User login
The following case was encountered and treated by Capt DellaVolpe during deployment with Operation Enduring Freedom-Trans Sahara on his tour as Special Operations Flight Surgeon with the Air Force Special Operations Command. The case highlights a rare but serious disease that can be particularly challenging to manage in military members serving overseas.
Case Presentation
After 2 days of worsening dyspnea on exertion, fever, and fatigue, a previously healthy 22-year-old man presented to a remote clinic established as part of a military deployment in central Africa. Despite having received azithromycin from a field medic, his condition continued to worsen. He had no cough, recent weight changes, or night sweats. The patient’s past medical history was unremarkable, including any prior history of pulmonary disorders.
The patient was a member of the U.S. Army Military Police Corps and had been deployed for 3 weeks. His job involved local patrols, and he had no history of airborne exposures, such as mold or chemical inhalants, or travel to sandy environments. Although he was previously a nonsmoker, he reported smoking local cigarettes to help him stay awake during night patrols over the past 2 weeks. The patient’s vaccination history included influenza, tetanus, measles/mumps/rubella, yellow fever, typhoid, hepatitis A/B, anthrax, meningococcus, and smallpox, all administered before deployment. At the time of evaluation, his temperature was 103.9°F, pulse 120 bpm, respiratory rate 32 breaths per minute, and blood pressure 110/70 mm Hg. His oxygen saturation was 80% on room air.
On examination, he was in significant distress and only able to speak in short sentences. There was no jugular venous distension or stridor. He was tachycardic, with a regular rhythm, without murmurs, rubs, or gallops. A pulmonary examination revealed decreased air movement bilaterally with bilateral inspiratory crackles at the bases. There was a tactile fremitus on the right side. He had no swelling or tenderness of the extremities, and no rashes were noted.
Laboratory capabilities were limited given the remote clinic location. Rapid malaria and rapid influenza were negative. A blood smear showed no organisms. A chest X-ray showed diffuse alveolar infiltrates and homogenous opacification of the right hemithorax.
The patient was placed on continuous oxygen by facemask and started on IV ceftriaxone and vancomycin. He was volume resuscitated with normal saline, with a modest effect on his heart rate. Attempts to wean his oxygen consumption were accompanied by an immediate oxygen desaturation to the low 80s. Because of the limited supply of oxygen available at the remote location as well as the patient’s poor response to broad-spectrum antibiotic coverage over the next 8 hours, he was evacuated by airborne casualty evacuation to the critical care team at Landstuhl Regional Medical Center in Germany.
Laboratory results revealed a peripheral leukocytosis with no eosinophilia. The patient underwent bronchoalveolar lavage (BAL), which showed 30% eosinophils. A diagnosis of acute eosinophilic pneumonia (AEP) was made, and the patient was started on IV methylprednisolone. He experienced a rapid resolution of symptoms and was completely weaned off oxygen 2 days later. The following week he was released from the hospital and able to return stateside to his unit.
Discussion
This case illustrates an uncommon but potentially life-threatening cause of respiratory failure, AEP. First described as a reversible, noninfectious cause of respiratory distress, AEP is now characterized as an uncommon yet severe febrile illness, which typically presents with hypoxia, pulmonary infiltrates, and increased eosinophilia on bronchoalveolar lavage in the absence of other causes.1-3 Untreated, AEP can progress to respiratory failure and death.
Acute eosinophilic pneumonia belongs to a heterogeneous group of disorders known as the eosinophilic lung syndromes. Although the pathophysiology has not been completely characterized, it is theorized that AEP is caused by a hypersensitivity reaction to an airborne antigen.2 Interleukin-5 has been implicated in the preferential activation of eosinophils and granule release and may be responsible for the eosinophilic alveolar exudates. In addition, interleukin-5 has been established as an eosinophil chemotactant.4-6
Acute eosinophilic pneumonia is relatively uncommon. Most epidemiologic studies have been limited to retrospective analyses and case series.7 However, the presentation of the disease suggests that it may be underdiagnosed.6,8 The most common presenting signs and symptoms are dyspnea, fever, cough, and crackles on inspiration. Acute eosinophilic pneumonia has been documented in men and women of all ages with a 2:1 male predominance, typically occurring in previously healthy individuals aged 18 to 40 years.9,10 Reports of AEP in infants also exist.11
Although idiopathic cases have been described in the literature, patients, on average, will have a history of airborne toxin exposure, including smoke, sand, dust, mold, or chemicals. However, a causal relationship has not been proven.12
Recent initiation of smoking has been associated with AEP, as was the case with this patient. This patient had no other history of airborne exposures, including burn pits, travel to sandy environments, mold, or chemical inhalants. Of all cases of AEP described to date, more than two-thirds have been associated with smoking.13,14 One Japanese case series further established the association through a “cigarette challenge test.”8,15,16 Typically, the patients described were in their mid-20s and rapidly developed symptoms consistent with AEP within 1 month of initiating smoking.
