User login
The Diagnosis: Primary Cutaneous Perivascular Epithelioid Cell Tumor
Perivascular epithelioid cell tumors (PEComas) were first described in 1996.1 They comprise a family of rare mesenchymal neoplasms that have a unique characteristic of staining positive for melanocytic and smooth muscle markers on immunohistochemistry.2 These neoplasms have been described in many areas of the body including the uterus, bladder, heart, pancreas, and prostate. The majority of PEComas are extracutaneous, with only 8% of reported cases originating on the skin.3 A case of primary cutaneous PEComa (pcPEComa) was described in 2003.4 The primary cutaneous form is extremely rare.3,5-7
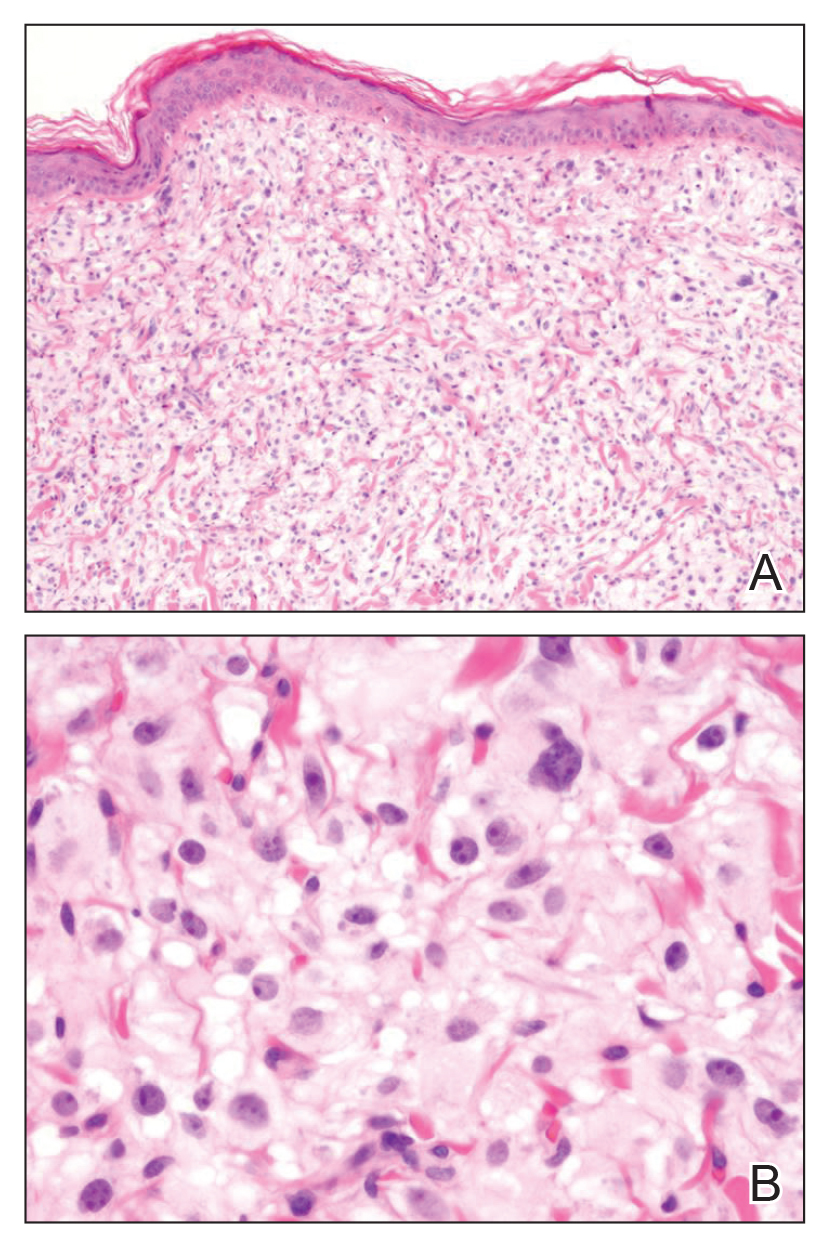
A broad deep shave biopsy was performed in our patient in an attempt to sample the entire lesion. Histopathologic examination of the nodule demonstrated a dermal neoplasm comprised of a diffuse proliferation of large polygonal cells with abundant clear cytoplasm, fine chromatin, and prominent nucleoli (Figure 1A). Higher-power magnification showed moderate nuclear pleomorphism and only rare mitotic figures (Figure 1B).
