User login
- Management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASC-US, but testing for HPV is preferred when the Pap test is liquid-based.
- The sensitivity of HPV triage for high-grade CIN is essentially equivalent to colposcopy, and reduces the need for colposcopy by half.
- HPV testing is a good option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization.
What’s the best management strategy for the roughly 2 to 3 million women each yearUpdate on Cervical Disease, for commentary by Thomas C. Wright, Jr, MD, Department of Pathology, College of Physicians and Surgeons of Columbia University.The difference is even more pronounced when the cumulative 2-year detection rate for CIN 2,3 is added in for women referred for HPV-positive ASCUS but not found to have CIN 2,3 at initial colposcopy. That rate rises from 20.1% at initial colposcopy to 26.9% at 2 years.8 Although many experts consider even HPV-positive ASCUS of minimal risk, few would consider a risk of high-grade disease exceeding 1 in 4 to be minimal. In fact, 39% of the total CIN 2,3 cases reported from a routine screening population were detected following triage of ASCUS, and fully 69% were from all equivocal and low-grade Pap diagnoses.9
TABLE 1
Risk of cervical intraepithelial neoplasia grade 2 or greater at initial colposcopy
| ASCUS | ||||
|---|---|---|---|---|
| STUDY | HPV TEST | HPV–POSITIVE | HPV–NEGATIVE | TOTAL RISK FOR ALL ASCUS |
| Cox6 | Hybrid capture 1 (expanded first–generation test) | 17% (14/81) | 0.74% (1/136) | 6.9% (15/217) |
| Manos7 | Hybrid capture 2 | 15% (45/300) | 1.2% (6/498) | 6.4% (51/798) |
| Solomon4 (ALTS) | Hybrid capture 2 | 18% (195/1,087) | 1.1% (13/1,175) | 9.2% (208/2,262) |
| ALTS = ASCUS/LSIL Triage Study; ASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus | ||||
Bethesda 3 redefines ASCUS
The third Bethesda System workshop took place in May 2001 with the aim of evaluating and updating earlier terminology.10 It began by eliminating the words “of undetermined significance” from the overall ASCUS category, which is now called simply “atypical squamous cells,” or ASC. Most subcategories of the former ASCUS were eliminated as well. (Note: Within this article, the acronyms ASCUS and ASC-US are both used to describe atypical squamous cells of undetermined significance. The latter acronym reflects usage and guidelines developed after the third Bethesda workshop.)
Now the ASC classification is broken down into 2 distinct groups:
Atypical squamous cells–undetermined significance, or ASC-US. This new subcategory includes cells previously termed “favor reactive” but not relegated by the pathologist to normal, as well as cells previously in the “unqualified” and “favor HPV” or “favor low-grade squamous intraepithelial lesion (LSIL)” subcategories.
Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesions, or ASC-H. This category includes atypical cells difficult to distinguish from high-grade cells but not definitive for that classification. Women with such Pap tests are at greater risk for high-risk HPV and histologic CIN 2,3 (TABLE 2).
Evidence-based guidelines reflect Bethesda 3 changes. By the time of Bethesda 3, extensive new data on the management of abnormal cytology was available, including but not limited to data from ALTS, making it possible to create evidence-based guidelines on management of abnormal cervical cytology and CIN. These guidelines were developed in 2001 at a consensus conference hosted by the American Society for Colposcopy and Cervical Pathology (ASCCP),11 with input from 29 professional organizations, federal agencies, and national and international health organizations.
The entire set recommendations for all types of abnormal Pap tests were published in the April 24, 2002 issue of the Journal of the American Medical Association, and management recommendations for histologically proven CIN were published in the July 2003 American Journal of Obstetrics and Gynecology and the July 2003 Journal of Lower Genital Tract Disease. The management algorithms for both cytology and histology can be downloaded from http://ASCCP.org.
TABLE 2
Comparison of risk for high-risk HPV and CIN grade 2,3, by Pap results
| HISTOLOGY | |||||
|---|---|---|---|---|---|
| PAP TEST | HIGH-RISK HPV | CIN 2 OR GREATER | CIN 3 | ||
| ASC-US | 63% | 12% | 5% | ||
| ASC-H | 86% | 40% | 24% | ||
| HSIL | 99% | 59% | 38% | ||
| Data from Sherman et al29 | |||||
| ASC-US = atypical squamous cells–undetermined significance; | |||||
| ASC-H = Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesion; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion | |||||
All 3 triage options safe, effective
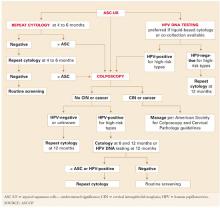
An evidence-based review found all 3 options safe and effective.11 Therefore, management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASCUS, but testing for HPV is preferred when the Pap test is liquid-based (FIGURE 1).
Liquid-based cytology (ThinPrep; Cytyc, Boxborough, Mass and SurePath, Raleigh-Durham, NC) has several advantages. For example, residual cells in the fluid can be tested for HPV, eliminating a return visit.
Immediate colposcopy: Low predictive value, high anxiety and expense. Proponents of immediate colposcopy for all women with ASC-US argue that this would theoretically detect all CIN 2,3 and cancer. However, the positive predictive value of this approach will always be extremely low due to the low rate (6.4% to 11.9%) of CIN 2,3 in women with ASCUS.4,6,7 What’s more, the cost and anxiety generated by immediate colposcopy are high.12
2 repeat cytologies: Sensitivity, cost issues. This approach requires at least 2 repeat, optimized (liquid-based) Pap tests to equal the sensitivity of a single HPV test. This, compounded with the high rate of repeat abnormal cytology requiring colposcopic evaluation, means repeat cytology is unlikely to be cost-competitive with HPV testing.4,13
- Cervical cytology as a triage option. Cytology has been a good screening test, but its comparatively low sensitivity (51% to 83%) and poor reproducibility reduces its value as a triage test.13-17 For example, in ALTS, of 1,473 repeat Paps originally read as ASCUS by good clinical pathologists, only 633 were reread as ASCUS when 2-of-3 agreement was obtained in a blinded review by an expert panel of pathologists.16 In other words, 840 (57%) were reread as something other than ASCUS. Most were downgraded to normal.
- The sensitivity of the HPV test in detecting CIN 2,3 was 92.4%. This rate was matched only by 2 repeat Pap tests, provided the threshold for referral to colposcopy was ASCUS or greater.17 At this threshold, 95% of the CIN 2,3 was detected with repeat Pap testing, but only after an average of 8 to 12 months. This contrasts with the immediate reassurance provided by the initial HPV test.
- ALTS did not evaluate repeat conventional Pap smears. Nor do the guidelines differentiate between conventional and liquid-based methods in the number of follow-up Pap tests required for reassurance, despite consensus that the sensitivity of liquid-based cytology is better than that of the conventional “dry slide.”
- Any woman with a repeat Pap result of ASC-US or greater should be referred to colposcopy. Referral at a threshold of LSIL or greater would result in far fewer colposcopies, but has not been shown to be sufficiently sensitive for CIN 2,3.17
HPV testing identifies clear risk. Any objective test that initially indicates which women with ASC-US are at risk for CIN 2,3 and which are not—either now or in the future—should confer a major advantage.
HPV-positive women are clearly at risk, justifying the anxiety and cost of colposcopic referral, while HPV-negative women may be reassured (FIGURE 2). Also, ALTS data showed HPV triage is essentially equivalent to immediate colposcopy in sensitivity for high-grade CIN, while halving colposcopic referrals.17,18
Because low-risk HPV types do not cause CIN 3 or cancer, the HPV test should document only high-risk types.11 The only HPV test approved by the US Food and Drug Administration (Hybrid Capture 2, Digene, Gaithersburg, Md) includes both low- and high-risk HPV panels. For cost savings, the laboratory can be asked to use only the high-risk panel. All positive high-risk HPV cases should be referred to colposcopy.