A high proportion of AEP has recently been described among newly deployed military personnel in Iraq.17 Out of 180,000 personnel deployed, 18 developed AEP with 14 having initiated smoking within 1 month of deployment. The authors concluded that compared with the controls, new-onset smokers had a significantly increased risk of developing AEP. Importantly, this case series describes 2 deaths associated with this disease, suggesting that this otherwise healthy population may have other environmental exposures that put them at a higher risk than that in the general population. Another case described a U.S. soldier in Korea with recent smoking history who was diagnosed with AEP on transbronchial lung biopsy.18
Given the nonspecific symptoms of the disease, early diagnosis relies on attention to clinical history, environmental exposures, and response to initial empiric treatment. Diagnosis is made based on pulmonary eosinophilia and exclusion of chronic causes of eosinophilic pulmonary disease, such as Churg-Strauss syndrome, chronic eosinophilic pneumonia, and tropical eosinophilia.9,19
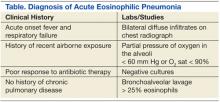
Criteria for diagnosis has evolved since AEP was initially reported. Current criteria include acute onset febrile respiratory manifestations < 1-month duration, bilateral diffuse infiltrates on chest radiograph, arterial oxygen pressure of < 60 mm Hg or pulse oximetry < 90% on room air, and BAL > 25% eosinophils. Blood, sputum, and BAL cultures must be negative for bacteria, fungi, and parasitic causes.7 Exposure to toxins known to cause eosinophilia should also be ruled out. Therefore, timely BAL is of paramount importance in any suspected case of AEP. Peripheral blood count will usually show a nonspecific leukocytosis, with a normal distribution of eosinophils; however, it is not uncommon for peripheral eosinophilia to occur late in the disease.14,20 Pulmonary eosinophilia in the absence of a peripheral eosinophilia is likely due to pulmonary eosinophil sequestration.
In one case series, the mean duration of time from symptom onset until diagnosis was 3.5 days.13 Diagnosis usually occurs after the patient’s clinical status worsens despite broad-spectrum antibiotic therapy. Acute eosinophilic pneumonia typically appears on a chest radiograph as bilateral reticular nodules with interstitial patterning. Variability can exist between alveolar, interstitial, and mixed infiltrate patterns.19 Pleural effusions may also be seen. These nonspecific patterns allow AEP to be easily mistaken for a variety of other pathologies, such as acute respiratory distress syndrome and community acquired pneumonia.
Corticosteroid therapy is the mainstay of treatment for AEP. Intravenous methylprednisolone is typically administered at dosages of 60 mg to 125 mg every 6 hours, followed by an oral prednisone taper.21 Relapses of AEP are not typical, and the prognosis is typically excellent if identified rapidly and treated appropriately. In one study of 127 individuals treated with corticosteroids, all survived, and most were transferred out of the ICU after 3 days of treatment. Dyspnea improved on treatment day 3, and all symptoms disappeared with an average of 7 days of treatment.7
Conclusion
Acute eosinophilic pneumonia is likely to be underreported clinically. Presently, AEP is largely a diagnosis of exclusion; the current criteria for diagnosis are fairly rigid and rely on BAL while ruling out other identifiable causes (Table). Clinical suspicion should be raised in patients with a history of new-onset smoking or other airborne toxin exposure. The broad spectrum of clinical presentations and diagnostic findings leave important questions unanswered regarding the mechanisms of the disease.
