User login
UPDATE ON CERVICAL DISEASE
Over the past year, we have gained further insight into the efficacy and safety of the human papillomavirus (HPV) vaccines; received new, practical cervical screening guidance from the Centers for Disease Control and Prevention (CDC); and gathered further evidence that colposcopy is not as sensitive at detecting high-grade cervical disease as we once thought.
In this article, I describe each of these developments in depth.
Both HPV vaccines are safe and effective—
and both offer cross-protection
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13.
HPV 16 accounts for about 55% of all cases of cervical cancer and HPV 18 for another 15%—and both HPV vaccines on the market provide coverage against these two types. Because vaccination stands to reduce the burden of cervical disease so dramatically, it behooves us to achieve the highest possible vaccination rate for girls and young women.
Regrettably, fewer than 40% of the eligible female population of the United States has received one or more injections of either the bivalent (HPV 16, 18) (Cervarix) or quadrivalent (HPV 6, 11, 16, 18) (Gardasil) vaccine—with the vaccination rate varying considerably by geographic location and socioeconomic status.1 Clearly, we have much work ahead of us to improve this rate.
What’s the big picture?
Each trial of the HPV vaccine to date has demonstrated high efficacy and safety. Drawing from the individual findings of these trials to develop a snapshot of overall efficacy
and safety has been difficult, however, owing to multiple clinical endpoints, differences in both the number of virus-like particle types and in the adjuvant used in each vaccine, variability of the populations, and different definitions of efficacy. These limitations have made it difficult for clinicians and patients to make an informed decision about which vaccine to choose.
To address these concerns, Lu and colleagues conducted a comprehensive systematic review and meta-analysis of seven unique randomized, controlled trials with a total enrollment of 44,141 females. Their goal: to assess the safety and efficacy of both vaccines against multiple virologic and clinical endpoints, including efficacy not only against the primary HPV vaccine types, but closely related types as well.
They focused on two groups of girls and women:
- The per protocol population (PPP) included females who were both DNA- and sero-negative to the HPV types contained in the vaccine at the start and end of the vaccination period. The PPP group received all three injections of the vaccine, with no protocol violations.
- The intention-to-treat cohort (ITT) included women and girls who had received one or more doses of the vaccine or placebo and who had follow-up data available, regardless of HPV status at enrollment.
The PPP more closely resembles the sexually naïve population that stands to benefit most from the full vaccination series, whereas the ITT is more similar to girls and women 18 to 26 years old who are seeking “catch-up” vaccination, most of them having initiated sexual activity or had less than perfect compliance with vaccination, or both.
In the ITT cohort, the pooled relative risk (RR) for HPV 16-related cervical intraepithelial neoplasia (CIN) grade 2 or worse was 0.47, corresponding to a pooled efficacy of 53%, a statistically significant benefit. In the PPP, the RR was 0.04, corresponding to a pooled efficacy of 96% for HPV 16-related CIN 2+. The RR was similar for HPV 18 (TABLE). The reduction in CIN 1 for women not previously infected with either of these high-risk HPV types was also high—95% for HPV 16 and 97% for HPV 18.
Effect of HPV vaccination on high-grade cervical disease
| Group | Relative risk of CIN 2+ | Reduction in CIN 2+ | ||
|---|---|---|---|---|
| HpV 16 | HpV 18 | HpV 16 | HpV 18 | |
| Intention to treat | 0.47 | 0.16 | 53% | 84% |
| Per protocol | 0.04 | 0.10 | 96% | 90% |
| CIN=cervical intraepithelial neoplasia SOURCE: Lu B, et al. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13. | ||||
Vaccines offer cross-protection against 3 additional HPV types
The possibility that the HPV vaccines provide cross-protection against closely related HPV types has generated considerable interest. Lu and colleagues assessed cross-protection against 6-month persistent infection related to five HPV types:
- HPV 31—relative risk (RR) of 0.47 and 0.30 in the ITT and PPP cohorts, respectively
- HPV 45—RR of 0.50 and 0.42 in the ITT and PPP cohorts, respectively. There was significant heterogeneity between the trials in efficacy against persistent HPV 45 infection.
- HPV 33—RR of 0.65 and 0.57 in the ITT and PPP cohorts, respectively
- HPV 52 and 58—no statistically significant cross-protection.
Adverse events are minimal
The most common systemic vaccine-related adverse events reported in all the trials were headache and fatigue, which were noted in 50% to 60% of participants. The most common serious adverse events were abnormal pregnancy outcomes, such as birth defects and spontaneous abortion, but the RR of 1.0 for all serious adverse events suggests a statistically insignificant difference in the risk of serious adverse events between vaccine and control groups. These findings are consistent with the most recent review by the CDC and FDA (October 2010), which concluded that Gardasil is safe and effective for the prevention of the four types covered in the vaccine.2 CDC updates on safety do not yet include the bivalent vaccine because of its more recent release to the US market.
