User login
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
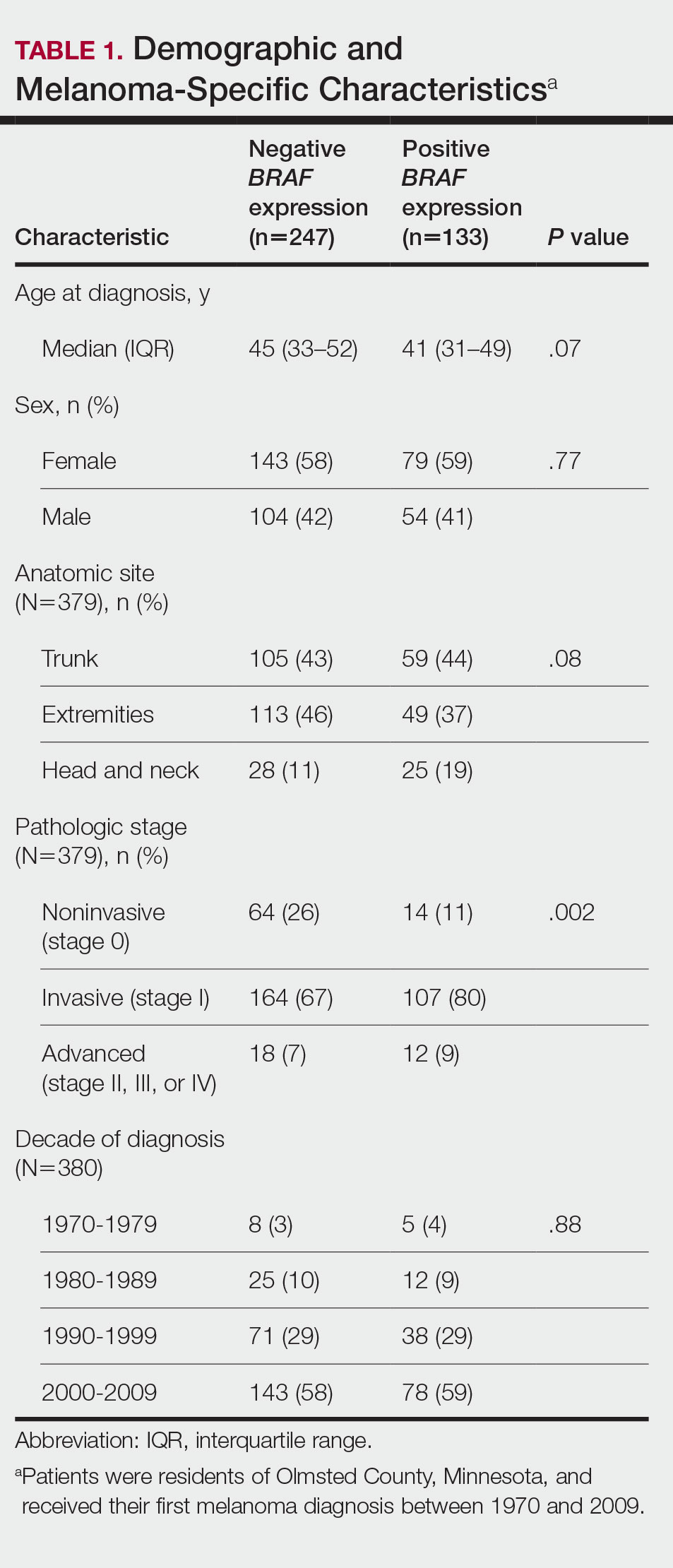
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results
Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
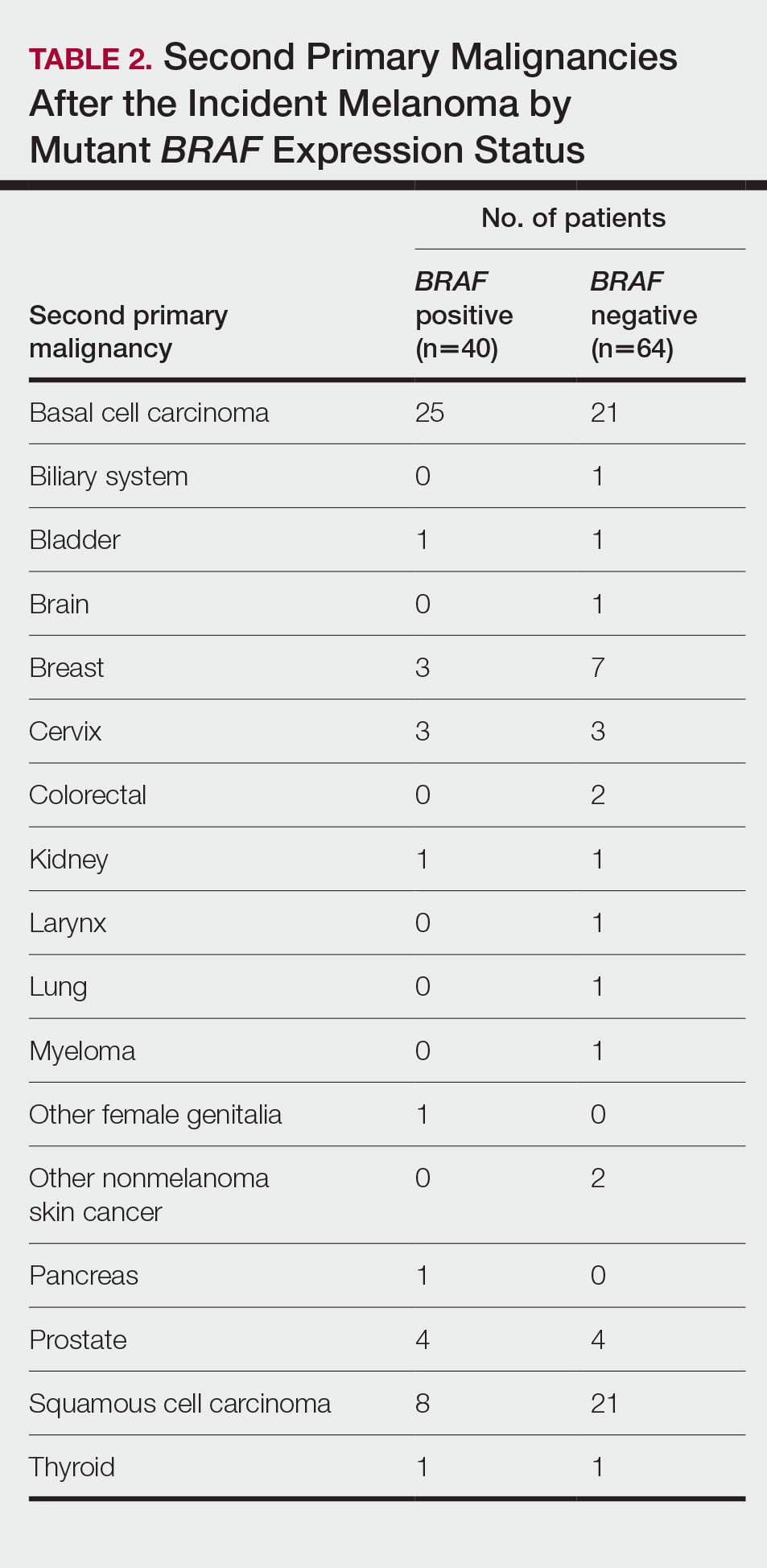
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.