This particular case illustrates the fundamental importance of taking a thorough history in any patient with a recent airborne exposure where AEP is suspected. Acute eosinophilic pneumonia should be considered in cases of pneumonia that continue to worsen despite the treatment of IV antibiotics; a BAL should be performed when appropriate.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989;321(9):569-574.
2. Badesch DB, King TE Jr, Schwarz MI. Acute eosinophilic pneumonia: A hypersensitivity phenomenon? Am Rev Respir Dis. 1989;139(1):249-252.
3. Buchheit J, Eid N, Rodgers G Jr, Feger T, Yakoub O. Acute eosinophilic pneumonia with respiratory failure: A new syndrome? Am Rev Respir Dis. 1992;145(3):716-718.
4. Okubo Y, Hossain M, Kai R, et al. Adhesion molecules on eosinophils in acute eosinophilic pneumonia. Am J Respir Crit Care Med. 1995;151(4):1259-1262.
5. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996;97(6):1366-1374.
6. Faustino L, da Fonseca DM, Takenaka MC, et al. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol. 2013;190(6):2614-2621.
7. Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41(2):402-409.
8. Shiota Y, Kawai T, Matsumoto H, et al. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000;39(10):830-833.
9. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75(6):334-342.
10. Rose DM, Hrncir DE. Primary eosinophilic lung diseases. Allergy Asthma Proc. 2013;34(1):19-25.
11. Park HN, Chung BH, Pyun JE, et al. Idiopathic acute eosinophilic pneumonia in a 14-month-old girl. Korean J Pediatr. 2013;56(1):37-41.
12. Kolb AG, Ives ST, Davies SF. Diagnosis in just over a minute: A case of chronic eosinophilic pneumonia. J Gen Intern Med. 2013;28(7):972-975.
13. Janz DR, O’Neal HR Jr, Ely EW. Acute eosinophilic pneumonia: A case report and review of the literature. Crit Care Med. 2009;37(4):1470-1474.
14. Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med. 2002;166(9):1235-1239.
15. Miki K, Miki M, Nakamura Y, et al. Early-phase neutrophilia in cigarette smoke-induced acute eosinophilic pneumonia. Intern Med. 2003;42(9):839-845.
16. Watanabe K, Fujimura M, Kasahara K, et al. Acute eosinophilic pneumonia following cigarette smoking: A case report including cigarette-smoking challenge test. Intern Med. 2002;41(11):1016-1020.
17. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA.2004;
292(24):2997-3005.
18. Lim SY, Suh GY, Jeon K. Acute eosinophilic pneumonia presenting as life-threatening hypoxaemia necessitating extracorporeal membrane oxygenation. Int J Tuberc Lung Dis. 2012;16(12):1711-1712.
19. Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150(5, pt 1):1423-1438.
20. Hayakawa H, Sato A, Toyoshima M, Imokawa S, Taniguchi M. A clinical study of idiopathic eosinophilic pneumonia. Chest. 1994;105(5):1462-1466.
21. Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med. 1999;160(4):1079-1100.
The following case was encountered and treated by Capt DellaVolpe during deployment with Operation Enduring Freedom-Trans Sahara on his tour as Special Operations Flight Surgeon with the Air Force Special Operations Command. The case highlights a rare but serious disease that can be particularly challenging to manage in military members serving overseas.
Case Presentation
After 2 days of worsening dyspnea on exertion, fever, and fatigue, a previously healthy 22-year-old man presented to a remote clinic established as part of a military deployment in central Africa. Despite having received azithromycin from a field medic, his condition continued to worsen. He had no cough, recent weight changes, or night sweats. The patient’s past medical history was unremarkable, including any prior history of pulmonary disorders.
The patient was a member of the U.S. Army Military Police Corps and had been deployed for 3 weeks. His job involved local patrols, and he had no history of airborne exposures, such as mold or chemical inhalants, or travel to sandy environments. Although he was previously a nonsmoker, he reported smoking local cigarettes to help him stay awake during night patrols over the past 2 weeks. The patient’s vaccination history included influenza, tetanus, measles/mumps/rubella, yellow fever, typhoid, hepatitis A/B, anthrax, meningococcus, and smallpox, all administered before deployment. At the time of evaluation, his temperature was 103.9°F, pulse 120 bpm, respiratory rate 32 breaths per minute, and blood pressure 110/70 mm Hg. His oxygen saturation was 80% on room air.