At every opportunity, encourage HPV vaccination for girls and women who are 9 to 26 years old.
New STD guidelines from the CDC include tips
on cervical cancer screening
Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
The CDC’s most recent sexually transmitted disease (STD) guidelines, released at the end of 2010, cover all sexually transmitted infections, including genital HPV infection. In general, the recommendations on cervical cancer screening are consistent with ACOG’s 2009 guidelines, which I discussed in the March 2010 Update on Cervical Disease. The CDC also offers concrete, useful suggestions on how to counsel patients who have genital warts or who test positive for an oncogenic strain of HPV. Although the guidelines are aimed at STD and public health clinics, they include many recommendations useful to all health care providers. For that reason, discussion of the highlights seems appropriate.
Like ACOG, CDC says screening should start at 21 years
Screening should begin when the patient is 21 years old and continue at 2-year intervals until she is 30 years old, at which time it should switch to every 3 years—provided she has had three consecutive normal Pap tests or one normal cotest (Pap and HPV test combined).
Because a woman may sometimes assume that she has undergone a Pap test by virtue of having had a pelvic examination, inaccuracies in self-reported screening intervals may arise. Therefore, it is imperative to devise a protocol for cervical cancer screening among women who do not have documentation, in their medical record, of a normal Pap test within the preceding 12 months. Although some women will undoubtedly undergo screening sooner than necessary, this approach will protect women lacking adequate documentation from being underscreened.
When to use the HPV test (and when to avoid it)
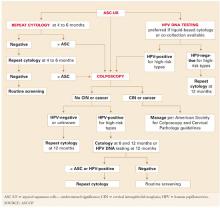
The guidelines confirm that the HPV test is an appropriate tool in the management of atypical squamous cells of undetermined significance (ASC–US) among women 21 years and older and as a cotest with the Pap for women who are 30 years and older.
The CDC recommends against the HPV test in the following situations:
- when deciding whether to vaccinate against HPV
- as part of a screen for STD
- in the triage of low-grade squamous intraepithelial lesion (LSIL) Pap results, although 2006 guidelines from the American Society for Colposcopy and Cervical Pathology and 2007 guidelines from ACOG recommend, as an option, the use of the HPV test in the triage of postmenopausal women who have LSIL
- in women younger than 21 years
- as a stand-alone primary cervical cancer screen (without the Pap test).
These recommendations are consistent with earlier conclusions.3
Perhaps the most important insights offered in the CDC’s 2010 STD guidelines are the counseling messages for women who undergo cotesting with both the HPV and Pap tests. It often is a challenge to communicate the indications for and findings of this screening approach. Here is guidance offered by the CDC:
- HPV is very common. It can infect the genital areas of both men and women. It usually has no signs or symptoms.
- Most sexually active persons get HPV at some time in their life, although few will ever know it. Even a person who has had only one lifetime sex partner can get HPV if the partner was infected.
- Although the immune system clears HPV infection most of the time, the infection fails to resolve in some people
- No clinically validated test exists for men to determine whether they have HPV infection. The most common manifestation of HPV infection in men is genital warts. High-risk HPV types seldom cause genital warts.
- Partners who are in a long-term relationship tend to share HPV. Sexual partners of HPV-infected people also likely have HPV, even though they may have no signs or symptoms of infection.
- Detection of high-risk HPV infection in a woman does not mean that she or her partner is engaging in sexual activity outside of a relationship. HPV infection can be present for many years before it is detected, and no method can accurately confirm when HPV infection was acquired.
The pap test is not a screening test for Std
Other findings that may be useful for all clinicians, as well as for those who practice in an STD clinic:
- The Pap test is not a screening test for STD
- All eligible women should undergo cervical cancer screening, regardless of sexual orientation (i.e., heterosexual, lesbian, or bisexual)
- Conventional cytology should be delayed if the patient is menstruating, and she should be advised to undergo a Pap test at the earliest opportunity
- If specific infections other than HPV are identified, the patient may need to undergo a repeat Pap test after appropriate treatment for those infections. However, in most instances, the Pap test will be reported as satisfactory for evaluation, and a reliable final report can be produced without the need to repeat the Pap test after treatment.
- The presence of a mucopurulent discharge should not delay the Pap test. The test can be performed after careful removal of the discharge with a saline-soaked cotton swab.
- When the Pap test is repeated because the previous test was interpreted as unsatisfactory, the patient should not be returned to regular screening intervals until the Pap test is reported as satisfactory and negative
- Cervical screening should not be accelerated for women who have genital warts and no other indication.
CDC recommendations on cervical cancer prevention and screening are consistent with those of other organizations, including ACOG. the counseling messages should be adopted universally.