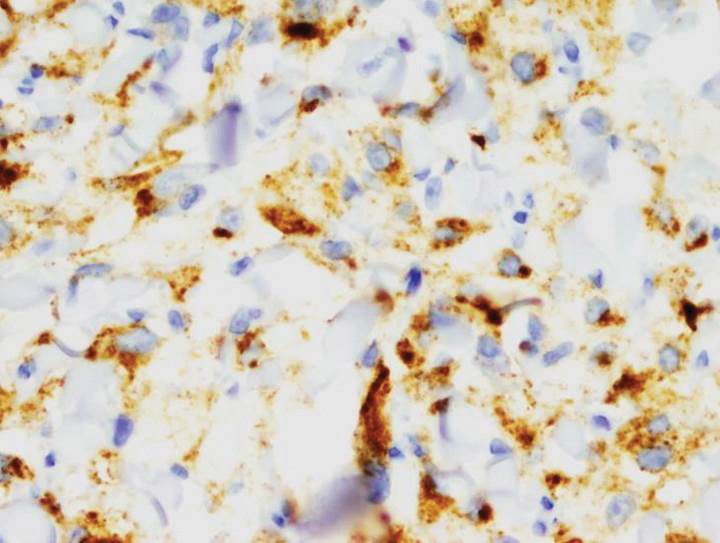
Immunohistochemical staining revealed positivity for myomelanocytic markers with positivity for human melanoma black 45 (HMB-45)(Figure 2) and desmin (not shown). Additionally, the tumor was positive for CD163 and negative for smooth muscle actin, cytokeratin, and S-100 protein.
Perivascular epithelioid cell tumors are characterized histologically as mesenchymal neoplasms containing large epithelioid to spindled cells with a slightly granular, vacuolated cytoplasm. These cells often are found in close proximity to vascular structures.3,5,8 The hallmark of PEComas is the expression of both melanocytic and muscle markers.3,8 A review of staining patterns of pcPEComas emphasized that immunophenotypes between visceral and primary cutaneous forms may vary considerably.3,5,8 The most consistent and sensitive melanocytic marker is HMB-45 (88%-92% positive).3,8 Positive Melan-A staining varies in the literature from 0% to 50% of cases.3 Our patient's neoplasm expressed the characteristic myomelanocytic immunophenotype with both HMB-45 and desmin positivity.
Given the histologic characteristics, these lesions can be mistaken for melanocytic and other nonmelanocytic tumors with a clear cell morphology such as balloon cell nevus, hypomelanotic blue nevus, and melanoma.2,3 A pigmented case of pcPEComa was reported in 2015 and was originally diagnosed as metastatic melanoma.6 Unlike pcPEComa, melanoma usually stains positive with S-100 protein in up to 99% of cases8 and is negative for muscle markers; however, a case series reported S-100 protein positivity in 38% of pcPEComas.3 Nonmelanocytic neoplasms in the histologic differential diagnosis include clear cell sarcoma and clear cell renal cell carcinoma, both of which show immunoreactivity for cytokeratin.9
Histologic criteria exist for establishing malignancy potential for visceral PEComas but not for pcPEComas, though it has been suggested that the same malignancy criteria should be applied to pcPEComas.3,9 Features associated with malignancy include size greater than 8 cm, mitotic activity greater than 1 mitosis per 50 high-power fields, infiltrative growth pattern, high nuclear grade, necrosis, and vascular invasion. Based on these criteria, fulfilling 2 or more features technically classifies the lesion as malignant, 1 feature classifies it as uncertain malignant potential, and a lack of these features renders the lesion benign.9
The overwhelming majority of pcPEComas are considered benign. One case of pcPEComa was considered malignant with a high mitotic rate (5 mitoses per 10 high-power fields) and nuclear atypia.10 Further workup with thoracic computed tomography and positron emission tomography-computed tomography was negative for metastasis. Treatment with wide excision and radiotherapy was performed with no sign of recurrence at 24-month follow-up.10
Although pcPEComas arising from the dermis seem to be benign overall, PEComas originating from the subcutaneous tissue may have greater malignancy potential. Two cases of subcutaneous PEComas presenting as nodules resulted in metastasis; one case had local nodal metastasis and another developed metastasis to the lungs months later.10,11
- Zamboni G, Pea M, Martignoni G, et al. Clear cell “sugar” tumorof the pancreas. a novel member of the family of lesions characterizedby the presence of perivascular epithelioid cells. Am J Surg Pathol.1996;20:722-730.