On examination, he was in significant distress and only able to speak in short sentences. There was no jugular venous distension or stridor. He was tachycardic, with a regular rhythm, without murmurs, rubs, or gallops. A pulmonary examination revealed decreased air movement bilaterally with bilateral inspiratory crackles at the bases. There was a tactile fremitus on the right side. He had no swelling or tenderness of the extremities, and no rashes were noted.
Laboratory capabilities were limited given the remote clinic location. Rapid malaria and rapid influenza were negative. A blood smear showed no organisms. A chest X-ray showed diffuse alveolar infiltrates and homogenous opacification of the right hemithorax.
The patient was placed on continuous oxygen by facemask and started on IV ceftriaxone and vancomycin. He was volume resuscitated with normal saline, with a modest effect on his heart rate. Attempts to wean his oxygen consumption were accompanied by an immediate oxygen desaturation to the low 80s. Because of the limited supply of oxygen available at the remote location as well as the patient’s poor response to broad-spectrum antibiotic coverage over the next 8 hours, he was evacuated by airborne casualty evacuation to the critical care team at Landstuhl Regional Medical Center in Germany.
Laboratory results revealed a peripheral leukocytosis with no eosinophilia. The patient underwent bronchoalveolar lavage (BAL), which showed 30% eosinophils. A diagnosis of acute eosinophilic pneumonia (AEP) was made, and the patient was started on IV methylprednisolone. He experienced a rapid resolution of symptoms and was completely weaned off oxygen 2 days later. The following week he was released from the hospital and able to return stateside to his unit.
Discussion
This case illustrates an uncommon but potentially life-threatening cause of respiratory failure, AEP. First described as a reversible, noninfectious cause of respiratory distress, AEP is now characterized as an uncommon yet severe febrile illness, which typically presents with hypoxia, pulmonary infiltrates, and increased eosinophilia on bronchoalveolar lavage in the absence of other causes.1-3 Untreated, AEP can progress to respiratory failure and death.
Acute eosinophilic pneumonia belongs to a heterogeneous group of disorders known as the eosinophilic lung syndromes. Although the pathophysiology has not been completely characterized, it is theorized that AEP is caused by a hypersensitivity reaction to an airborne antigen.2 Interleukin-5 has been implicated in the preferential activation of eosinophils and granule release and may be responsible for the eosinophilic alveolar exudates. In addition, interleukin-5 has been established as an eosinophil chemotactant.4-6
Acute eosinophilic pneumonia is relatively uncommon. Most epidemiologic studies have been limited to retrospective analyses and case series.7 However, the presentation of the disease suggests that it may be underdiagnosed.6,8 The most common presenting signs and symptoms are dyspnea, fever, cough, and crackles on inspiration. Acute eosinophilic pneumonia has been documented in men and women of all ages with a 2:1 male predominance, typically occurring in previously healthy individuals aged 18 to 40 years.9,10 Reports of AEP in infants also exist.11
Although idiopathic cases have been described in the literature, patients, on average, will have a history of airborne toxin exposure, including smoke, sand, dust, mold, or chemicals. However, a causal relationship has not been proven.12
Recent initiation of smoking has been associated with AEP, as was the case with this patient. This patient had no other history of airborne exposures, including burn pits, travel to sandy environments, mold, or chemical inhalants. Of all cases of AEP described to date, more than two-thirds have been associated with smoking.13,14 One Japanese case series further established the association through a “cigarette challenge test.”8,15,16 Typically, the patients described were in their mid-20s and rapidly developed symptoms consistent with AEP within 1 month of initiating smoking.