FIGURE 1 3 triage options for management of ASC-US
FIGURE 2 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
Some high-grade lesions are still overlooked
A single HPV test or 2 repeat liquid-based Pap tests with a colposcopy-referral threshold of any findings of ASC-US or greater have similar sensitivity for CIN 2,3.17,18
The guidelines state that women who undergo immediate colposcopy with negative results or who have a negative initial HPV test should undergo a follow-up Pap test in 12 months. Note that the guidelines do not state that these women can return directly to routine screening. The reason: In some settings, “routine” screening is at 2- or 3-year intervals, and some risk still exists—albeit minimal—for missed CIN 2,3.
For example, 1 of 83 cases of CIN 2,3 were missed by HPV testing in the study by Manos et al.7 In ALTS, that number was 1 in 90,4 and in the study by Cox et al6 it was 1 in 136. Further, colposcopy did not initially detect 25% of the cumulative high-grade lesions detected over 2 years of follow-up in ALTS.17
In contrast, the recommendation for women with 2 repeat normal Pap tests is to return to “routine screening.” This inexplicably departs from the 12-month repeat Pap testing urged for women with negative results on the other 2 triage options, despite a similar risk of missed high-grade disease.
- In my opinion, all 3 scenarios should be managed by repeat Pap testing in 12 months.
Reducing referrals to colposcopy
If all women returned as directed for repeat cytology, more of them would be referred to colposcopy by repeat abnormal Pap tests at the ASC-US threshold than by testing positive for high-risk HPV types. In ALTS, 53% tested positive for high-risk HPV and were referred to colposcopy, compared with 67% who had an abnormal Pap test on the first or second repeat (these women also had 1 or 2 more office visits prior to referral to colposcopy.).
No difference for conventional smears. All the advantages of HPV testing in the triage of women with ASC-US persist when the initial referral Pap test is a conventional smear. The only exception is that HPV testing would require the patient to return for a repeat office visit. An alternative would be co-collecting an HPV-test sample at the time of the primary screening Pap test.
One major health-maintenance organization collects a separate sample from all women when the routine conventional Pap test is obtained using a standard Hybrid Capture 2 HPV test kit. The HPV-testing samples are then held until the results of the Pap smear are reported. For women reported to have ASCUS, the samples are sent to the lab for HPV testing; the remaining samples (approximately 95% in most practices) are discarded as medical waste. The cost of each discarded kit is approximately $1. Modeling has found this approach to be cost-effective.19
Postcolposcopy management
Many clinicians are concerned that women referred for the evaluation of HPV-positive ASC-US and found not to have CIN or other manifestations of HPV at colposcopy have a “false-positive” HPV test. However, although there are occasional HPV tests that misclassify a low-risk HPV type as high-risk, actual false-positive tests are very rare.
The 2-year ALTS longitudinal data provide the best information on what to expect when a woman with HPV-positive ASC-US or LSIL is found at colposcopy to have no CIN or to have only CIN 1 that is subsequently managed expectantly.8
The cumulative risk of CIN 2,3 over the 2 years was nearly equivalent for women referred initially for LSIL (27.6%) and for women referred for HPV-positive ASCUS (26.7%), further verifying that management should be similar. Two thirds of the CIN 2,3 was detected at initial colposcopy, and the remaining one third during the postcolposcopy 2-year follow-up.
The risk for subsequent detection of high-grade CIN was nearly identical for all women initially found not to have CIN 2,3 regardless of whether CIN 1 was detected at initial colposcopy, whether the colposcopy was initially completely normal, or whether there were changes that were biopsied and found not to have CIN (risk for CIN 2,3 was 13%, 11.3%, and 11.7% respectively).
Hence, all women referred for evaluation of HPV-positive ASC-US or LSIL and not treated for CIN 2,3 require similar diligent follow-up.
A single HPV test at 12 months detected 92% of all CIN 2,3 found over the 24-month follow-up; 55% tested HPV-positive and were referred to colposcopy.20 Repeat liquid-based cytology at 6 and 12 months referred to colposcopy 63% of women (using a threshold of a repeat Pap test of ASCUS or greater). Cumulative sensitivity of 2 repeat cytologies for CIN 2,3 was slightly less (88%). Combining a repeat Pap test with an HPV test did not increase sensitivity, but did significantly increase referral to colposcopy.
An HPV test alone at 12 months might be the most efficient test for identifying women with CIN 2,3 after colposcopy.20 Further support for this approach can be found in the substantial body of evidence showing that only persistent HPV progresses to CIN 321 and that testing for high-risk HPV detects most CIN 3.4,17,20
The ASCCP guidelines for women referred for either HPV-positive ASC-US or LSIL and found not to have CIN 2,3 or greater at initial colposcopy recommend either HPV testing at 12 months or repeat cytology at 6 and 12 months (FIGURE 1).11,22
Posttreatment follow-up. The ASCCP treatment guidelines also list HPV testing as an acceptable option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization,22 since there is substantial evidence that women successfully treated for CIN become HPV-negative, whereas women with persistent disease remain HPV-positive.23-25
A posttreatment HPV test should be performed no sooner than 6 months following the procedure, as it takes time for the patient to return to HPV-negative status. A positive HPV test is an indication for colposcopy. However, the guidelines advise against basing repeat treatment on a positive HPV test alone without documentation of persistent CIN.22
Other options for posttreatment surveillance include either repeat cytology or a combination of Pap testing and colposcopy at 4- to 6-month intervals until at least 3 cytologic results are “negative for squamous intraepithelial lesion or malignancy.”22
Annual cytologic follow-up is recommended thereafter. During that follow-up, any abnormal Pap test (ASC-US or greater) should be referred to colposcopy.
Managing ASC-US in special populations
Management of ASC-US may differ from the general recommendations when the patient is postmenopausal or immunosuppressed. However, there are no differences in the management guidelines during pregnancy.
HPV-negative postmenopausal patients. All 3 management options—immediate colposcopy, repeat cytology, and HPV DNA testing—are acceptable for postmenopausal women with ASC-US.11 However, estrogen deficiency is a common cause of ASC-US and is responsible for increasing rates of HPV-negative ASC-US in this age group despite high sensitivity of HPV testing for CIN 2 and 3.5
- Treatment with vaginal estrogen cream followed by repeat cytology approximately 1 week after completing the regimen is an option for postmenopausal women with ASC-US. This approach also may be helpful for perimenopausal women and for women of any age on progestin-only contraception who have clinical or cytologic evidence of atrophy.
- Women with ASC-US or greater on repeat cytology should be referred for colposcopy, whereas women with normal repeat cytology should have a second Pap test in 4 to 6 months. Repeating the course of vaginal estrogen prior to each Pap test may be helpful when atrophy is likely to persist. After 2 normal repeat Pap tests, the patient can return to routine screening.
Refer all immunosuppressed women for colposcopy. The management of ASC-US in HIV-infected women is particularly problematic because the rates of ASC-US and HPV detection are 2 to 3 times greater than in HIV-negative women. In addition, the risk of CIN 2 and 3 is much higher.26 HPV testing as a triage for ASC-US is not efficient in immunosuppressed women because the majority of ASC-US Pap tests in these women are HPV-positive.
ASCCP recommends colposcopy referral of all immunosuppressed women with ASCUS Pap test, regardless of their CD4 count, HIV viral load, or anti-retroviral therapy.26
Managing ASC-H: First, colposcopy
Clearly, women with ASC-H test results face a greater risk for CIN 2,3 and should be referred for immediate colposcopy.
The ASC-H designation is uncommon, reported in 0.27% to 0.6% of all Pap tests,27,28 or approximately 1 in 10 Pap smears read as ASC.
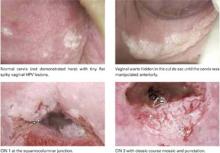
In ALTS, HPV testing and histology results were compared for women with Pap tests categorized as equivocal LSIL (ASCUSL), ASCUS-H, and high-grade squamous intraepithelial lesion (HSIL) (TABLE 2).29 High-risk HPV DNA was detected in 86% of ASCUS-H liquid-based Pap tests and 69.8% of ASCUS-H conventional smears. CIN 2,3 was found in 40% of liquid-based ASCUS-H smears and in 27.2% of conventional ASCUSH smears. A 3-year retrospective review of ASC-H with follow-up at Johns Hopkins Medical Institutions determined that 49% of patients had no CIN or glandular lesions.28 Of the 51% with CIN, approximately half the lesions were CIN 1 and half were CIN 2,3.