- Folpe AK, Wiatkowski D. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1-15.
- Charli-Joseph Y, Saggini A, Vemula S, et al. Primary cutaneous perivascularepithelioid cell tumor: a clinicopathological and molecular reappraisal. J Am Acad Dermatol. 2014;71:1127-1136.
- Crowson AN, Taylor JR, Magro CM. Cutaneous clear cell myomelanocytictumor-perivascular epithelioid cell tumor: first reported case. Mod Pathol. 2003;16:90A.
- Chaplin A, Conrad D, Tatlidil C, et al. Primary cutaneous PEComa. Am J Dermatopathol. 2010;32:310-312.
- Navale P, Asgari M, Chen S. Pigmented perivascular epithelioid cell tumor of the skin. Am J Dermatopathol. 2015;37:866-869.
- Ieremia E, Robson A. Cutaneous PEComa. Am J Dermatopathol. 2014;36:E198-E201.
- Calder K, Schlauder S, Morgan M. Malignant perivascularepithelioid cell tumor (‘PEComa’): a case report and literature review of cutaneous/subcutaneous presentations. J Cutan Pathol. 2008;35:499-503.
- Folpe A, Mentzel T, Lehr H, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Dermatopathol. 2005; 29:1558-1575.
- Greveling K, Winnepenninckx V, Nagtzaam I, et al. Malignant perivascular epithelioid cell tumor: a case report of a cutaneous tumor on the cheek of a male patient. J Am Acad Dermatol. 2013;69:E262-E264.
- Shon W, Kim J, Sukov W, et al. Malignant TFE3-rearranged perivascular epithelioid cell neoplasm (PEComa) presenting as a subcutaneous mass. Br J Dermatol. 2015;174:617-620.
The Diagnosis: Primary Cutaneous Perivascular Epithelioid Cell Tumor
Perivascular epithelioid cell tumors (PEComas) were first described in 1996.1 They comprise a family of rare mesenchymal neoplasms that have a unique characteristic of staining positive for melanocytic and smooth muscle markers on immunohistochemistry.2 These neoplasms have been described in many areas of the body including the uterus, bladder, heart, pancreas, and prostate. The majority of PEComas are extracutaneous, with only 8% of reported cases originating on the skin.3 A case of primary cutaneous PEComa (pcPEComa) was described in 2003.4 The primary cutaneous form is extremely rare.3,5-7
A broad deep shave biopsy was performed in our patient in an attempt to sample the entire lesion. Histopathologic examination of the nodule demonstrated a dermal neoplasm comprised of a diffuse proliferation of large polygonal cells with abundant clear cytoplasm, fine chromatin, and prominent nucleoli (Figure 1A). Higher-power magnification showed moderate nuclear pleomorphism and only rare mitotic figures (Figure 1B).
Immunohistochemical staining revealed positivity for myomelanocytic markers with positivity for human melanoma black 45 (HMB-45)(Figure 2) and desmin (not shown). Additionally, the tumor was positive for CD163 and negative for smooth muscle actin, cytokeratin, and S-100 protein.
