User login
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
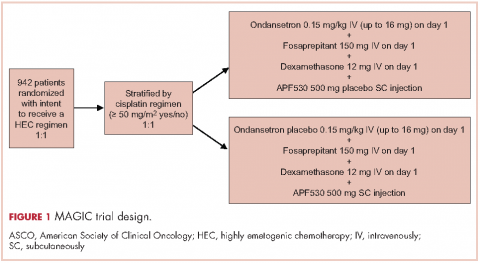
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
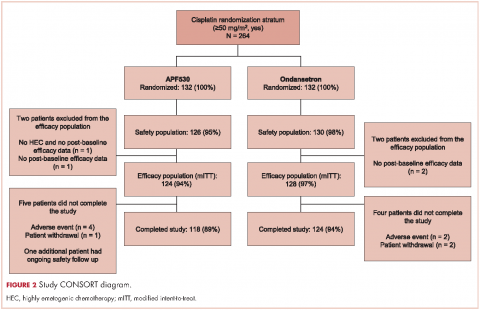
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
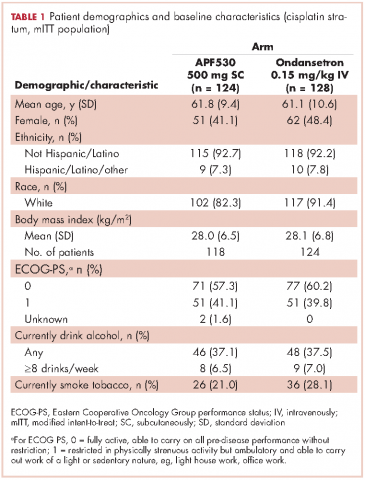
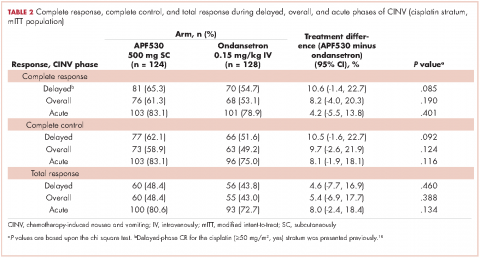
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
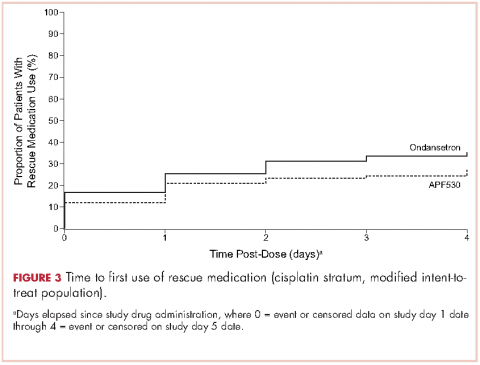
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
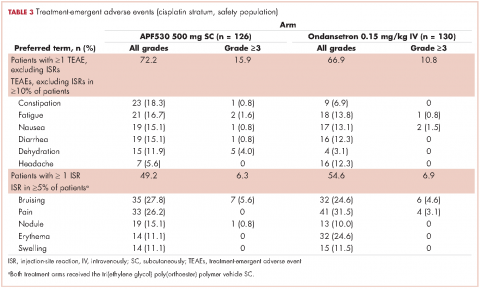
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.