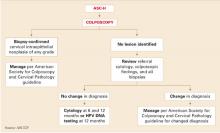
Further management depends on whether CIN is detected (FIGURE 3). If no CIN is found, the ASCCP guidelines recommend that cytology, colposcopy, and histology be reviewed. If there is a change in the diagnosis—eg, if the Pap interpretation is revised to HSIL—the patient should be managed accordingly.11
If there is no change, the patient should be followed with repeat cytology at 6-and 12-month intervals or HPV testing at 12 months. Women having any repeat abnormal Pap test at a threshold of ASC-US or greater or a positive HPV test should undergo repeat colposcopy.
ASC-H is of greater risk than ASC-US, but it is not as risky as HSIL. Therefore, a surgical excision procedure in the absence of documented CIN 2,3 would not normally be indicated.11
FIGURE 3 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
HPV test as triage option would mean retooling the system
Cytologic management systems have traditionally involved follow-up by repeat cytology, colposcopy, and, when necessary, treatment. Adding another triage option—HPV testing—requires that this system be retooled.
The labs. This is not difficult when the laboratory interpreting the liquid-based Pap test is the same lab that performs the “reflex” HPV test, as this allows the ASC-US Pap test to be reported as HPV-negative or HPV-positive. However, if the HPV test must be performed in a separate reference laboratory, the results of the Pap and HPV tests will arrive separately, and the clinician must collate the 2 reports before relaying the result to the patient.
The patients. Remember than an HPV test is a test for a sexually transmitted disease. (So is the Pap test, although it has not traditionally been considered as such.) For that reason, I give all patients a written explanation of the rationale behind testing ASC-US Pap tests for HPV. This explanation includes 2 check-off options at the bottom of the sheet where patients can indicate whether they would prefer HPV testing or one of the other follow-up options.
Most patients elect the HPV option. Our Pap test requisitions also have a check-off portion that allows us to notify the lab of patients who wants an HPV test if the Pap is interpreted as ASC-US.
The office staff. Whenever a new test or procedure is introduced, it is of primary importance that the office staff responsible for completing critical information on the requisition form is adequately trained. This involves knowing when and how to order the test and how to complete insurance information and clinical history on the Pap requisition—including the correct International Classification of Diseases, Ninth Revision code—to ensure that the HPV test is covered by the patient’s insurer.
Clinicians must understand the usually benign nature of HPV infection. Reporting a positive HPV test in a manner that is not unduly concerning requires reassuring and nonjudgmental communication of the results based on a broad understanding of the usually low-risk natural history of the virus, yet fosters responsible follow-up.
Why all HPV-positive ASC-US requires diligent follow-up
The recently released ASCCP guidelines recognize HPV testing as an option in the management of ASC results, including:
- initial management of ASC-US,
- postcolposcopy management of ASC-H or HPV-positive ASC Pap tests found to be normal or to have CIN 1, and
- posttreatment follow-up.
For each indication, the HPV test identifies women most likely to have CIN (HPV-positive) and those likely to have benign processes not related to HPV (HPV-negative).
New longitudinal data verify that women with HPV-positive ASC-US continue at risk for detection of CIN 2,3 (about 12% overall), whether the original colposcopic finding was normal or CIN 1.8 Therefore, they need continued diligent follow-up.
Dr. Cox serves on the Speaker’s Bureaus of Cytyc Corporation and Digene Corporation.
1. Kurman RJ, Henson DE, Berbst AL, Noller KL, Schiffman MH. Interim guidelines for the management of abnormal cervical cytology. JAMA. 1994;271:1866-1869.
2. Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials. Int J Gynecol Cancer. 1995;5:321-328.
3. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726-742.
4. Solomon D, Schiffman M, Tarone R, et al. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293-299.
5. Sherman ME, Schiffman M, Cox JT, et al. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-107.
6. Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172:946-954.
7. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
8. Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406-1412.
9. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of the minimally abnormal Papanicolaou diagnosis. Obstet Gynecol. 1998;91:973-976.
10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119.
11. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
12. Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient awareness and choice of management. J Royal Soc Med. 1996;89:257.-
13. Wright TC, Lorincz AT, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Pap smears. Am J Obstet Gynecol. 1998;178:926-966.
14. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
15. Agency for Health Care Policy and Research. Evidence Report/Technology Assessment #5: Evaluation of Cervical Cytology. Rockville, Md: AHCPR; January 1999.
16. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance—Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505.
17. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383-1392.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003;127:946-949.
19. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382-2390.
20. Guido R, Schiffman M, Solomon D, et al. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401-1405.
21. Nobbenhuis M, Walboomers JM, Helmerhorst TI, Rozendaal L. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20.-
22. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
23. Paraskevaidis E, Koliopoulos G, Alamanos Y, Malamou-Mitsi V, Lolis ED, Kitchener HC. Human papillomavirus testing and the outcome of treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2001;98:833-836.
24. Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84:796-801.
25. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001;82:177-180.
26. Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432-442.
27. Selvaggi SM. Reporting of atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H) on cervical samples: is it significant? Diagn Cytopathol. 2003;29:38-41.
28. Alli PM, Ali SZ. Atypical squamous cells of undetermined significance—rule out high-grade squamous intraepithelial lesion: cytopathologic characteristics and clinical correlates. Diagn Cytopathol. 2003;28:308-312.
29. Sherman ME, Solomon D, Schiffman M, et al. A comparison of equivocal LSIL and equivocal HSIL cervical cytology in the ASCUS LSIL Triage Study. Am J Clin Pathol. 2001;116:386-394.
- Management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASC-US, but testing for HPV is preferred when the Pap test is liquid-based.
- The sensitivity of HPV triage for high-grade CIN is essentially equivalent to colposcopy, and reduces the need for colposcopy by half.
- HPV testing is a good option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization.
What’s the best management strategy for the roughly 2 to 3 million women each yearUpdate on Cervical Disease, for commentary by Thomas C. Wright, Jr, MD, Department of Pathology, College of Physicians and Surgeons of Columbia University.The difference is even more pronounced when the cumulative 2-year detection rate for CIN 2,3 is added in for women referred for HPV-positive ASCUS but not found to have CIN 2,3 at initial colposcopy. That rate rises from 20.1% at initial colposcopy to 26.9% at 2 years.8 Although many experts consider even HPV-positive ASCUS of minimal risk, few would consider a risk of high-grade disease exceeding 1 in 4 to be minimal. In fact, 39% of the total CIN 2,3 cases reported from a routine screening population were detected following triage of ASCUS, and fully 69% were from all equivocal and low-grade Pap diagnoses.9
TABLE 1
Risk of cervical intraepithelial neoplasia grade 2 or greater at initial colposcopy
| ASCUS | ||||
|---|---|---|---|---|
| STUDY | HPV TEST | HPV–POSITIVE | HPV–NEGATIVE | TOTAL RISK FOR ALL ASCUS |
| Cox6 | Hybrid capture 1 (expanded first–generation test) | 17% (14/81) | 0.74% (1/136) | 6.9% (15/217) |
| Manos7 | Hybrid capture 2 | 15% (45/300) | 1.2% (6/498) | 6.4% (51/798) |
| Solomon4 (ALTS) | Hybrid capture 2 | 18% (195/1,087) | 1.1% (13/1,175) | 9.2% (208/2,262) |
| ALTS = ASCUS/LSIL Triage Study; ASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus | ||||
Bethesda 3 redefines ASCUS
The third Bethesda System workshop took place in May 2001 with the aim of evaluating and updating earlier terminology.10 It began by eliminating the words “of undetermined significance” from the overall ASCUS category, which is now called simply “atypical squamous cells,” or ASC. Most subcategories of the former ASCUS were eliminated as well. (Note: Within this article, the acronyms ASCUS and ASC-US are both used to describe atypical squamous cells of undetermined significance. The latter acronym reflects usage and guidelines developed after the third Bethesda workshop.)