Perivascular epithelioid cell tumors are characterized histologically as mesenchymal neoplasms containing large epithelioid to spindled cells with a slightly granular, vacuolated cytoplasm. These cells often are found in close proximity to vascular structures.3,5,8 The hallmark of PEComas is the expression of both melanocytic and muscle markers.3,8 A review of staining patterns of pcPEComas emphasized that immunophenotypes between visceral and primary cutaneous forms may vary considerably.3,5,8 The most consistent and sensitive melanocytic marker is HMB-45 (88%-92% positive).3,8 Positive Melan-A staining varies in the literature from 0% to 50% of cases.3 Our patient's neoplasm expressed the characteristic myomelanocytic immunophenotype with both HMB-45 and desmin positivity.
Given the histologic characteristics, these lesions can be mistaken for melanocytic and other nonmelanocytic tumors with a clear cell morphology such as balloon cell nevus, hypomelanotic blue nevus, and melanoma.2,3 A pigmented case of pcPEComa was reported in 2015 and was originally diagnosed as metastatic melanoma.6 Unlike pcPEComa, melanoma usually stains positive with S-100 protein in up to 99% of cases8 and is negative for muscle markers; however, a case series reported S-100 protein positivity in 38% of pcPEComas.3 Nonmelanocytic neoplasms in the histologic differential diagnosis include clear cell sarcoma and clear cell renal cell carcinoma, both of which show immunoreactivity for cytokeratin.9
Histologic criteria exist for establishing malignancy potential for visceral PEComas but not for pcPEComas, though it has been suggested that the same malignancy criteria should be applied to pcPEComas.3,9 Features associated with malignancy include size greater than 8 cm, mitotic activity greater than 1 mitosis per 50 high-power fields, infiltrative growth pattern, high nuclear grade, necrosis, and vascular invasion. Based on these criteria, fulfilling 2 or more features technically classifies the lesion as malignant, 1 feature classifies it as uncertain malignant potential, and a lack of these features renders the lesion benign.9
The overwhelming majority of pcPEComas are considered benign. One case of pcPEComa was considered malignant with a high mitotic rate (5 mitoses per 10 high-power fields) and nuclear atypia.10 Further workup with thoracic computed tomography and positron emission tomography-computed tomography was negative for metastasis. Treatment with wide excision and radiotherapy was performed with no sign of recurrence at 24-month follow-up.10
Although pcPEComas arising from the dermis seem to be benign overall, PEComas originating from the subcutaneous tissue may have greater malignancy potential. Two cases of subcutaneous PEComas presenting as nodules resulted in metastasis; one case had local nodal metastasis and another developed metastasis to the lungs months later.10,11
The Diagnosis: Primary Cutaneous Perivascular Epithelioid Cell Tumor
Perivascular epithelioid cell tumors (PEComas) were first described in 1996.1 They comprise a family of rare mesenchymal neoplasms that have a unique characteristic of staining positive for melanocytic and smooth muscle markers on immunohistochemistry.2 These neoplasms have been described in many areas of the body including the uterus, bladder, heart, pancreas, and prostate. The majority of PEComas are extracutaneous, with only 8% of reported cases originating on the skin.3 A case of primary cutaneous PEComa (pcPEComa) was described in 2003.4 The primary cutaneous form is extremely rare.3,5-7
A broad deep shave biopsy was performed in our patient in an attempt to sample the entire lesion. Histopathologic examination of the nodule demonstrated a dermal neoplasm comprised of a diffuse proliferation of large polygonal cells with abundant clear cytoplasm, fine chromatin, and prominent nucleoli (Figure 1A). Higher-power magnification showed moderate nuclear pleomorphism and only rare mitotic figures (Figure 1B).
Immunohistochemical staining revealed positivity for myomelanocytic markers with positivity for human melanoma black 45 (HMB-45)(Figure 2) and desmin (not shown). Additionally, the tumor was positive for CD163 and negative for smooth muscle actin, cytokeratin, and S-100 protein.