A high proportion of AEP has recently been described among newly deployed military personnel in Iraq.17 Out of 180,000 personnel deployed, 18 developed AEP with 14 having initiated smoking within 1 month of deployment. The authors concluded that compared with the controls, new-onset smokers had a significantly increased risk of developing AEP. Importantly, this case series describes 2 deaths associated with this disease, suggesting that this otherwise healthy population may have other environmental exposures that put them at a higher risk than that in the general population. Another case described a U.S. soldier in Korea with recent smoking history who was diagnosed with AEP on transbronchial lung biopsy.18
Given the nonspecific symptoms of the disease, early diagnosis relies on attention to clinical history, environmental exposures, and response to initial empiric treatment. Diagnosis is made based on pulmonary eosinophilia and exclusion of chronic causes of eosinophilic pulmonary disease, such as Churg-Strauss syndrome, chronic eosinophilic pneumonia, and tropical eosinophilia.9,19
Criteria for diagnosis has evolved since AEP was initially reported. Current criteria include acute onset febrile respiratory manifestations < 1-month duration, bilateral diffuse infiltrates on chest radiograph, arterial oxygen pressure of < 60 mm Hg or pulse oximetry < 90% on room air, and BAL > 25% eosinophils. Blood, sputum, and BAL cultures must be negative for bacteria, fungi, and parasitic causes.7 Exposure to toxins known to cause eosinophilia should also be ruled out. Therefore, timely BAL is of paramount importance in any suspected case of AEP. Peripheral blood count will usually show a nonspecific leukocytosis, with a normal distribution of eosinophils; however, it is not uncommon for peripheral eosinophilia to occur late in the disease.14,20 Pulmonary eosinophilia in the absence of a peripheral eosinophilia is likely due to pulmonary eosinophil sequestration.
In one case series, the mean duration of time from symptom onset until diagnosis was 3.5 days.13 Diagnosis usually occurs after the patient’s clinical status worsens despite broad-spectrum antibiotic therapy. Acute eosinophilic pneumonia typically appears on a chest radiograph as bilateral reticular nodules with interstitial patterning. Variability can exist between alveolar, interstitial, and mixed infiltrate patterns.19 Pleural effusions may also be seen. These nonspecific patterns allow AEP to be easily mistaken for a variety of other pathologies, such as acute respiratory distress syndrome and community acquired pneumonia.
Corticosteroid therapy is the mainstay of treatment for AEP. Intravenous methylprednisolone is typically administered at dosages of 60 mg to 125 mg every 6 hours, followed by an oral prednisone taper.21 Relapses of AEP are not typical, and the prognosis is typically excellent if identified rapidly and treated appropriately. In one study of 127 individuals treated with corticosteroids, all survived, and most were transferred out of the ICU after 3 days of treatment. Dyspnea improved on treatment day 3, and all symptoms disappeared with an average of 7 days of treatment.7
Conclusion
Acute eosinophilic pneumonia is likely to be underreported clinically. Presently, AEP is largely a diagnosis of exclusion; the current criteria for diagnosis are fairly rigid and rely on BAL while ruling out other identifiable causes (Table). Clinical suspicion should be raised in patients with a history of new-onset smoking or other airborne toxin exposure. The broad spectrum of clinical presentations and diagnostic findings leave important questions unanswered regarding the mechanisms of the disease.
This particular case illustrates the fundamental importance of taking a thorough history in any patient with a recent airborne exposure where AEP is suspected. Acute eosinophilic pneumonia should be considered in cases of pneumonia that continue to worsen despite the treatment of IV antibiotics; a BAL should be performed when appropriate.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The following case was encountered and treated by Capt DellaVolpe during deployment with Operation Enduring Freedom-Trans Sahara on his tour as Special Operations Flight Surgeon with the Air Force Special Operations Command. The case highlights a rare but serious disease that can be particularly challenging to manage in military members serving overseas.
Case Presentation
After 2 days of worsening dyspnea on exertion, fever, and fatigue, a previously healthy 22-year-old man presented to a remote clinic established as part of a military deployment in central Africa. Despite having received azithromycin from a field medic, his condition continued to worsen. He had no cough, recent weight changes, or night sweats. The patient’s past medical history was unremarkable, including any prior history of pulmonary disorders.
The patient was a member of the U.S. Army Military Police Corps and had been deployed for 3 weeks. His job involved local patrols, and he had no history of airborne exposures, such as mold or chemical inhalants, or travel to sandy environments. Although he was previously a nonsmoker, he reported smoking local cigarettes to help him stay awake during night patrols over the past 2 weeks. The patient’s vaccination history included influenza, tetanus, measles/mumps/rubella, yellow fever, typhoid, hepatitis A/B, anthrax, meningococcus, and smallpox, all administered before deployment. At the time of evaluation, his temperature was 103.9°F, pulse 120 bpm, respiratory rate 32 breaths per minute, and blood pressure 110/70 mm Hg. His oxygen saturation was 80% on room air.
