, the kit includes valves of increasing levels of expiration resistance: low (nights 1 and 2), medium (nights 3 and 4), and normal (nights 5–30).
Outcomes of nasal EPAP therapy
A multicenter 30-day in-home trial evaluated efficacy and compliance of nasal EPAP therapy.35 The AHI was reduced by 50% or more in 14 of 34 (41%) patients using nasal EPAP compared with the control group at the 30-day follow-up. The patient-reported compliance with nasal EPAP was 94%. Patients in this study had mild to moderate OSA and did not have obesity or other comorbidities such as pulmonary hypertension or cardiovascular disease.
A randomized controlled trial compared nasal EPAP with a sham device in patients with newly diagnosed or untreated OSA (N = 250) for 3 months.36 A median reduction of 52% in the AHI was noted in the intention-to-treat group (N = 229) during rapid eye movement (REM) and non-REM sleep, though it was statistically significant only during REM sleep and supine sleep. At 3 months, improved OSA was maintained in 42% of the patients using nasal EPAP compared with 10% of patients using a sham device. Improvements in daytime sleepiness and adherence with 88% using EPAP the entire night were also noted.
In a 12-month study of nasal EPAP, 67% of patients (34 of 51) used nasal EPAP for the full trial duration.37 Of patients using nasal EPAP for 12 months, the median AHI was reduced by 71%, the ESS improved, and adherence to full-night use was 89%.
Patient considerations for nasal EPAP
In clinical practice, nasal EPAP therapy requires nasal patency and use of a chin strap in patients with mouth leakage. Nasal EPAP may be recommended for patients who travel frequently and can go without continuous PAP or bilevel PAP for short periods of time, and for patients who do not have significant medical comorbidities.
Side effects and limitations of nasal EPAP
Reported side effects of nasal EPAP include difficulty with exhalation, nasal discomfort, dry mouth, and headache. Nasal EPAP therapy is of limited use in patients with severe OSA and severe oxygen desaturation. The efficacy of nasal EPAP beyond 12 months is unknown. Use of nasal EPAP in patients with prior upper-airway surgery and in combination with other therapies is yet to be evaluated.
- Tuomilehto HPI, Seppä JM, Partinen MM, et al; Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179(4):320–327.
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192(1):175–184.
- de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015; 11(2):131–137.
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45(4):353–359.
- Rowley JA, Badr MS. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th edition. Philadelphia, PA: Elsevier; 2017:1076–1087.
- Camacho M, Certal V, Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2013; 79(6):780–788.
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33(10):1408–1413.
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33(10):1396–1407.
- Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc 2009; 84(9):795–800.
- Larson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994; 104(11 Pt 1):1362–1368.
- Aboussouan LS, Golish JA, Wood BG, Mehta AC, Wood DE, Dinner DS. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest 1995; 107(4):946–951.
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9(5):433–438.
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124(3):797–802.
- Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop 2014; 146(4):477–492.
- Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016; 142(1):58–66.
- Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013; 23(3):414–423.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):139–149.
- Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg 2016; 154(5):970–977.
- Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015; 45(1):129–138.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018; 159(1):194–202.
- Woodson BT, Soose RJ, Gillespie MB; STAR Trial Investigators. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 2016; 154(1):181–188.
- Woodson BT, Gillespie MB, Soose RJ, et al; STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short-and long-term effect. Otolaryngol Head Neck Surg 2014; 151(5):880–887.
- Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016; 155(1):188–193.
- Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017; 13(9):1075–1079.
- Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018; 39(3):266–270.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015—an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015; 11(7):773–827.
- Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011; 34(4):469–477.
- Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake caliber of the velopharynx. Thorax 1999; 54(11):972–977.
- Tsuiki S, Ono T, Kuroda T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000; 4(2):53–58.
- Lowe AA. Oral appliances for sleep breathing disorders. Principles and Practice of Sleep Medicine. 3rd edition. In: Kryger MH, Roth T, Dement WE, eds. Philadelphia: Saunders; 2000:929–939.
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129(2):214–221.
- Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132(6):806–814.
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep 1999; 22(2):237–240.
- Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med 2008; 4(5):426–433.
- Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med 2009; 5(6):532–537.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34(4):479–485.
- Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011; 7(5):449–453.
- Tuomilehto HPI, Seppä JM, Partinen MM, et al; Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179(4):320–327.
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192(1):175–184.
- de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015; 11(2):131–137.
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45(4):353–359.
- Rowley JA, Badr MS. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th edition. Philadelphia, PA: Elsevier; 2017:1076–1087.
- Camacho M, Certal V, Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2013; 79(6):780–788.
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33(10):1408–1413.
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33(10):1396–1407.
- Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc 2009; 84(9):795–800.
- Larson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994; 104(11 Pt 1):1362–1368.
- Aboussouan LS, Golish JA, Wood BG, Mehta AC, Wood DE, Dinner DS. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest 1995; 107(4):946–951.
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9(5):433–438.
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124(3):797–802.
- Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop 2014; 146(4):477–492.
- Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016; 142(1):58–66.
- Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013; 23(3):414–423.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):139–149.
- Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg 2016; 154(5):970–977.
- Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015; 45(1):129–138.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018; 159(1):194–202.
- Woodson BT, Soose RJ, Gillespie MB; STAR Trial Investigators. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 2016; 154(1):181–188.
- Woodson BT, Gillespie MB, Soose RJ, et al; STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short-and long-term effect. Otolaryngol Head Neck Surg 2014; 151(5):880–887.
- Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016; 155(1):188–193.
- Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017; 13(9):1075–1079.
- Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018; 39(3):266–270.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015—an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015; 11(7):773–827.
- Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011; 34(4):469–477.
- Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake caliber of the velopharynx. Thorax 1999; 54(11):972–977.
- Tsuiki S, Ono T, Kuroda T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000; 4(2):53–58.
- Lowe AA. Oral appliances for sleep breathing disorders. Principles and Practice of Sleep Medicine. 3rd edition. In: Kryger MH, Roth T, Dement WE, eds. Philadelphia: Saunders; 2000:929–939.
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129(2):214–221.
- Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132(6):806–814.
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep 1999; 22(2):237–240.
- Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med 2008; 4(5):426–433.
- Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med 2009; 5(6):532–537.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34(4):479–485.
- Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011; 7(5):449–453.
KEY POINTS
- Alternative interventions for OSA are available for patients who cannot use PAP therapy.
- Lifestyle interventions that may benefit patients with OSA are weight loss, exercise, change in sleep position, alcohol avoidance, and a review of concomitant medications.
- Surgical interventions for OSA target the airway obstruction and include uvulopalatopharyngoplasty, maxillomandibular advancement, and bariatric surgery. Drug-induced sleep endoscopy is increasingly used to locate airway obstruction in patients with OSA.
- Alternative device therapies for OSA are the implanted hypoglossal nerve stimulation system, oral appliances, and nasal expiratory PAP therapy valves.