Perivascular epithelioid cell tumors are characterized histologically as mesenchymal neoplasms containing large epithelioid to spindled cells with a slightly granular, vacuolated cytoplasm. These cells often are found in close proximity to vascular structures.3,5,8 The hallmark of PEComas is the expression of both melanocytic and muscle markers.3,8 A review of staining patterns of pcPEComas emphasized that immunophenotypes between visceral and primary cutaneous forms may vary considerably.3,5,8 The most consistent and sensitive melanocytic marker is HMB-45 (88%-92% positive).3,8 Positive Melan-A staining varies in the literature from 0% to 50% of cases.3 Our patient's neoplasm expressed the characteristic myomelanocytic immunophenotype with both HMB-45 and desmin positivity.
Given the histologic characteristics, these lesions can be mistaken for melanocytic and other nonmelanocytic tumors with a clear cell morphology such as balloon cell nevus, hypomelanotic blue nevus, and melanoma.2,3 A pigmented case of pcPEComa was reported in 2015 and was originally diagnosed as metastatic melanoma.6 Unlike pcPEComa, melanoma usually stains positive with S-100 protein in up to 99% of cases8 and is negative for muscle markers; however, a case series reported S-100 protein positivity in 38% of pcPEComas.3 Nonmelanocytic neoplasms in the histologic differential diagnosis include clear cell sarcoma and clear cell renal cell carcinoma, both of which show immunoreactivity for cytokeratin.9
Histologic criteria exist for establishing malignancy potential for visceral PEComas but not for pcPEComas, though it has been suggested that the same malignancy criteria should be applied to pcPEComas.3,9 Features associated with malignancy include size greater than 8 cm, mitotic activity greater than 1 mitosis per 50 high-power fields, infiltrative growth pattern, high nuclear grade, necrosis, and vascular invasion. Based on these criteria, fulfilling 2 or more features technically classifies the lesion as malignant, 1 feature classifies it as uncertain malignant potential, and a lack of these features renders the lesion benign.9
The overwhelming majority of pcPEComas are considered benign. One case of pcPEComa was considered malignant with a high mitotic rate (5 mitoses per 10 high-power fields) and nuclear atypia.10 Further workup with thoracic computed tomography and positron emission tomography-computed tomography was negative for metastasis. Treatment with wide excision and radiotherapy was performed with no sign of recurrence at 24-month follow-up.10
Although pcPEComas arising from the dermis seem to be benign overall, PEComas originating from the subcutaneous tissue may have greater malignancy potential. Two cases of subcutaneous PEComas presenting as nodules resulted in metastasis; one case had local nodal metastasis and another developed metastasis to the lungs months later.10,11
- Zamboni G, Pea M, Martignoni G, et al. Clear cell “sugar” tumorof the pancreas. a novel member of the family of lesions characterizedby the presence of perivascular epithelioid cells. Am J Surg Pathol.1996;20:722-730.
- Folpe AK, Wiatkowski D. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1-15.
- Charli-Joseph Y, Saggini A, Vemula S, et al. Primary cutaneous perivascularepithelioid cell tumor: a clinicopathological and molecular reappraisal. J Am Acad Dermatol. 2014;71:1127-1136.
- Crowson AN, Taylor JR, Magro CM. Cutaneous clear cell myomelanocytictumor-perivascular epithelioid cell tumor: first reported case. Mod Pathol. 2003;16:90A.
- Chaplin A, Conrad D, Tatlidil C, et al. Primary cutaneous PEComa. Am J Dermatopathol. 2010;32:310-312.
- Navale P, Asgari M, Chen S. Pigmented perivascular epithelioid cell tumor of the skin. Am J Dermatopathol. 2015;37:866-869.
- Ieremia E, Robson A. Cutaneous PEComa. Am J Dermatopathol. 2014;36:E198-E201.
- Calder K, Schlauder S, Morgan M. Malignant perivascularepithelioid cell tumor (‘PEComa’): a case report and literature review of cutaneous/subcutaneous presentations. J Cutan Pathol. 2008;35:499-503.
- Folpe A, Mentzel T, Lehr H, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Dermatopathol. 2005; 29:1558-1575.
- Greveling K, Winnepenninckx V, Nagtzaam I, et al. Malignant perivascular epithelioid cell tumor: a case report of a cutaneous tumor on the cheek of a male patient. J Am Acad Dermatol. 2013;69:E262-E264.