On examination, he was in significant distress and only able to speak in short sentences. There was no jugular venous distension or stridor. He was tachycardic, with a regular rhythm, without murmurs, rubs, or gallops. A pulmonary examination revealed decreased air movement bilaterally with bilateral inspiratory crackles at the bases. There was a tactile fremitus on the right side. He had no swelling or tenderness of the extremities, and no rashes were noted.
Laboratory capabilities were limited given the remote clinic location. Rapid malaria and rapid influenza were negative. A blood smear showed no organisms. A chest X-ray showed diffuse alveolar infiltrates and homogenous opacification of the right hemithorax.
The patient was placed on continuous oxygen by facemask and started on IV ceftriaxone and vancomycin. He was volume resuscitated with normal saline, with a modest effect on his heart rate. Attempts to wean his oxygen consumption were accompanied by an immediate oxygen desaturation to the low 80s. Because of the limited supply of oxygen available at the remote location as well as the patient’s poor response to broad-spectrum antibiotic coverage over the next 8 hours, he was evacuated by airborne casualty evacuation to the critical care team at Landstuhl Regional Medical Center in Germany.
Laboratory results revealed a peripheral leukocytosis with no eosinophilia. The patient underwent bronchoalveolar lavage (BAL), which showed 30% eosinophils. A diagnosis of acute eosinophilic pneumonia (AEP) was made, and the patient was started on IV methylprednisolone. He experienced a rapid resolution of symptoms and was completely weaned off oxygen 2 days later. The following week he was released from the hospital and able to return stateside to his unit.
Discussion
This case illustrates an uncommon but potentially life-threatening cause of respiratory failure, AEP. First described as a reversible, noninfectious cause of respiratory distress, AEP is now characterized as an uncommon yet severe febrile illness, which typically presents with hypoxia, pulmonary infiltrates, and increased eosinophilia on bronchoalveolar lavage in the absence of other causes.1-3 Untreated, AEP can progress to respiratory failure and death.
Acute eosinophilic pneumonia belongs to a heterogeneous group of disorders known as the eosinophilic lung syndromes. Although the pathophysiology has not been completely characterized, it is theorized that AEP is caused by a hypersensitivity reaction to an airborne antigen.2 Interleukin-5 has been implicated in the preferential activation of eosinophils and granule release and may be responsible for the eosinophilic alveolar exudates. In addition, interleukin-5 has been established as an eosinophil chemotactant.4-6
Acute eosinophilic pneumonia is relatively uncommon. Most epidemiologic studies have been limited to retrospective analyses and case series.7 However, the presentation of the disease suggests that it may be underdiagnosed.6,8 The most common presenting signs and symptoms are dyspnea, fever, cough, and crackles on inspiration. Acute eosinophilic pneumonia has been documented in men and women of all ages with a 2:1 male predominance, typically occurring in previously healthy individuals aged 18 to 40 years.9,10 Reports of AEP in infants also exist.11
Although idiopathic cases have been described in the literature, patients, on average, will have a history of airborne toxin exposure, including smoke, sand, dust, mold, or chemicals. However, a causal relationship has not been proven.12
Recent initiation of smoking has been associated with AEP, as was the case with this patient. This patient had no other history of airborne exposures, including burn pits, travel to sandy environments, mold, or chemical inhalants. Of all cases of AEP described to date, more than two-thirds have been associated with smoking.13,14 One Japanese case series further established the association through a “cigarette challenge test.”8,15,16 Typically, the patients described were in their mid-20s and rapidly developed symptoms consistent with AEP within 1 month of initiating smoking.