Now the ASC classification is broken down into 2 distinct groups:
Atypical squamous cells–undetermined significance, or ASC-US. This new subcategory includes cells previously termed “favor reactive” but not relegated by the pathologist to normal, as well as cells previously in the “unqualified” and “favor HPV” or “favor low-grade squamous intraepithelial lesion (LSIL)” subcategories.
Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesions, or ASC-H. This category includes atypical cells difficult to distinguish from high-grade cells but not definitive for that classification. Women with such Pap tests are at greater risk for high-risk HPV and histologic CIN 2,3 (TABLE 2).
Evidence-based guidelines reflect Bethesda 3 changes. By the time of Bethesda 3, extensive new data on the management of abnormal cytology was available, including but not limited to data from ALTS, making it possible to create evidence-based guidelines on management of abnormal cervical cytology and CIN. These guidelines were developed in 2001 at a consensus conference hosted by the American Society for Colposcopy and Cervical Pathology (ASCCP),11 with input from 29 professional organizations, federal agencies, and national and international health organizations.
The entire set recommendations for all types of abnormal Pap tests were published in the April 24, 2002 issue of the Journal of the American Medical Association, and management recommendations for histologically proven CIN were published in the July 2003 American Journal of Obstetrics and Gynecology and the July 2003 Journal of Lower Genital Tract Disease. The management algorithms for both cytology and histology can be downloaded from http://ASCCP.org.
TABLE 2
Comparison of risk for high-risk HPV and CIN grade 2,3, by Pap results
| HISTOLOGY | |||||
|---|---|---|---|---|---|
| PAP TEST | HIGH-RISK HPV | CIN 2 OR GREATER | CIN 3 | ||
| ASC-US | 63% | 12% | 5% | ||
| ASC-H | 86% | 40% | 24% | ||
| HSIL | 99% | 59% | 38% | ||
| Data from Sherman et al29 | |||||
| ASC-US = atypical squamous cells–undetermined significance; | |||||
| ASC-H = Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesion; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion | |||||
All 3 triage options safe, effective
An evidence-based review found all 3 options safe and effective.11 Therefore, management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASCUS, but testing for HPV is preferred when the Pap test is liquid-based (FIGURE 1).
Liquid-based cytology (ThinPrep; Cytyc, Boxborough, Mass and SurePath, Raleigh-Durham, NC) has several advantages. For example, residual cells in the fluid can be tested for HPV, eliminating a return visit.
Immediate colposcopy: Low predictive value, high anxiety and expense. Proponents of immediate colposcopy for all women with ASC-US argue that this would theoretically detect all CIN 2,3 and cancer. However, the positive predictive value of this approach will always be extremely low due to the low rate (6.4% to 11.9%) of CIN 2,3 in women with ASCUS.4,6,7 What’s more, the cost and anxiety generated by immediate colposcopy are high.12
2 repeat cytologies: Sensitivity, cost issues. This approach requires at least 2 repeat, optimized (liquid-based) Pap tests to equal the sensitivity of a single HPV test. This, compounded with the high rate of repeat abnormal cytology requiring colposcopic evaluation, means repeat cytology is unlikely to be cost-competitive with HPV testing.4,13
- Cervical cytology as a triage option. Cytology has been a good screening test, but its comparatively low sensitivity (51% to 83%) and poor reproducibility reduces its value as a triage test.13-17 For example, in ALTS, of 1,473 repeat Paps originally read as ASCUS by good clinical pathologists, only 633 were reread as ASCUS when 2-of-3 agreement was obtained in a blinded review by an expert panel of pathologists.16 In other words, 840 (57%) were reread as something other than ASCUS. Most were downgraded to normal.
- The sensitivity of the HPV test in detecting CIN 2,3 was 92.4%. This rate was matched only by 2 repeat Pap tests, provided the threshold for referral to colposcopy was ASCUS or greater.17 At this threshold, 95% of the CIN 2,3 was detected with repeat Pap testing, but only after an average of 8 to 12 months. This contrasts with the immediate reassurance provided by the initial HPV test.
- ALTS did not evaluate repeat conventional Pap smears. Nor do the guidelines differentiate between conventional and liquid-based methods in the number of follow-up Pap tests required for reassurance, despite consensus that the sensitivity of liquid-based cytology is better than that of the conventional “dry slide.”
- Any woman with a repeat Pap result of ASC-US or greater should be referred to colposcopy. Referral at a threshold of LSIL or greater would result in far fewer colposcopies, but has not been shown to be sufficiently sensitive for CIN 2,3.17
HPV testing identifies clear risk. Any objective test that initially indicates which women with ASC-US are at risk for CIN 2,3 and which are not—either now or in the future—should confer a major advantage.
HPV-positive women are clearly at risk, justifying the anxiety and cost of colposcopic referral, while HPV-negative women may be reassured (FIGURE 2). Also, ALTS data showed HPV triage is essentially equivalent to immediate colposcopy in sensitivity for high-grade CIN, while halving colposcopic referrals.17,18
Because low-risk HPV types do not cause CIN 3 or cancer, the HPV test should document only high-risk types.11 The only HPV test approved by the US Food and Drug Administration (Hybrid Capture 2, Digene, Gaithersburg, Md) includes both low- and high-risk HPV panels. For cost savings, the laboratory can be asked to use only the high-risk panel. All positive high-risk HPV cases should be referred to colposcopy.
FIGURE 1 3 triage options for management of ASC-US
FIGURE 2 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
Some high-grade lesions are still overlooked
A single HPV test or 2 repeat liquid-based Pap tests with a colposcopy-referral threshold of any findings of ASC-US or greater have similar sensitivity for CIN 2,3.17,18
The guidelines state that women who undergo immediate colposcopy with negative results or who have a negative initial HPV test should undergo a follow-up Pap test in 12 months. Note that the guidelines do not state that these women can return directly to routine screening. The reason: In some settings, “routine” screening is at 2- or 3-year intervals, and some risk still exists—albeit minimal—for missed CIN 2,3.
For example, 1 of 83 cases of CIN 2,3 were missed by HPV testing in the study by Manos et al.7 In ALTS, that number was 1 in 90,4 and in the study by Cox et al6 it was 1 in 136. Further, colposcopy did not initially detect 25% of the cumulative high-grade lesions detected over 2 years of follow-up in ALTS.17
In contrast, the recommendation for women with 2 repeat normal Pap tests is to return to “routine screening.” This inexplicably departs from the 12-month repeat Pap testing urged for women with negative results on the other 2 triage options, despite a similar risk of missed high-grade disease.
- In my opinion, all 3 scenarios should be managed by repeat Pap testing in 12 months.
Reducing referrals to colposcopy
If all women returned as directed for repeat cytology, more of them would be referred to colposcopy by repeat abnormal Pap tests at the ASC-US threshold than by testing positive for high-risk HPV types. In ALTS, 53% tested positive for high-risk HPV and were referred to colposcopy, compared with 67% who had an abnormal Pap test on the first or second repeat (these women also had 1 or 2 more office visits prior to referral to colposcopy.).
No difference for conventional smears. All the advantages of HPV testing in the triage of women with ASC-US persist when the initial referral Pap test is a conventional smear. The only exception is that HPV testing would require the patient to return for a repeat office visit. An alternative would be co-collecting an HPV-test sample at the time of the primary screening Pap test.
One major health-maintenance organization collects a separate sample from all women when the routine conventional Pap test is obtained using a standard Hybrid Capture 2 HPV test kit. The HPV-testing samples are then held until the results of the Pap smear are reported. For women reported to have ASCUS, the samples are sent to the lab for HPV testing; the remaining samples (approximately 95% in most practices) are discarded as medical waste. The cost of each discarded kit is approximately $1. Modeling has found this approach to be cost-effective.19
Postcolposcopy management
Many clinicians are concerned that women referred for the evaluation of HPV-positive ASC-US and found not to have CIN or other manifestations of HPV at colposcopy have a “false-positive” HPV test. However, although there are occasional HPV tests that misclassify a low-risk HPV type as high-risk, actual false-positive tests are very rare.
