User login
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI) as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is 5 or more per hour with 1 or more of the following features: sleepiness; nonrestorative sleep; fatigue or insomnia; waking up with breath-holding spells, gasping, or choking; snoring or breathing interruptions; or a coexisting diagnosis of hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type II diabetes.1
Many patients with an AHI less than 15 may also have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation, and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease, and cognitive impairment.
QUALITY OF LIFE
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention, and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020 (healthypeople.gov).
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a 4-part process: use evidence-based therapy, monitor the therapy, assess symptoms with a validated tool such as Epworth Sleepiness Scale, and assess the incidence of motor vehicle accidents. Information from these 4 processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
DROWSY DRIVING
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (5-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is 6 times greater than in the general population.7
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of 5 years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are 2 main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
DEPRESSION
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
A one-level increase in the severity of OSA (ie, from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal, and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After 3 months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
METABOLIC SYNDROME
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these 2 conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has 3 or more of the following features or conditions:
- Waist circumference greater than 40 inches (men), greater than 35 inches (women)
- Triglycerides 150 mg/dL or greater or treatment for hypertriglyceridemia
- High-density lipoprotein cholesterol less than 40 mg/dL (men), less than 50 mg/dL (women), or treatment for cholesterol
- Blood pressure 130/85 mm Hg or greater, or treatment for hypertension
- Fasting blood glucose 100 mg/dL or greater, or treatment for hyperglycemia.19
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension
Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over 4 years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (ie, uncontrolled hypertension despite use of 3 or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a 4 times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA
Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver, and fat cells resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism, and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome
Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use 5 hours) reported an increase in the insulin sensitivity index after 2 days, and a further increase after 3 months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level, and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides, and possibly systolic blood pressure and triglycerides.60
COGNITIVE IMPAIRMENT
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a 2 times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage, and the impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a 4 times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition
One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition
White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention, and executive function paralleled the changes in white matter after the treatment.
CONCLUSION
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease, and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
- Sateia MJ. International Classification of Sleep Disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11(3):357–383.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002; 347(7): 498–504.
- Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med; 2017;13(11):1255–1263. doi:10.5664/jcsm.6792
- McDaid C, Dureé KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med Rev 2009; 13(6):427–436.
- Arita A, Sasanabe R, Hasegawa R, et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep Breath 2015; 19(4):1229–1234.
- Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J; the Cooperative Group Burgos–Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med 1999; 340(11):847–851.
- NHTSA. Drowsy driving. Washington, DC: National Highway Traffic Safety Administration. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/811449. Published March 2011. Accessed August 19, 2019.
- Vakulin A, D’Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016; 127(2):1428–1435.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20(8):608–613.
- George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56(7):508–512.
- Walia HK, Thompson N, Pascoe M, et al. Impact of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med (in press).
- Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8(8):17–25.
- Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9(5):417–423.
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166(16):1709–1715.
- Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest; 2004;125(4):1279–1285.
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11(9):1029–1038.
- Relia S, Thompson NR, Mehra R, et al. Depression score changes in response to sleep disordered breathing treatment with positive airway pressure in a large clinic-based cohort. Sleep Breath 2018; 22(1):195–203.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287(3):356–359.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ 2009; 108(5):263–265.
- Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med 2015; 15:105.
- Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009; 32(5):615–622.
- Sadasivam K, Chinnasami B, Ayyavo S, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 2015; 9(4):CC07–CC10.
- Chang TI, Tanner JM, Harada ND, Garrett NR, Friedlander AH. Prevalence of calcified carotid artery atheromas on the panoramic images of patients with syndrome Z, coexisting obstructive sleep apnea, and metabolic syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(1):134–141.
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208(2):490–495.
- Nakanishi-Minami T, Kishida K, Nakagawa Y, et al. Metabolic syndrome correlates intracoronary stenosis detected by multislice computed tomography in male subjects with sleep-disordered breathing. Diabetol Metab Syndr 2012; 4:6.
- Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath 2012; 16(3):677–684.
- Nieto FJ, Young TB, Lind BK, et al; for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283(14):1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342(19):1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307(20):2169–2176.
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007; 132(6):1858–1862.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10(8):835–843.
- Braincon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 2015; 7:25. doi:10.1186/s13098-015-0018-3
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137(1):95–101.
- Spiegel K. Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005; 99(5):2008–2019.
- Pépin J-L, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax 2012; 67(12):1025–1027.
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol (Lausanne) 2018; 9:440.
- Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg 2009; 64(4):329–334.
- Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 2011; 15(4):665–672.
- Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134(4):686–692.
- Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diag Research 2015; 9(4):CC07–CC10.