A high proportion of AEP has recently been described among newly deployed military personnel in Iraq.17 Out of 180,000 personnel deployed, 18 developed AEP with 14 having initiated smoking within 1 month of deployment. The authors concluded that compared with the controls, new-onset smokers had a significantly increased risk of developing AEP. Importantly, this case series describes 2 deaths associated with this disease, suggesting that this otherwise healthy population may have other environmental exposures that put them at a higher risk than that in the general population. Another case described a U.S. soldier in Korea with recent smoking history who was diagnosed with AEP on transbronchial lung biopsy.18
Given the nonspecific symptoms of the disease, early diagnosis relies on attention to clinical history, environmental exposures, and response to initial empiric treatment. Diagnosis is made based on pulmonary eosinophilia and exclusion of chronic causes of eosinophilic pulmonary disease, such as Churg-Strauss syndrome, chronic eosinophilic pneumonia, and tropical eosinophilia.9,19
Criteria for diagnosis has evolved since AEP was initially reported. Current criteria include acute onset febrile respiratory manifestations < 1-month duration, bilateral diffuse infiltrates on chest radiograph, arterial oxygen pressure of < 60 mm Hg or pulse oximetry < 90% on room air, and BAL > 25% eosinophils. Blood, sputum, and BAL cultures must be negative for bacteria, fungi, and parasitic causes.7 Exposure to toxins known to cause eosinophilia should also be ruled out. Therefore, timely BAL is of paramount importance in any suspected case of AEP. Peripheral blood count will usually show a nonspecific leukocytosis, with a normal distribution of eosinophils; however, it is not uncommon for peripheral eosinophilia to occur late in the disease.14,20 Pulmonary eosinophilia in the absence of a peripheral eosinophilia is likely due to pulmonary eosinophil sequestration.
In one case series, the mean duration of time from symptom onset until diagnosis was 3.5 days.13 Diagnosis usually occurs after the patient’s clinical status worsens despite broad-spectrum antibiotic therapy. Acute eosinophilic pneumonia typically appears on a chest radiograph as bilateral reticular nodules with interstitial patterning. Variability can exist between alveolar, interstitial, and mixed infiltrate patterns.19 Pleural effusions may also be seen. These nonspecific patterns allow AEP to be easily mistaken for a variety of other pathologies, such as acute respiratory distress syndrome and community acquired pneumonia.
Corticosteroid therapy is the mainstay of treatment for AEP. Intravenous methylprednisolone is typically administered at dosages of 60 mg to 125 mg every 6 hours, followed by an oral prednisone taper.21 Relapses of AEP are not typical, and the prognosis is typically excellent if identified rapidly and treated appropriately. In one study of 127 individuals treated with corticosteroids, all survived, and most were transferred out of the ICU after 3 days of treatment. Dyspnea improved on treatment day 3, and all symptoms disappeared with an average of 7 days of treatment.7
Conclusion
Acute eosinophilic pneumonia is likely to be underreported clinically. Presently, AEP is largely a diagnosis of exclusion; the current criteria for diagnosis are fairly rigid and rely on BAL while ruling out other identifiable causes (Table). Clinical suspicion should be raised in patients with a history of new-onset smoking or other airborne toxin exposure. The broad spectrum of clinical presentations and diagnostic findings leave important questions unanswered regarding the mechanisms of the disease.
This particular case illustrates the fundamental importance of taking a thorough history in any patient with a recent airborne exposure where AEP is suspected. Acute eosinophilic pneumonia should be considered in cases of pneumonia that continue to worsen despite the treatment of IV antibiotics; a BAL should be performed when appropriate.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989;321(9):569-574.
2. Badesch DB, King TE Jr, Schwarz MI. Acute eosinophilic pneumonia: A hypersensitivity phenomenon? Am Rev Respir Dis. 1989;139(1):249-252.
3. Buchheit J, Eid N, Rodgers G Jr, Feger T, Yakoub O. Acute eosinophilic pneumonia with respiratory failure: A new syndrome? Am Rev Respir Dis. 1992;145(3):716-718.
4. Okubo Y, Hossain M, Kai R, et al. Adhesion molecules on eosinophils in acute eosinophilic pneumonia. Am J Respir Crit Care Med. 1995;151(4):1259-1262.
5. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996;97(6):1366-1374.
6. Faustino L, da Fonseca DM, Takenaka MC, et al. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol. 2013;190(6):2614-2621.
7. Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41(2):402-409.
8. Shiota Y, Kawai T, Matsumoto H, et al. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000;39(10):830-833.
9. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75(6):334-342.
10. Rose DM, Hrncir DE. Primary eosinophilic lung diseases. Allergy Asthma Proc. 2013;34(1):19-25.
11. Park HN, Chung BH, Pyun JE, et al. Idiopathic acute eosinophilic pneumonia in a 14-month-old girl. Korean J Pediatr. 2013;56(1):37-41.