The 2-year ALTS longitudinal data provide the best information on what to expect when a woman with HPV-positive ASC-US or LSIL is found at colposcopy to have no CIN or to have only CIN 1 that is subsequently managed expectantly.8
The cumulative risk of CIN 2,3 over the 2 years was nearly equivalent for women referred initially for LSIL (27.6%) and for women referred for HPV-positive ASCUS (26.7%), further verifying that management should be similar. Two thirds of the CIN 2,3 was detected at initial colposcopy, and the remaining one third during the postcolposcopy 2-year follow-up.
The risk for subsequent detection of high-grade CIN was nearly identical for all women initially found not to have CIN 2,3 regardless of whether CIN 1 was detected at initial colposcopy, whether the colposcopy was initially completely normal, or whether there were changes that were biopsied and found not to have CIN (risk for CIN 2,3 was 13%, 11.3%, and 11.7% respectively).
Hence, all women referred for evaluation of HPV-positive ASC-US or LSIL and not treated for CIN 2,3 require similar diligent follow-up.
A single HPV test at 12 months detected 92% of all CIN 2,3 found over the 24-month follow-up; 55% tested HPV-positive and were referred to colposcopy.20 Repeat liquid-based cytology at 6 and 12 months referred to colposcopy 63% of women (using a threshold of a repeat Pap test of ASCUS or greater). Cumulative sensitivity of 2 repeat cytologies for CIN 2,3 was slightly less (88%). Combining a repeat Pap test with an HPV test did not increase sensitivity, but did significantly increase referral to colposcopy.
An HPV test alone at 12 months might be the most efficient test for identifying women with CIN 2,3 after colposcopy.20 Further support for this approach can be found in the substantial body of evidence showing that only persistent HPV progresses to CIN 321 and that testing for high-risk HPV detects most CIN 3.4,17,20
The ASCCP guidelines for women referred for either HPV-positive ASC-US or LSIL and found not to have CIN 2,3 or greater at initial colposcopy recommend either HPV testing at 12 months or repeat cytology at 6 and 12 months (FIGURE 1).11,22
Posttreatment follow-up. The ASCCP treatment guidelines also list HPV testing as an acceptable option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization,22 since there is substantial evidence that women successfully treated for CIN become HPV-negative, whereas women with persistent disease remain HPV-positive.23-25
A posttreatment HPV test should be performed no sooner than 6 months following the procedure, as it takes time for the patient to return to HPV-negative status. A positive HPV test is an indication for colposcopy. However, the guidelines advise against basing repeat treatment on a positive HPV test alone without documentation of persistent CIN.22
Other options for posttreatment surveillance include either repeat cytology or a combination of Pap testing and colposcopy at 4- to 6-month intervals until at least 3 cytologic results are “negative for squamous intraepithelial lesion or malignancy.”22
Annual cytologic follow-up is recommended thereafter. During that follow-up, any abnormal Pap test (ASC-US or greater) should be referred to colposcopy.
Managing ASC-US in special populations
Management of ASC-US may differ from the general recommendations when the patient is postmenopausal or immunosuppressed. However, there are no differences in the management guidelines during pregnancy.
HPV-negative postmenopausal patients. All 3 management options—immediate colposcopy, repeat cytology, and HPV DNA testing—are acceptable for postmenopausal women with ASC-US.11 However, estrogen deficiency is a common cause of ASC-US and is responsible for increasing rates of HPV-negative ASC-US in this age group despite high sensitivity of HPV testing for CIN 2 and 3.5
- Treatment with vaginal estrogen cream followed by repeat cytology approximately 1 week after completing the regimen is an option for postmenopausal women with ASC-US. This approach also may be helpful for perimenopausal women and for women of any age on progestin-only contraception who have clinical or cytologic evidence of atrophy.
- Women with ASC-US or greater on repeat cytology should be referred for colposcopy, whereas women with normal repeat cytology should have a second Pap test in 4 to 6 months. Repeating the course of vaginal estrogen prior to each Pap test may be helpful when atrophy is likely to persist. After 2 normal repeat Pap tests, the patient can return to routine screening.
Refer all immunosuppressed women for colposcopy. The management of ASC-US in HIV-infected women is particularly problematic because the rates of ASC-US and HPV detection are 2 to 3 times greater than in HIV-negative women. In addition, the risk of CIN 2 and 3 is much higher.26 HPV testing as a triage for ASC-US is not efficient in immunosuppressed women because the majority of ASC-US Pap tests in these women are HPV-positive.
ASCCP recommends colposcopy referral of all immunosuppressed women with ASCUS Pap test, regardless of their CD4 count, HIV viral load, or anti-retroviral therapy.26
Managing ASC-H: First, colposcopy
Clearly, women with ASC-H test results face a greater risk for CIN 2,3 and should be referred for immediate colposcopy.
The ASC-H designation is uncommon, reported in 0.27% to 0.6% of all Pap tests,27,28 or approximately 1 in 10 Pap smears read as ASC.
In ALTS, HPV testing and histology results were compared for women with Pap tests categorized as equivocal LSIL (ASCUSL), ASCUS-H, and high-grade squamous intraepithelial lesion (HSIL) (TABLE 2).29 High-risk HPV DNA was detected in 86% of ASCUS-H liquid-based Pap tests and 69.8% of ASCUS-H conventional smears. CIN 2,3 was found in 40% of liquid-based ASCUS-H smears and in 27.2% of conventional ASCUSH smears. A 3-year retrospective review of ASC-H with follow-up at Johns Hopkins Medical Institutions determined that 49% of patients had no CIN or glandular lesions.28 Of the 51% with CIN, approximately half the lesions were CIN 1 and half were CIN 2,3.
Further management depends on whether CIN is detected (FIGURE 3). If no CIN is found, the ASCCP guidelines recommend that cytology, colposcopy, and histology be reviewed. If there is a change in the diagnosis—eg, if the Pap interpretation is revised to HSIL—the patient should be managed accordingly.11
If there is no change, the patient should be followed with repeat cytology at 6-and 12-month intervals or HPV testing at 12 months. Women having any repeat abnormal Pap test at a threshold of ASC-US or greater or a positive HPV test should undergo repeat colposcopy.
ASC-H is of greater risk than ASC-US, but it is not as risky as HSIL. Therefore, a surgical excision procedure in the absence of documented CIN 2,3 would not normally be indicated.11
FIGURE 3 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
HPV test as triage option would mean retooling the system
Cytologic management systems have traditionally involved follow-up by repeat cytology, colposcopy, and, when necessary, treatment. Adding another triage option—HPV testing—requires that this system be retooled.
The labs. This is not difficult when the laboratory interpreting the liquid-based Pap test is the same lab that performs the “reflex” HPV test, as this allows the ASC-US Pap test to be reported as HPV-negative or HPV-positive. However, if the HPV test must be performed in a separate reference laboratory, the results of the Pap and HPV tests will arrive separately, and the clinician must collate the 2 reports before relaying the result to the patient.
The patients. Remember than an HPV test is a test for a sexually transmitted disease. (So is the Pap test, although it has not traditionally been considered as such.) For that reason, I give all patients a written explanation of the rationale behind testing ASC-US Pap tests for HPV. This explanation includes 2 check-off options at the bottom of the sheet where patients can indicate whether they would prefer HPV testing or one of the other follow-up options.
Most patients elect the HPV option. Our Pap test requisitions also have a check-off portion that allows us to notify the lab of patients who wants an HPV test if the Pap is interpreted as ASC-US.
The office staff. Whenever a new test or procedure is introduced, it is of primary importance that the office staff responsible for completing critical information on the requisition form is adequately trained. This involves knowing when and how to order the test and how to complete insurance information and clinical history on the Pap requisition—including the correct International Classification of Diseases, Ninth Revision code—to ensure that the HPV test is covered by the patient’s insurer.
Clinicians must understand the usually benign nature of HPV infection. Reporting a positive HPV test in a manner that is not unduly concerning requires reassuring and nonjudgmental communication of the results based on a broad understanding of the usually low-risk natural history of the virus, yet fosters responsible follow-up.