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169(2):156–162.
- Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9(5):679–687.
- Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analysis of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11(4):475–485.
- Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22(2):287–295.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006; 129(6):1459–1467.
- Durán-Cantolla J, Aizpuru F, Montserrat JM, et al; on behalf of the Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ 2010; 341:c5991.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370(24):2276–2285.
- Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006; 61(12):1083–1090.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310(22):2407–2415.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359(9302):204–210.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006; 27(6):1229–1235.
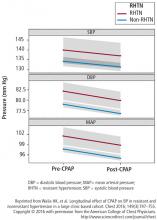
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149(3):747–755.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012; 8(5):587–596.
- Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, Li X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol 2017;43(5):373–379. doi:10.1590/S1806-37562016000000190. [Article in English, Portuguese]
- Foster GD, Borradaile KE, Sanders MH; for the Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169(17):1619–1626.
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014; 370(24):2265–2275.
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6):613–619.
- Kim H, Yun C-H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013; 36(5):709–715B.
- Chen H-L, Lu C-H, Lin H-C, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015; 38(3):361–370.
- Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015; 84(3):296–303.
- Osorio RS, Gumb T, Pirraglia E, et al; for the Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015; 84(19):1964–1971.
- Bu X-L, Liu Y-H, Wang Q-H, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015; 5:13917.
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein e 4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70(12):1544–1551.
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014; 35(suppl 2):S29–S34.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156):373–377.
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018; 86:142–149.
- Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2):115–119.
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014; 37(9):1465–1475.
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI) as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is 5 or more per hour with 1 or more of the following features: sleepiness; nonrestorative sleep; fatigue or insomnia; waking up with breath-holding spells, gasping, or choking; snoring or breathing interruptions; or a coexisting diagnosis of hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type II diabetes.1
Many patients with an AHI less than 15 may also have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation, and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease, and cognitive impairment.
QUALITY OF LIFE
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention, and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020 (healthypeople.gov).
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a 4-part process: use evidence-based therapy, monitor the therapy, assess symptoms with a validated tool such as Epworth Sleepiness Scale, and assess the incidence of motor vehicle accidents. Information from these 4 processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
DROWSY DRIVING
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (5-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is 6 times greater than in the general population.7
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of 5 years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are 2 main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
DEPRESSION
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
A one-level increase in the severity of OSA (ie, from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal, and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After 3 months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
METABOLIC SYNDROME
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these 2 conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has 3 or more of the following features or conditions:
- Waist circumference greater than 40 inches (men), greater than 35 inches (women)
- Triglycerides 150 mg/dL or greater or treatment for hypertriglyceridemia
- High-density lipoprotein cholesterol less than 40 mg/dL (men), less than 50 mg/dL (women), or treatment for cholesterol
- Blood pressure 130/85 mm Hg or greater, or treatment for hypertension
- Fasting blood glucose 100 mg/dL or greater, or treatment for hyperglycemia.19
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension
Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over 4 years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (ie, uncontrolled hypertension despite use of 3 or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a 4 times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA
Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver, and fat cells resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism, and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome
Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use 5 hours) reported an increase in the insulin sensitivity index after 2 days, and a further increase after 3 months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level, and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides, and possibly systolic blood pressure and triglycerides.60
COGNITIVE IMPAIRMENT
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a 2 times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage, and the impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a 4 times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition
One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition
White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention, and executive function paralleled the changes in white matter after the treatment.
CONCLUSION
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease, and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI) as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is 5 or more per hour with 1 or more of the following features: sleepiness; nonrestorative sleep; fatigue or insomnia; waking up with breath-holding spells, gasping, or choking; snoring or breathing interruptions; or a coexisting diagnosis of hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type II diabetes.1
Many patients with an AHI less than 15 may also have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation, and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease, and cognitive impairment.
QUALITY OF LIFE
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention, and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020 (healthypeople.gov).