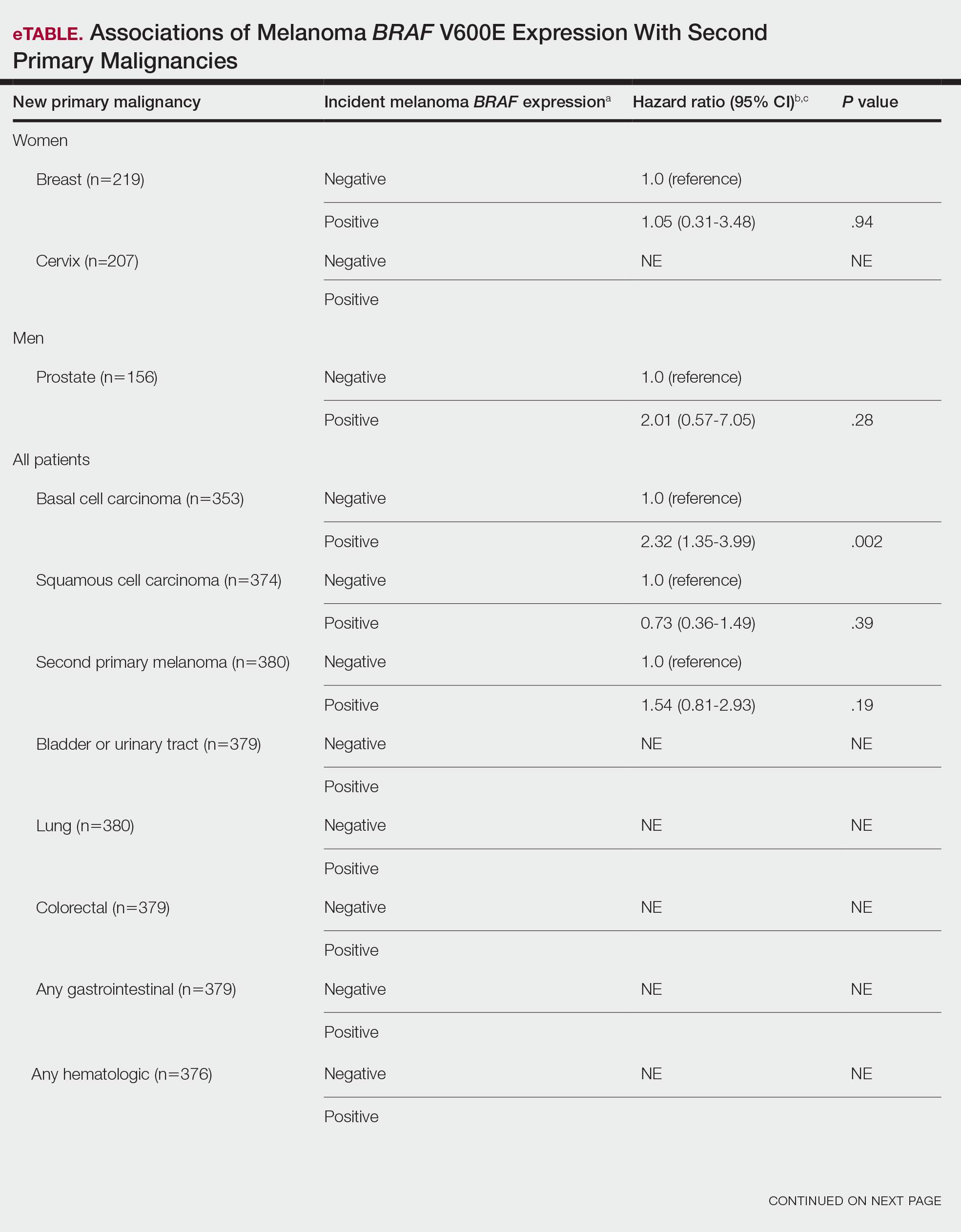
BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.
Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.