Why all HPV-positive ASC-US requires diligent follow-up
The recently released ASCCP guidelines recognize HPV testing as an option in the management of ASC results, including:
- initial management of ASC-US,
- postcolposcopy management of ASC-H or HPV-positive ASC Pap tests found to be normal or to have CIN 1, and
- posttreatment follow-up.
For each indication, the HPV test identifies women most likely to have CIN (HPV-positive) and those likely to have benign processes not related to HPV (HPV-negative).
New longitudinal data verify that women with HPV-positive ASC-US continue at risk for detection of CIN 2,3 (about 12% overall), whether the original colposcopic finding was normal or CIN 1.8 Therefore, they need continued diligent follow-up.
Dr. Cox serves on the Speaker’s Bureaus of Cytyc Corporation and Digene Corporation.
- Management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASC-US, but testing for HPV is preferred when the Pap test is liquid-based.
- The sensitivity of HPV triage for high-grade CIN is essentially equivalent to colposcopy, and reduces the need for colposcopy by half.
- HPV testing is a good option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization.
What’s the best management strategy for the roughly 2 to 3 million women each yearUpdate on Cervical Disease, for commentary by Thomas C. Wright, Jr, MD, Department of Pathology, College of Physicians and Surgeons of Columbia University.The difference is even more pronounced when the cumulative 2-year detection rate for CIN 2,3 is added in for women referred for HPV-positive ASCUS but not found to have CIN 2,3 at initial colposcopy. That rate rises from 20.1% at initial colposcopy to 26.9% at 2 years.8 Although many experts consider even HPV-positive ASCUS of minimal risk, few would consider a risk of high-grade disease exceeding 1 in 4 to be minimal. In fact, 39% of the total CIN 2,3 cases reported from a routine screening population were detected following triage of ASCUS, and fully 69% were from all equivocal and low-grade Pap diagnoses.9
TABLE 1
Risk of cervical intraepithelial neoplasia grade 2 or greater at initial colposcopy
| ASCUS | ||||
|---|---|---|---|---|
| STUDY | HPV TEST | HPV–POSITIVE | HPV–NEGATIVE | TOTAL RISK FOR ALL ASCUS |
| Cox6 | Hybrid capture 1 (expanded first–generation test) | 17% (14/81) | 0.74% (1/136) | 6.9% (15/217) |
| Manos7 | Hybrid capture 2 | 15% (45/300) | 1.2% (6/498) | 6.4% (51/798) |
| Solomon4 (ALTS) | Hybrid capture 2 | 18% (195/1,087) | 1.1% (13/1,175) | 9.2% (208/2,262) |
| ALTS = ASCUS/LSIL Triage Study; ASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus | ||||
Bethesda 3 redefines ASCUS
The third Bethesda System workshop took place in May 2001 with the aim of evaluating and updating earlier terminology.10 It began by eliminating the words “of undetermined significance” from the overall ASCUS category, which is now called simply “atypical squamous cells,” or ASC. Most subcategories of the former ASCUS were eliminated as well. (Note: Within this article, the acronyms ASCUS and ASC-US are both used to describe atypical squamous cells of undetermined significance. The latter acronym reflects usage and guidelines developed after the third Bethesda workshop.)
Now the ASC classification is broken down into 2 distinct groups:
Atypical squamous cells–undetermined significance, or ASC-US. This new subcategory includes cells previously termed “favor reactive” but not relegated by the pathologist to normal, as well as cells previously in the “unqualified” and “favor HPV” or “favor low-grade squamous intraepithelial lesion (LSIL)” subcategories.
Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesions, or ASC-H. This category includes atypical cells difficult to distinguish from high-grade cells but not definitive for that classification. Women with such Pap tests are at greater risk for high-risk HPV and histologic CIN 2,3 (TABLE 2).
Evidence-based guidelines reflect Bethesda 3 changes. By the time of Bethesda 3, extensive new data on the management of abnormal cytology was available, including but not limited to data from ALTS, making it possible to create evidence-based guidelines on management of abnormal cervical cytology and CIN. These guidelines were developed in 2001 at a consensus conference hosted by the American Society for Colposcopy and Cervical Pathology (ASCCP),11 with input from 29 professional organizations, federal agencies, and national and international health organizations.
The entire set recommendations for all types of abnormal Pap tests were published in the April 24, 2002 issue of the Journal of the American Medical Association, and management recommendations for histologically proven CIN were published in the July 2003 American Journal of Obstetrics and Gynecology and the July 2003 Journal of Lower Genital Tract Disease. The management algorithms for both cytology and histology can be downloaded from http://ASCCP.org.
TABLE 2
Comparison of risk for high-risk HPV and CIN grade 2,3, by Pap results
| HISTOLOGY | |||||
|---|---|---|---|---|---|
| PAP TEST | HIGH-RISK HPV | CIN 2 OR GREATER | CIN 3 | ||
| ASC-US | 63% | 12% | 5% | ||
| ASC-H | 86% | 40% | 24% | ||
| HSIL | 99% | 59% | 38% | ||
| Data from Sherman et al29 | |||||
| ASC-US = atypical squamous cells–undetermined significance; | |||||
| ASC-H = Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesion; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion | |||||
All 3 triage options safe, effective
An evidence-based review found all 3 options safe and effective.11 Therefore, management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASCUS, but testing for HPV is preferred when the Pap test is liquid-based (FIGURE 1).
Liquid-based cytology (ThinPrep; Cytyc, Boxborough, Mass and SurePath, Raleigh-Durham, NC) has several advantages. For example, residual cells in the fluid can be tested for HPV, eliminating a return visit.
Immediate colposcopy: Low predictive value, high anxiety and expense. Proponents of immediate colposcopy for all women with ASC-US argue that this would theoretically detect all CIN 2,3 and cancer. However, the positive predictive value of this approach will always be extremely low due to the low rate (6.4% to 11.9%) of CIN 2,3 in women with ASCUS.4,6,7 What’s more, the cost and anxiety generated by immediate colposcopy are high.12
2 repeat cytologies: Sensitivity, cost issues. This approach requires at least 2 repeat, optimized (liquid-based) Pap tests to equal the sensitivity of a single HPV test. This, compounded with the high rate of repeat abnormal cytology requiring colposcopic evaluation, means repeat cytology is unlikely to be cost-competitive with HPV testing.4,13
- Cervical cytology as a triage option. Cytology has been a good screening test, but its comparatively low sensitivity (51% to 83%) and poor reproducibility reduces its value as a triage test.13-17 For example, in ALTS, of 1,473 repeat Paps originally read as ASCUS by good clinical pathologists, only 633 were reread as ASCUS when 2-of-3 agreement was obtained in a blinded review by an expert panel of pathologists.16 In other words, 840 (57%) were reread as something other than ASCUS. Most were downgraded to normal.
- The sensitivity of the HPV test in detecting CIN 2,3 was 92.4%. This rate was matched only by 2 repeat Pap tests, provided the threshold for referral to colposcopy was ASCUS or greater.17 At this threshold, 95% of the CIN 2,3 was detected with repeat Pap testing, but only after an average of 8 to 12 months. This contrasts with the immediate reassurance provided by the initial HPV test.
- ALTS did not evaluate repeat conventional Pap smears. Nor do the guidelines differentiate between conventional and liquid-based methods in the number of follow-up Pap tests required for reassurance, despite consensus that the sensitivity of liquid-based cytology is better than that of the conventional “dry slide.”
- Any woman with a repeat Pap result of ASC-US or greater should be referred to colposcopy. Referral at a threshold of LSIL or greater would result in far fewer colposcopies, but has not been shown to be sufficiently sensitive for CIN 2,3.17
HPV testing identifies clear risk. Any objective test that initially indicates which women with ASC-US are at risk for CIN 2,3 and which are not—either now or in the future—should confer a major advantage.
HPV-positive women are clearly at risk, justifying the anxiety and cost of colposcopic referral, while HPV-negative women may be reassured (FIGURE 2). Also, ALTS data showed HPV triage is essentially equivalent to immediate colposcopy in sensitivity for high-grade CIN, while halving colposcopic referrals.17,18
Because low-risk HPV types do not cause CIN 3 or cancer, the HPV test should document only high-risk types.11 The only HPV test approved by the US Food and Drug Administration (Hybrid Capture 2, Digene, Gaithersburg, Md) includes both low- and high-risk HPV panels. For cost savings, the laboratory can be asked to use only the high-risk panel. All positive high-risk HPV cases should be referred to colposcopy.