- Shon W, Kim J, Sukov W, et al. Malignant TFE3-rearranged perivascular epithelioid cell neoplasm (PEComa) presenting as a subcutaneous mass. Br J Dermatol. 2015;174:617-620.
- Zamboni G, Pea M, Martignoni G, et al. Clear cell “sugar” tumorof the pancreas. a novel member of the family of lesions characterizedby the presence of perivascular epithelioid cells. Am J Surg Pathol.1996;20:722-730.
- Folpe AK, Wiatkowski D. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1-15.
- Charli-Joseph Y, Saggini A, Vemula S, et al. Primary cutaneous perivascularepithelioid cell tumor: a clinicopathological and molecular reappraisal. J Am Acad Dermatol. 2014;71:1127-1136.
- Crowson AN, Taylor JR, Magro CM. Cutaneous clear cell myomelanocytictumor-perivascular epithelioid cell tumor: first reported case. Mod Pathol. 2003;16:90A.
- Chaplin A, Conrad D, Tatlidil C, et al. Primary cutaneous PEComa. Am J Dermatopathol. 2010;32:310-312.
- Navale P, Asgari M, Chen S. Pigmented perivascular epithelioid cell tumor of the skin. Am J Dermatopathol. 2015;37:866-869.
- Ieremia E, Robson A. Cutaneous PEComa. Am J Dermatopathol. 2014;36:E198-E201.
- Calder K, Schlauder S, Morgan M. Malignant perivascularepithelioid cell tumor (‘PEComa’): a case report and literature review of cutaneous/subcutaneous presentations. J Cutan Pathol. 2008;35:499-503.
- Folpe A, Mentzel T, Lehr H, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Dermatopathol. 2005; 29:1558-1575.
- Greveling K, Winnepenninckx V, Nagtzaam I, et al. Malignant perivascular epithelioid cell tumor: a case report of a cutaneous tumor on the cheek of a male patient. J Am Acad Dermatol. 2013;69:E262-E264.
- Shon W, Kim J, Sukov W, et al. Malignant TFE3-rearranged perivascular epithelioid cell neoplasm (PEComa) presenting as a subcutaneous mass. Br J Dermatol. 2015;174:617-620.
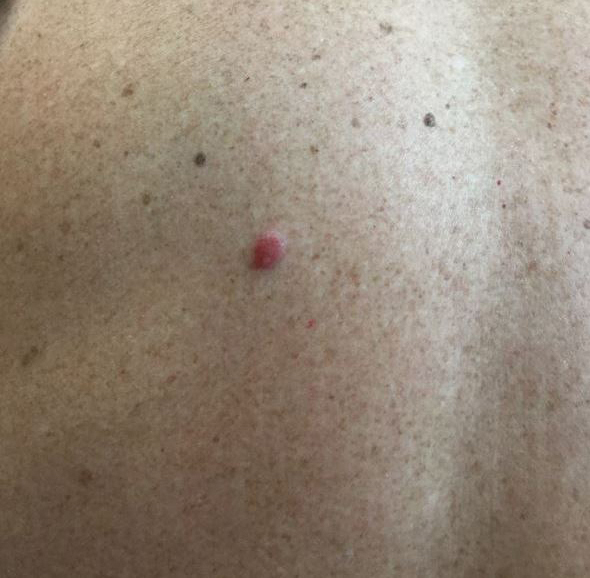
A 54-year-old man presented with an asymptomatic nodule on the left side of the mid back that had been slowly growing in size over the last 12 months. The patient had 2 other lesions on the nasal supratip and left upper arm that were concerning for basal cell carcinoma. The patient’s medical history was notable for stage IV mantle cell lymphoma diagnosed 8 years prior by lymph node biopsy. He completed multiple rounds of methotrexate and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy over 2 years and later received a stem cell transplant; he had been in clinical remission for the last 6 years. On review of symptoms he denied any fevers, chills, fatigue, night sweats, or constitutional symptoms. The remainder of the review of symptoms was negative. Physical examination showed a 1.5×1.0-cm pink, firm, nontender nodule on the left side of the mid back.