Article
The clinical utility of newly approved angiogenic markers for identifying patients at risk for adverse outcomes due to preeclampsia
- Author:
- Robert L. Barbieri, MD
Preeclampsia is one of the biggest clinical challenges in obstetric practice. A normal ratio of soluble fms-like tyrosine kinase-...
Article

Breakthroughs in the prevention of RSV disease among infants
- Author:
- Robert L. Barbieri, MD
RSV disease is a major public health problem among infants younger than age 8 months, causing 100 to 300 deaths annually. In 2023, two new...
Article

Nonhormonal medication treatment of VMS
- Author:
- Robert L. Barbieri, MD
For patients with bothersome vasomotor symptoms (VMS) who cannot or choose not to take estrogen, nonhormonal medication options include...
Article

Medication treatment of opioid use disorder in primary care practice: Opportunities and limitations
- Author:
- Robert L. Barbieri, MD
Restrictions on prescribing buprenorphine (BUP) for opioid use disorder (OUD) have been rescinded, increasing the opportunity for primary care...
Article
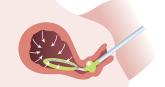
Intrauterine vacuum device treatment of postpartum hemorrhage
- Author:
- Robert L. Barbieri, MD
First-line, conservative treatment of postpartum hemorrhage includes uterotonic medications and, if indicated, uterine balloon tamponade or...
Article
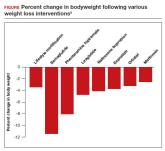
Anti-obesity medications: Breakthroughs and limitations
- Author:
- Robert L. Barbieri, MD
Semaglutide is a breakthrough, practice-changing weight loss medication that reliably causes significant weight loss; however, weight gain is...
Article

Therapeutic hypothermia to treat neonatal encephalopathy improves childhood outcomes
- Author:
- Robert L. Barbieri, MD
Whole-body cooling, initiated within 6 hours of birth for a newborn with neonatal encephalopathy, improves childhood neurodevelopmental outcomes,...
Article
Would you prescribe antenatal steroids to a pregnant patient at high risk for delivering at 22 weeks’ gestation?
- Author:
- Robert L. Barbieri, MD
For infants born at 22 to 23 weeks’ gestation, the combination of a completed course of antenatal steroids plus active support of the newborn at...
Article

What is the most effective management of first trimester miscarriage?
- Author:
- Robert L. Barbieri, MD
A Cochrane network meta-analysis reported that both uterine aspiration and medication management are more effective than expectant management for...
Article
Advances in the treatment of fetal demise in the second and third trimester
- Author:
- Robert L. Barbieri, MD
Treatment of fetal demise is best managed as a 2-step process, beginning with cervical preparation using cervical dilators,...
Article
Is it time to reconsider Rh testing and Rh D immune globulin treatment for miscarriage and abortion care in early pregnancy?
- Author:
- Robert L. Barbieri, MD
New guidelines propose to reduce the use of Rh testing and Rh D immune globulin administration for miscarriage and abortion care in early...
Article

35 years in service to you, our community of reproductive health care clinicians
- Author:
- Robert L. Barbieri, MD
Article
Simplify your approach to the diagnosis and treatment of PCOS
- Author:
- Robert L. Barbieri, MD
Simplify the diagnosis of polycystic ovary syndrome (PCOS) by focusing on 2 core criteria: hyperandrogenism and oligo-ovulation....
Article

Should every scheduled cesarean birth use an Enhanced Recovery after Surgery (ERAS) pathway?
- Author:
- Julianna Schantz-Dunn, MD, MPH
- Robert L. Barbieri, MD
Standardization of surgical processes, with adjustment based on unique individual characteristics, improves outcomes
Article
Dietary sodium and potassium consumption and cardiovascular health
- Author:
- Robert L. Barbieri, MD
Diets with high amounts of potassium and low amounts of sodium are associated with the best cardiovascular outcomes
...