FIGURE 1 3 triage options for management of ASC-US
FIGURE 2 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
Some high-grade lesions are still overlooked
A single HPV test or 2 repeat liquid-based Pap tests with a colposcopy-referral threshold of any findings of ASC-US or greater have similar sensitivity for CIN 2,3.17,18
The guidelines state that women who undergo immediate colposcopy with negative results or who have a negative initial HPV test should undergo a follow-up Pap test in 12 months. Note that the guidelines do not state that these women can return directly to routine screening. The reason: In some settings, “routine” screening is at 2- or 3-year intervals, and some risk still exists—albeit minimal—for missed CIN 2,3.
For example, 1 of 83 cases of CIN 2,3 were missed by HPV testing in the study by Manos et al.7 In ALTS, that number was 1 in 90,4 and in the study by Cox et al6 it was 1 in 136. Further, colposcopy did not initially detect 25% of the cumulative high-grade lesions detected over 2 years of follow-up in ALTS.17
In contrast, the recommendation for women with 2 repeat normal Pap tests is to return to “routine screening.” This inexplicably departs from the 12-month repeat Pap testing urged for women with negative results on the other 2 triage options, despite a similar risk of missed high-grade disease.
- In my opinion, all 3 scenarios should be managed by repeat Pap testing in 12 months.
Reducing referrals to colposcopy
If all women returned as directed for repeat cytology, more of them would be referred to colposcopy by repeat abnormal Pap tests at the ASC-US threshold than by testing positive for high-risk HPV types. In ALTS, 53% tested positive for high-risk HPV and were referred to colposcopy, compared with 67% who had an abnormal Pap test on the first or second repeat (these women also had 1 or 2 more office visits prior to referral to colposcopy.).
No difference for conventional smears. All the advantages of HPV testing in the triage of women with ASC-US persist when the initial referral Pap test is a conventional smear. The only exception is that HPV testing would require the patient to return for a repeat office visit. An alternative would be co-collecting an HPV-test sample at the time of the primary screening Pap test.
One major health-maintenance organization collects a separate sample from all women when the routine conventional Pap test is obtained using a standard Hybrid Capture 2 HPV test kit. The HPV-testing samples are then held until the results of the Pap smear are reported. For women reported to have ASCUS, the samples are sent to the lab for HPV testing; the remaining samples (approximately 95% in most practices) are discarded as medical waste. The cost of each discarded kit is approximately $1. Modeling has found this approach to be cost-effective.19
Postcolposcopy management
Many clinicians are concerned that women referred for the evaluation of HPV-positive ASC-US and found not to have CIN or other manifestations of HPV at colposcopy have a “false-positive” HPV test. However, although there are occasional HPV tests that misclassify a low-risk HPV type as high-risk, actual false-positive tests are very rare.
The 2-year ALTS longitudinal data provide the best information on what to expect when a woman with HPV-positive ASC-US or LSIL is found at colposcopy to have no CIN or to have only CIN 1 that is subsequently managed expectantly.8
The cumulative risk of CIN 2,3 over the 2 years was nearly equivalent for women referred initially for LSIL (27.6%) and for women referred for HPV-positive ASCUS (26.7%), further verifying that management should be similar. Two thirds of the CIN 2,3 was detected at initial colposcopy, and the remaining one third during the postcolposcopy 2-year follow-up.
The risk for subsequent detection of high-grade CIN was nearly identical for all women initially found not to have CIN 2,3 regardless of whether CIN 1 was detected at initial colposcopy, whether the colposcopy was initially completely normal, or whether there were changes that were biopsied and found not to have CIN (risk for CIN 2,3 was 13%, 11.3%, and 11.7% respectively).
Hence, all women referred for evaluation of HPV-positive ASC-US or LSIL and not treated for CIN 2,3 require similar diligent follow-up.
A single HPV test at 12 months detected 92% of all CIN 2,3 found over the 24-month follow-up; 55% tested HPV-positive and were referred to colposcopy.20 Repeat liquid-based cytology at 6 and 12 months referred to colposcopy 63% of women (using a threshold of a repeat Pap test of ASCUS or greater). Cumulative sensitivity of 2 repeat cytologies for CIN 2,3 was slightly less (88%). Combining a repeat Pap test with an HPV test did not increase sensitivity, but did significantly increase referral to colposcopy.
An HPV test alone at 12 months might be the most efficient test for identifying women with CIN 2,3 after colposcopy.20 Further support for this approach can be found in the substantial body of evidence showing that only persistent HPV progresses to CIN 321 and that testing for high-risk HPV detects most CIN 3.4,17,20
The ASCCP guidelines for women referred for either HPV-positive ASC-US or LSIL and found not to have CIN 2,3 or greater at initial colposcopy recommend either HPV testing at 12 months or repeat cytology at 6 and 12 months (FIGURE 1).11,22
Posttreatment follow-up. The ASCCP treatment guidelines also list HPV testing as an acceptable option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization,22 since there is substantial evidence that women successfully treated for CIN become HPV-negative, whereas women with persistent disease remain HPV-positive.23-25
A posttreatment HPV test should be performed no sooner than 6 months following the procedure, as it takes time for the patient to return to HPV-negative status. A positive HPV test is an indication for colposcopy. However, the guidelines advise against basing repeat treatment on a positive HPV test alone without documentation of persistent CIN.22
Other options for posttreatment surveillance include either repeat cytology or a combination of Pap testing and colposcopy at 4- to 6-month intervals until at least 3 cytologic results are “negative for squamous intraepithelial lesion or malignancy.”22
Annual cytologic follow-up is recommended thereafter. During that follow-up, any abnormal Pap test (ASC-US or greater) should be referred to colposcopy.
Managing ASC-US in special populations
Management of ASC-US may differ from the general recommendations when the patient is postmenopausal or immunosuppressed. However, there are no differences in the management guidelines during pregnancy.
HPV-negative postmenopausal patients. All 3 management options—immediate colposcopy, repeat cytology, and HPV DNA testing—are acceptable for postmenopausal women with ASC-US.11 However, estrogen deficiency is a common cause of ASC-US and is responsible for increasing rates of HPV-negative ASC-US in this age group despite high sensitivity of HPV testing for CIN 2 and 3.5
- Treatment with vaginal estrogen cream followed by repeat cytology approximately 1 week after completing the regimen is an option for postmenopausal women with ASC-US. This approach also may be helpful for perimenopausal women and for women of any age on progestin-only contraception who have clinical or cytologic evidence of atrophy.
- Women with ASC-US or greater on repeat cytology should be referred for colposcopy, whereas women with normal repeat cytology should have a second Pap test in 4 to 6 months. Repeating the course of vaginal estrogen prior to each Pap test may be helpful when atrophy is likely to persist. After 2 normal repeat Pap tests, the patient can return to routine screening.
Refer all immunosuppressed women for colposcopy. The management of ASC-US in HIV-infected women is particularly problematic because the rates of ASC-US and HPV detection are 2 to 3 times greater than in HIV-negative women. In addition, the risk of CIN 2 and 3 is much higher.26 HPV testing as a triage for ASC-US is not efficient in immunosuppressed women because the majority of ASC-US Pap tests in these women are HPV-positive.
ASCCP recommends colposcopy referral of all immunosuppressed women with ASCUS Pap test, regardless of their CD4 count, HIV viral load, or anti-retroviral therapy.26
Managing ASC-H: First, colposcopy
Clearly, women with ASC-H test results face a greater risk for CIN 2,3 and should be referred for immediate colposcopy.
The ASC-H designation is uncommon, reported in 0.27% to 0.6% of all Pap tests,27,28 or approximately 1 in 10 Pap smears read as ASC.
