User login
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
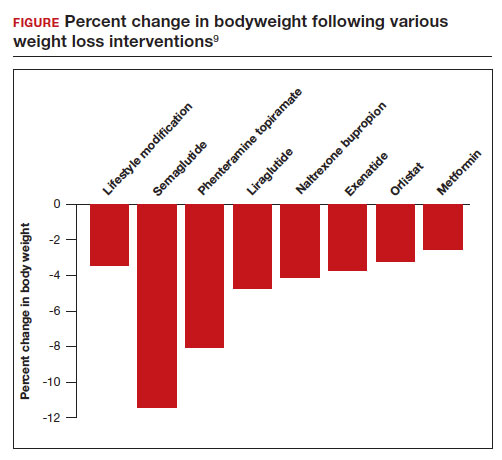
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.
GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9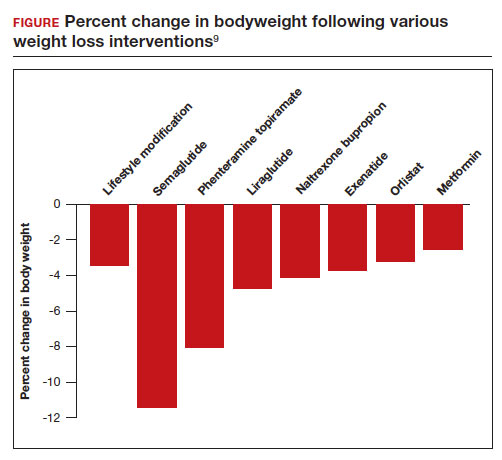
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.

GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.

GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.