User login

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
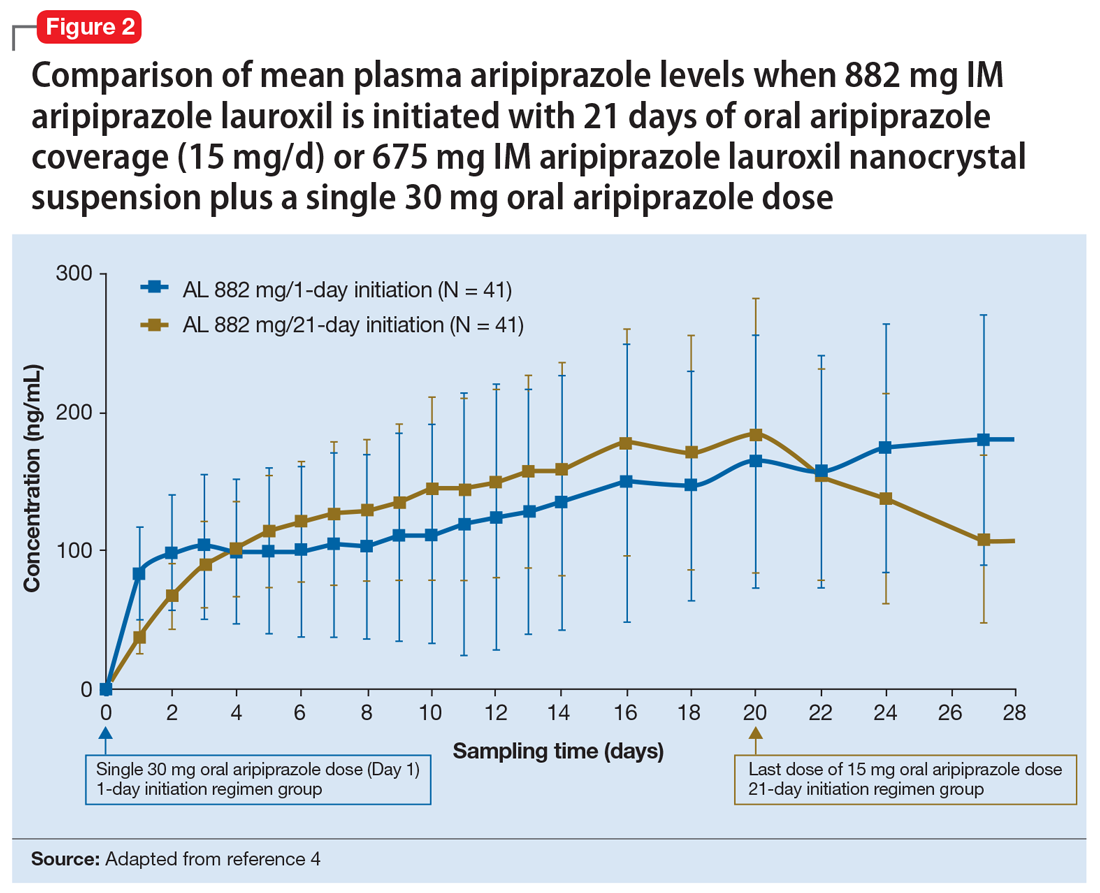
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL
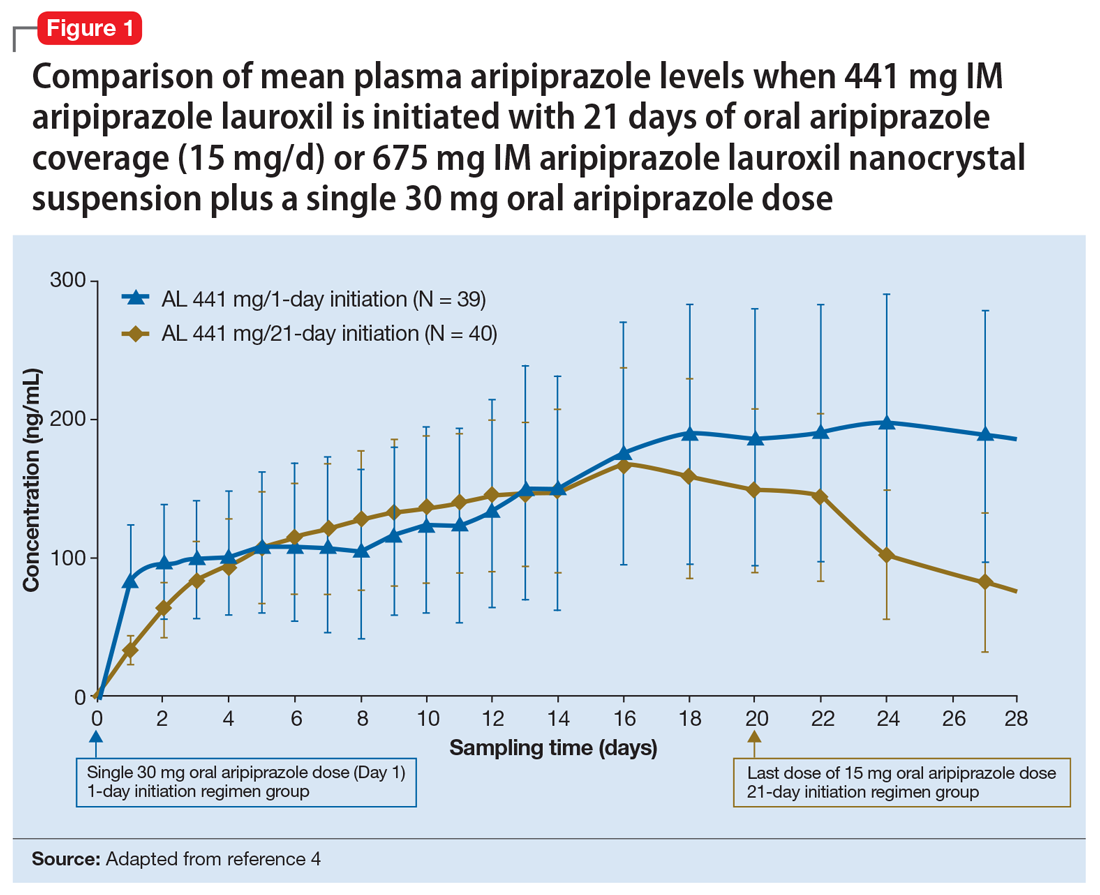
Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL
Continue to: Clinical considerations
Clinical considerations
AL
Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
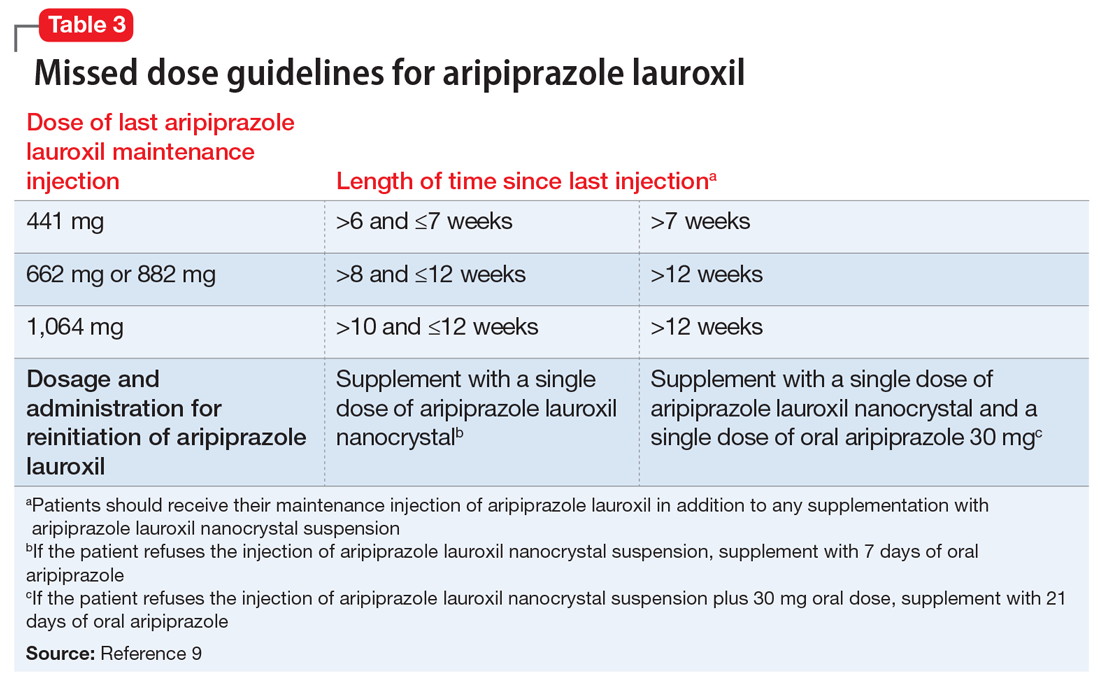
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL

Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL

Continue to: Clinical considerations
Clinical considerations
AL

Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL

Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL

Continue to: Clinical considerations
Clinical considerations
AL

Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.