In ALTS, HPV testing and histology results were compared for women with Pap tests categorized as equivocal LSIL (ASCUSL), ASCUS-H, and high-grade squamous intraepithelial lesion (HSIL) (TABLE 2).29 High-risk HPV DNA was detected in 86% of ASCUS-H liquid-based Pap tests and 69.8% of ASCUS-H conventional smears. CIN 2,3 was found in 40% of liquid-based ASCUS-H smears and in 27.2% of conventional ASCUSH smears. A 3-year retrospective review of ASC-H with follow-up at Johns Hopkins Medical Institutions determined that 49% of patients had no CIN or glandular lesions.28 Of the 51% with CIN, approximately half the lesions were CIN 1 and half were CIN 2,3.
Further management depends on whether CIN is detected (FIGURE 3). If no CIN is found, the ASCCP guidelines recommend that cytology, colposcopy, and histology be reviewed. If there is a change in the diagnosis—eg, if the Pap interpretation is revised to HSIL—the patient should be managed accordingly.11
If there is no change, the patient should be followed with repeat cytology at 6-and 12-month intervals or HPV testing at 12 months. Women having any repeat abnormal Pap test at a threshold of ASC-US or greater or a positive HPV test should undergo repeat colposcopy.
ASC-H is of greater risk than ASC-US, but it is not as risky as HSIL. Therefore, a surgical excision procedure in the absence of documented CIN 2,3 would not normally be indicated.11
FIGURE 3 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
HPV test as triage option would mean retooling the system
Cytologic management systems have traditionally involved follow-up by repeat cytology, colposcopy, and, when necessary, treatment. Adding another triage option—HPV testing—requires that this system be retooled.
The labs. This is not difficult when the laboratory interpreting the liquid-based Pap test is the same lab that performs the “reflex” HPV test, as this allows the ASC-US Pap test to be reported as HPV-negative or HPV-positive. However, if the HPV test must be performed in a separate reference laboratory, the results of the Pap and HPV tests will arrive separately, and the clinician must collate the 2 reports before relaying the result to the patient.
The patients. Remember than an HPV test is a test for a sexually transmitted disease. (So is the Pap test, although it has not traditionally been considered as such.) For that reason, I give all patients a written explanation of the rationale behind testing ASC-US Pap tests for HPV. This explanation includes 2 check-off options at the bottom of the sheet where patients can indicate whether they would prefer HPV testing or one of the other follow-up options.
Most patients elect the HPV option. Our Pap test requisitions also have a check-off portion that allows us to notify the lab of patients who wants an HPV test if the Pap is interpreted as ASC-US.
The office staff. Whenever a new test or procedure is introduced, it is of primary importance that the office staff responsible for completing critical information on the requisition form is adequately trained. This involves knowing when and how to order the test and how to complete insurance information and clinical history on the Pap requisition—including the correct International Classification of Diseases, Ninth Revision code—to ensure that the HPV test is covered by the patient’s insurer.
Clinicians must understand the usually benign nature of HPV infection. Reporting a positive HPV test in a manner that is not unduly concerning requires reassuring and nonjudgmental communication of the results based on a broad understanding of the usually low-risk natural history of the virus, yet fosters responsible follow-up.
Why all HPV-positive ASC-US requires diligent follow-up
The recently released ASCCP guidelines recognize HPV testing as an option in the management of ASC results, including:
- initial management of ASC-US,
- postcolposcopy management of ASC-H or HPV-positive ASC Pap tests found to be normal or to have CIN 1, and
- posttreatment follow-up.
For each indication, the HPV test identifies women most likely to have CIN (HPV-positive) and those likely to have benign processes not related to HPV (HPV-negative).
New longitudinal data verify that women with HPV-positive ASC-US continue at risk for detection of CIN 2,3 (about 12% overall), whether the original colposcopic finding was normal or CIN 1.8 Therefore, they need continued diligent follow-up.
Dr. Cox serves on the Speaker’s Bureaus of Cytyc Corporation and Digene Corporation.
1. Kurman RJ, Henson DE, Berbst AL, Noller KL, Schiffman MH. Interim guidelines for the management of abnormal cervical cytology. JAMA. 1994;271:1866-1869.
2. Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials. Int J Gynecol Cancer. 1995;5:321-328.
3. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726-742.
4. Solomon D, Schiffman M, Tarone R, et al. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293-299.
5. Sherman ME, Schiffman M, Cox JT, et al. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-107.
6. Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172:946-954.
7. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
8. Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406-1412.
9. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of the minimally abnormal Papanicolaou diagnosis. Obstet Gynecol. 1998;91:973-976.
10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119.
11. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
12. Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient awareness and choice of management. J Royal Soc Med. 1996;89:257.-
13. Wright TC, Lorincz AT, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Pap smears. Am J Obstet Gynecol. 1998;178:926-966.
14. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
15. Agency for Health Care Policy and Research. Evidence Report/Technology Assessment #5: Evaluation of Cervical Cytology. Rockville, Md: AHCPR; January 1999.
16. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance—Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505.
17. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383-1392.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003;127:946-949.
19. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382-2390.
20. Guido R, Schiffman M, Solomon D, et al. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401-1405.
21. Nobbenhuis M, Walboomers JM, Helmerhorst TI, Rozendaal L. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20.-
22. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
23. Paraskevaidis E, Koliopoulos G, Alamanos Y, Malamou-Mitsi V, Lolis ED, Kitchener HC. Human papillomavirus testing and the outcome of treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2001;98:833-836.
24. Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84:796-801.
25. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001;82:177-180.
26. Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432-442.
27. Selvaggi SM. Reporting of atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H) on cervical samples: is it significant? Diagn Cytopathol. 2003;29:38-41.
28. Alli PM, Ali SZ. Atypical squamous cells of undetermined significance—rule out high-grade squamous intraepithelial lesion: cytopathologic characteristics and clinical correlates. Diagn Cytopathol. 2003;28:308-312.
29. Sherman ME, Solomon D, Schiffman M, et al. A comparison of equivocal LSIL and equivocal HSIL cervical cytology in the ASCUS LSIL Triage Study. Am J Clin Pathol. 2001;116:386-394.
1. Kurman RJ, Henson DE, Berbst AL, Noller KL, Schiffman MH. Interim guidelines for the management of abnormal cervical cytology. JAMA. 1994;271:1866-1869.
2. Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials. Int J Gynecol Cancer. 1995;5:321-328.
3. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726-742.
4. Solomon D, Schiffman M, Tarone R, et al. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293-299.
5. Sherman ME, Schiffman M, Cox JT, et al. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-107.
6. Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172:946-954.
7. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
8. Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406-1412.
9. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of the minimally abnormal Papanicolaou diagnosis. Obstet Gynecol. 1998;91:973-976.
10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119.
11. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
12. Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient awareness and choice of management. J Royal Soc Med. 1996;89:257.-
13. Wright TC, Lorincz AT, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Pap smears. Am J Obstet Gynecol. 1998;178:926-966.
14. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
15. Agency for Health Care Policy and Research. Evidence Report/Technology Assessment #5: Evaluation of Cervical Cytology. Rockville, Md: AHCPR; January 1999.
16. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance—Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505.
17. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383-1392.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003;127:946-949.
19. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382-2390.
20. Guido R, Schiffman M, Solomon D, et al. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401-1405.
21. Nobbenhuis M, Walboomers JM, Helmerhorst TI, Rozendaal L. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20.-
22. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
23. Paraskevaidis E, Koliopoulos G, Alamanos Y, Malamou-Mitsi V, Lolis ED, Kitchener HC. Human papillomavirus testing and the outcome of treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2001;98:833-836.
24. Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84:796-801.
25. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001;82:177-180.
26. Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432-442.
27. Selvaggi SM. Reporting of atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H) on cervical samples: is it significant? Diagn Cytopathol. 2003;29:38-41.
28. Alli PM, Ali SZ. Atypical squamous cells of undetermined significance—rule out high-grade squamous intraepithelial lesion: cytopathologic characteristics and clinical correlates. Diagn Cytopathol. 2003;28:308-312.
29. Sherman ME, Solomon D, Schiffman M, et al. A comparison of equivocal LSIL and equivocal HSIL cervical cytology in the ASCUS LSIL Triage Study. Am J Clin Pathol. 2001;116:386-394.