12. Kolb AG, Ives ST, Davies SF. Diagnosis in just over a minute: A case of chronic eosinophilic pneumonia. J Gen Intern Med. 2013;28(7):972-975.
13. Janz DR, O’Neal HR Jr, Ely EW. Acute eosinophilic pneumonia: A case report and review of the literature. Crit Care Med. 2009;37(4):1470-1474.
14. Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med. 2002;166(9):1235-1239.
15. Miki K, Miki M, Nakamura Y, et al. Early-phase neutrophilia in cigarette smoke-induced acute eosinophilic pneumonia. Intern Med. 2003;42(9):839-845.
16. Watanabe K, Fujimura M, Kasahara K, et al. Acute eosinophilic pneumonia following cigarette smoking: A case report including cigarette-smoking challenge test. Intern Med. 2002;41(11):1016-1020.
17. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA.2004;
292(24):2997-3005.
18. Lim SY, Suh GY, Jeon K. Acute eosinophilic pneumonia presenting as life-threatening hypoxaemia necessitating extracorporeal membrane oxygenation. Int J Tuberc Lung Dis. 2012;16(12):1711-1712.
19. Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150(5, pt 1):1423-1438.
20. Hayakawa H, Sato A, Toyoshima M, Imokawa S, Taniguchi M. A clinical study of idiopathic eosinophilic pneumonia. Chest. 1994;105(5):1462-1466.
21. Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med. 1999;160(4):1079-1100.
1. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989;321(9):569-574.
2. Badesch DB, King TE Jr, Schwarz MI. Acute eosinophilic pneumonia: A hypersensitivity phenomenon? Am Rev Respir Dis. 1989;139(1):249-252.
3. Buchheit J, Eid N, Rodgers G Jr, Feger T, Yakoub O. Acute eosinophilic pneumonia with respiratory failure: A new syndrome? Am Rev Respir Dis. 1992;145(3):716-718.
4. Okubo Y, Hossain M, Kai R, et al. Adhesion molecules on eosinophils in acute eosinophilic pneumonia. Am J Respir Crit Care Med. 1995;151(4):1259-1262.
5. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996;97(6):1366-1374.
6. Faustino L, da Fonseca DM, Takenaka MC, et al. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol. 2013;190(6):2614-2621.
7. Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41(2):402-409.
8. Shiota Y, Kawai T, Matsumoto H, et al. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000;39(10):830-833.
9. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75(6):334-342.
10. Rose DM, Hrncir DE. Primary eosinophilic lung diseases. Allergy Asthma Proc. 2013;34(1):19-25.
11. Park HN, Chung BH, Pyun JE, et al. Idiopathic acute eosinophilic pneumonia in a 14-month-old girl. Korean J Pediatr. 2013;56(1):37-41.
12. Kolb AG, Ives ST, Davies SF. Diagnosis in just over a minute: A case of chronic eosinophilic pneumonia. J Gen Intern Med. 2013;28(7):972-975.
13. Janz DR, O’Neal HR Jr, Ely EW. Acute eosinophilic pneumonia: A case report and review of the literature. Crit Care Med. 2009;37(4):1470-1474.
14. Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med. 2002;166(9):1235-1239.
15. Miki K, Miki M, Nakamura Y, et al. Early-phase neutrophilia in cigarette smoke-induced acute eosinophilic pneumonia. Intern Med. 2003;42(9):839-845.
16. Watanabe K, Fujimura M, Kasahara K, et al. Acute eosinophilic pneumonia following cigarette smoking: A case report including cigarette-smoking challenge test. Intern Med. 2002;41(11):1016-1020.
17. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA.2004;
292(24):2997-3005.
18. Lim SY, Suh GY, Jeon K. Acute eosinophilic pneumonia presenting as life-threatening hypoxaemia necessitating extracorporeal membrane oxygenation. Int J Tuberc Lung Dis. 2012;16(12):1711-1712.
19. Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150(5, pt 1):1423-1438.
20. Hayakawa H, Sato A, Toyoshima M, Imokawa S, Taniguchi M. A clinical study of idiopathic eosinophilic pneumonia. Chest. 1994;105(5):1462-1466.
21. Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med. 1999;160(4):1079-1100.