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a 4-part process: use evidence-based therapy, monitor the therapy, assess symptoms with a validated tool such as Epworth Sleepiness Scale, and assess the incidence of motor vehicle accidents. Information from these 4 processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
DROWSY DRIVING
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (5-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is 6 times greater than in the general population.7
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of 5 years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are 2 main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
DEPRESSION
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
A one-level increase in the severity of OSA (ie, from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal, and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After 3 months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
METABOLIC SYNDROME
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these 2 conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has 3 or more of the following features or conditions:
- Waist circumference greater than 40 inches (men), greater than 35 inches (women)
- Triglycerides 150 mg/dL or greater or treatment for hypertriglyceridemia
- High-density lipoprotein cholesterol less than 40 mg/dL (men), less than 50 mg/dL (women), or treatment for cholesterol
- Blood pressure 130/85 mm Hg or greater, or treatment for hypertension
- Fasting blood glucose 100 mg/dL or greater, or treatment for hyperglycemia.19
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension
Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over 4 years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (ie, uncontrolled hypertension despite use of 3 or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a 4 times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA
Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver, and fat cells resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism, and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome
Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use 5 hours) reported an increase in the insulin sensitivity index after 2 days, and a further increase after 3 months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level, and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides, and possibly systolic blood pressure and triglycerides.60
COGNITIVE IMPAIRMENT
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a 2 times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage, and the impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a 4 times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition
One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition
White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention, and executive function paralleled the changes in white matter after the treatment.
CONCLUSION
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease, and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
- Sateia MJ. International Classification of Sleep Disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11(3):357–383.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002; 347(7): 498–504.
- Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med; 2017;13(11):1255–1263. doi:10.5664/jcsm.6792
- McDaid C, Dureé KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med Rev 2009; 13(6):427–436.
- Arita A, Sasanabe R, Hasegawa R, et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep Breath 2015; 19(4):1229–1234.
- Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J; the Cooperative Group Burgos–Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med 1999; 340(11):847–851.
- NHTSA. Drowsy driving. Washington, DC: National Highway Traffic Safety Administration. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/811449. Published March 2011. Accessed August 19, 2019.
- Vakulin A, D’Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016; 127(2):1428–1435.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20(8):608–613.
- George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56(7):508–512.
- Walia HK, Thompson N, Pascoe M, et al. Impact of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med (in press).
- Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8(8):17–25.
- Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9(5):417–423.
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166(16):1709–1715.
- Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest; 2004;125(4):1279–1285.
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11(9):1029–1038.
- Relia S, Thompson NR, Mehra R, et al. Depression score changes in response to sleep disordered breathing treatment with positive airway pressure in a large clinic-based cohort. Sleep Breath 2018; 22(1):195–203.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287(3):356–359.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ 2009; 108(5):263–265.
- Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med 2015; 15:105.
- Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009; 32(5):615–622.
- Sadasivam K, Chinnasami B, Ayyavo S, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 2015; 9(4):CC07–CC10.
- Chang TI, Tanner JM, Harada ND, Garrett NR, Friedlander AH. Prevalence of calcified carotid artery atheromas on the panoramic images of patients with syndrome Z, coexisting obstructive sleep apnea, and metabolic syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(1):134–141.
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208(2):490–495.
- Nakanishi-Minami T, Kishida K, Nakagawa Y, et al. Metabolic syndrome correlates intracoronary stenosis detected by multislice computed tomography in male subjects with sleep-disordered breathing. Diabetol Metab Syndr 2012; 4:6.
- Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath 2012; 16(3):677–684.
- Nieto FJ, Young TB, Lind BK, et al; for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283(14):1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342(19):1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307(20):2169–2176.
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007; 132(6):1858–1862.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10(8):835–843.
- Braincon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 2015; 7:25. doi:10.1186/s13098-015-0018-3
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137(1):95–101.
- Spiegel K. Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005; 99(5):2008–2019.
- Pépin J-L, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax 2012; 67(12):1025–1027.
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol (Lausanne) 2018; 9:440.
- Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg 2009; 64(4):329–334.
- Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 2011; 15(4):665–672.
- Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134(4):686–692.
- Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diag Research 2015; 9(4):CC07–CC10.
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169(2):156–162.
- Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9(5):679–687.
- Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analysis of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11(4):475–485.
- Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22(2):287–295.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006; 129(6):1459–1467.
- Durán-Cantolla J, Aizpuru F, Montserrat JM, et al; on behalf of the Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ 2010; 341:c5991.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370(24):2276–2285.
- Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006; 61(12):1083–1090.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310(22):2407–2415.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359(9302):204–210.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006; 27(6):1229–1235.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149(3):747–755.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012; 8(5):587–596.
- Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, Li X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol 2017;43(5):373–379. doi:10.1590/S1806-37562016000000190. [Article in English, Portuguese]
- Foster GD, Borradaile KE, Sanders MH; for the Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169(17):1619–1626.
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014; 370(24):2265–2275.
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6):613–619.
- Kim H, Yun C-H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013; 36(5):709–715B.
- Chen H-L, Lu C-H, Lin H-C, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015; 38(3):361–370.
- Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015; 84(3):296–303.
- Osorio RS, Gumb T, Pirraglia E, et al; for the Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015; 84(19):1964–1971.
- Bu X-L, Liu Y-H, Wang Q-H, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015; 5:13917.
