User login
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
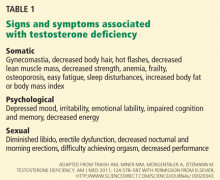
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
KEY POINTS
- General health benefits and safety of TRT in asymptomatic patients are not clearly defined by current data.
- Treatment of low testosterone is discouraged in the absence of clinical symptoms.
- A morning serum testosterone should be obtained after ruling out other causes of symptoms. It should also be repeated to confirm androgen deficiency in older men.
- Androgen deficiency in older men is associated with metabolic syndrome, type 2 diabetes mellitus, obesity, osteoporosis, renal failure, anemia, and previous treatment with steroids or opiates.
- TRT in men with a history of prostate cancer remains controversial. The existing limited data suggest that TRT is safe after curative therapy for prostate cancer. Patients treated should be monitored closely and informed of the risks of cancer progression and recurrence while they are on TRT.