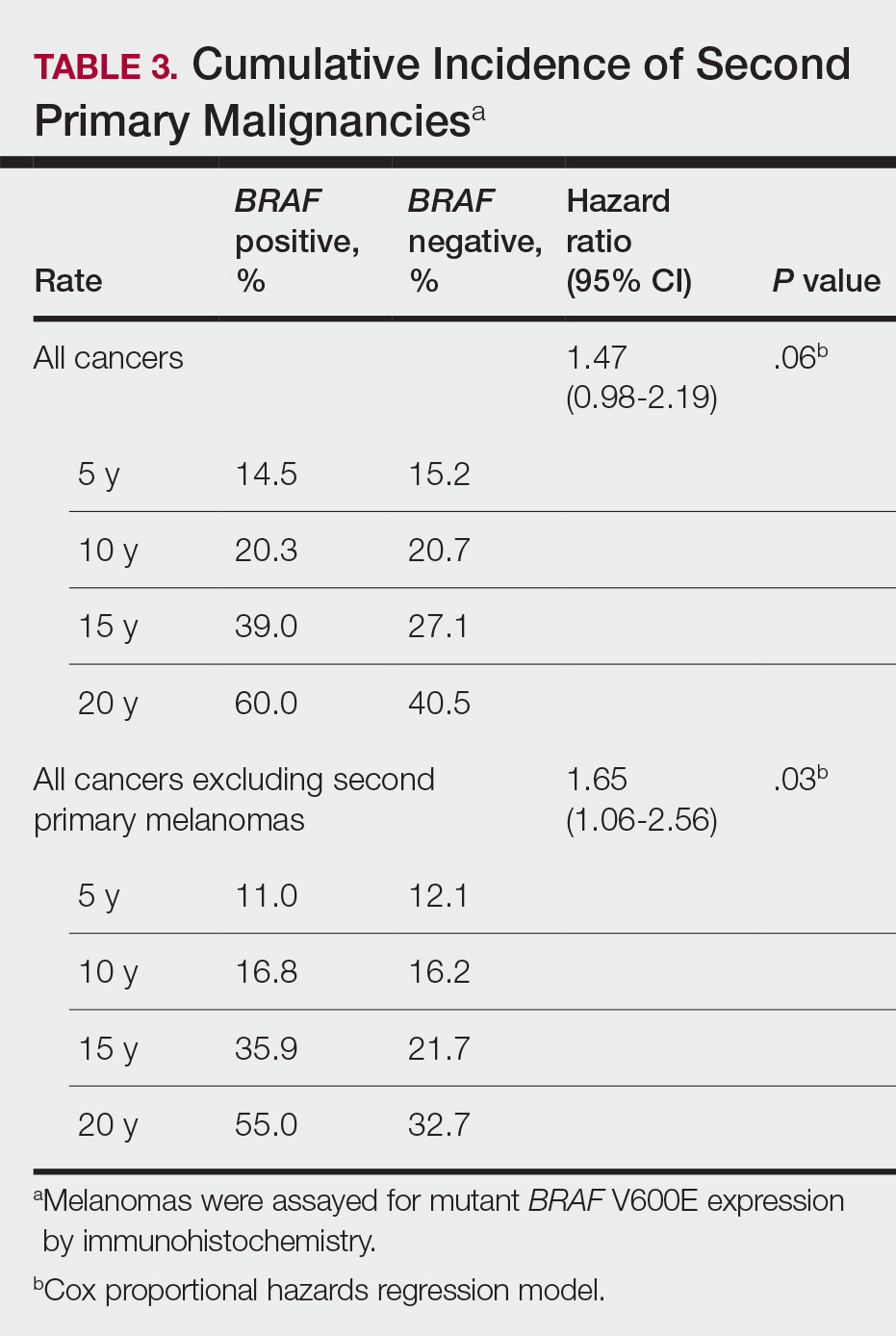
The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
Practice Points
- Dermatologists should be aware of the long-term risk of second primary malignancies after an incident melanoma.
- BRAF mutations occur in melanomas and several other cancers. Our study found that melanoma BRAF V600E expression is associated with an increased risk for basal cell carcinomas.