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein e 4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70(12):1544–1551.
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014; 35(suppl 2):S29–S34.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156):373–377.
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018; 86:142–149.
- Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2):115–119.
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014; 37(9):1465–1475.
- Sateia MJ. International Classification of Sleep Disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11(3):357–383.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002; 347(7): 498–504.
- Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med; 2017;13(11):1255–1263. doi:10.5664/jcsm.6792
- McDaid C, Dureé KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med Rev 2009; 13(6):427–436.
- Arita A, Sasanabe R, Hasegawa R, et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep Breath 2015; 19(4):1229–1234.
- Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J; the Cooperative Group Burgos–Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med 1999; 340(11):847–851.
- NHTSA. Drowsy driving. Washington, DC: National Highway Traffic Safety Administration. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/811449. Published March 2011. Accessed August 19, 2019.
- Vakulin A, D’Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016; 127(2):1428–1435.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20(8):608–613.
- George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56(7):508–512.
- Walia HK, Thompson N, Pascoe M, et al. Impact of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med (in press).
- Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8(8):17–25.
- Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9(5):417–423.
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166(16):1709–1715.
- Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest; 2004;125(4):1279–1285.
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11(9):1029–1038.
- Relia S, Thompson NR, Mehra R, et al. Depression score changes in response to sleep disordered breathing treatment with positive airway pressure in a large clinic-based cohort. Sleep Breath 2018; 22(1):195–203.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287(3):356–359.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ 2009; 108(5):263–265.
- Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med 2015; 15:105.
- Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009; 32(5):615–622.
- Sadasivam K, Chinnasami B, Ayyavo S, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 2015; 9(4):CC07–CC10.
- Chang TI, Tanner JM, Harada ND, Garrett NR, Friedlander AH. Prevalence of calcified carotid artery atheromas on the panoramic images of patients with syndrome Z, coexisting obstructive sleep apnea, and metabolic syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(1):134–141.
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208(2):490–495.
- Nakanishi-Minami T, Kishida K, Nakagawa Y, et al. Metabolic syndrome correlates intracoronary stenosis detected by multislice computed tomography in male subjects with sleep-disordered breathing. Diabetol Metab Syndr 2012; 4:6.
- Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath 2012; 16(3):677–684.
- Nieto FJ, Young TB, Lind BK, et al; for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283(14):1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342(19):1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307(20):2169–2176.
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007; 132(6):1858–1862.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10(8):835–843.
- Braincon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 2015; 7:25. doi:10.1186/s13098-015-0018-3
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137(1):95–101.
- Spiegel K. Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005; 99(5):2008–2019.
- Pépin J-L, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax 2012; 67(12):1025–1027.
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol (Lausanne) 2018; 9:440.
- Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg 2009; 64(4):329–334.
- Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 2011; 15(4):665–672.
- Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134(4):686–692.
- Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diag Research 2015; 9(4):CC07–CC10.
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169(2):156–162.
- Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9(5):679–687.
- Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analysis of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11(4):475–485.
- Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22(2):287–295.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006; 129(6):1459–1467.
- Durán-Cantolla J, Aizpuru F, Montserrat JM, et al; on behalf of the Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ 2010; 341:c5991.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370(24):2276–2285.
- Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006; 61(12):1083–1090.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310(22):2407–2415.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359(9302):204–210.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006; 27(6):1229–1235.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149(3):747–755.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012; 8(5):587–596.
- Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, Li X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol 2017;43(5):373–379. doi:10.1590/S1806-37562016000000190. [Article in English, Portuguese]
- Foster GD, Borradaile KE, Sanders MH; for the Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169(17):1619–1626.
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014; 370(24):2265–2275.
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6):613–619.
- Kim H, Yun C-H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013; 36(5):709–715B.
- Chen H-L, Lu C-H, Lin H-C, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015; 38(3):361–370.
- Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015; 84(3):296–303.
- Osorio RS, Gumb T, Pirraglia E, et al; for the Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015; 84(19):1964–1971.
- Bu X-L, Liu Y-H, Wang Q-H, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015; 5:13917.
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein e 4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70(12):1544–1551.
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014; 35(suppl 2):S29–S34.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156):373–377.
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018; 86:142–149.
- Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2):115–119.
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014; 37(9):1465–1475.
KEY POINTS
- OSA is associated with negative health consequences such as depression, drowsy driving, metabolic disease, and cognitive decline.
- Several possible mechanisms to explain the health consequences of OSA have been explored.
- Treatment of patients with OSA may improve outcomes for many of the health consequences associated with OSA.
- Screening for OSA is important to identify and treat patients to reduce the associated health risks.