When colposcopic biopsy is indicated, take more than one sample
Stoler MH, Vichnin MD, Ferenczy A, et al; the FUTURE I, II and III Investigators. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–1362.
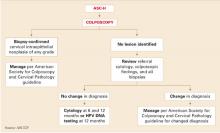
The progress we have made in cervical cancer prevention is largely due to our ability to detect and treat precancer, particularly CIN 3, before it gains the capacity to invade. Until recently, few experts would have questioned the value of the partnership between cervical cytology screening and treatment of lesions detected on colposcopically directed biopsy.4 However, over the past decade, the accuracy of colposcopy for detection of high-grade lesions has been widely questioned, first by studies assessing static digitized cervigrams or colposcopy photo images, and more recently by studies comparing “real-time” colposcopy to histology obtained during colposcopy or excisional biopsy, or both.
The largest of these studies was conducted by Stoler and colleagues to compare the results of colposcopically directed biopsy and subsequent cervical excision among 737 women (16 to 45 years old) in the placebo arm of the quadrivalent HPV vaccine (FUTURE) randomized, controlled trials. In these trials, all women were referred for colposcopy according to a Pap triage algorithm, and one or more biopsies was taken from the area with the greatest apparent abnormality, as viewed by colposcopy. When excisional treatment was indicated, a biopsy of the worst-appearing area was taken again just before the excision.
Each patient’s most severe pathology-panel diagnosis for the excisional specimen was compared with:
- the most severe biopsy result from the preceding 6 months (excluding the biopsy taken on the same day as the excisional procedure) (Analysis 1)
- the biopsy taken on the same day as the definitive excisional procedure (Analysis 2).
When CIN 2 and CIN 3 are managed similarly, a discrepancy of one degree between colposcopically directed biopsy and the excisional specimen is considered sufficient agreement. Therefore, in this study, a difference of one degree in histologic diagnosis was considered agreement.
High-grade disease was more likely to be underestimated
on the same-day biopsy
Colposcopically directed biopsies obtained within 6 months before definitive treatment (Analysis 1) had lower overall agreement with the excisional specimen than biopsies collected on the same day as definitive treatment (Analysis 2). However, underestimation of high-grade disease was lower (26% overall underestimation of CIN 2 or 3 or adenocarcinoma in situ [AIS]) on earlier biopsy specimens than on those collected on the same day as definitive treatment (57% overall underestimation of CIN 2 or 3 or AIS).
Conversely, overestimation, or removal, of disease was higher (36%) in biopsies collected within 6 months before the excisional treatment, compared with biopsies collected on the same day as definitive treatment (5%).
The investigators suggested that any discrepancy in accuracy between the biopsy obtained at treatment and the biopsy obtained earlier might be the result of less diligent colposcopic evaluation and biopsy placement when the colposcopist knew that definitive therapy would immediately follow. Another possibility, they noted, is that lesions biopsied as early as 6 months before definitive treatment may have regressed in the process of tissue repair or were completely removed by the biopsy.
When all biopsies were compared with the final diagnosis of the excisional specimen, the colposcopically directed biopsy was less severe 42% to 66% of the time when the excisional histology was read as CIN 3 or AIS. However, when one degree of discrepancy was allowed, as it is in clinical practice, agreement was 92%. This suggests that women in the FUTURE trials, as well as those in real clinical practice, are typically managed appropriately under current protocols that combine cytology and colposcopy results to properly identify women who have cervical lesions that require surgical intervention.
Most CIN 3 lesions were small
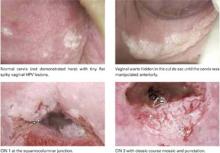
Many of the CIN 3 lesions in this trial were small, as they were in the ASCUS LSIL Triage Study (ALTS), in which the median length of CIN 3 lesions was only 6.5 mm. Also in ALTS, lesions in one third of patients were so small that colposcopically directed biopsy did not leave any residual disease to be detected in the loop electrosurgical excision specimen.5 The size of a CIN 3 lesion that has associated invasion is, on average, seven times larger than without invasion.6 Although colposcopy is much less likely to miss large lesions, it is important to miss as little high-grade disease as possible because the risk of invasion is cumulative over time and unpredictable in a given patient.

Multiple biopsies boost detection
Sampling more than one area improves the accuracy of colposcopically directed biopsy, even when one area looks most abnormal. This colposcopy photo shows potential biopsy sites (within the ovals), although other choices may also be reasonable. Several studies have shown that colposcopically directed biopsy of even normal-appearing areas at the squamocolumnar junction or within large ectopies can improve detection of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.Studies have shown that it is possible to increase the accuracy of detection of CIN 2+ by increasing the number of biopsies. In this study by Stoler and colleagues, the sensitivity of initial colposcopy improved from 47% (for one biopsy) to 65% (two biopsies) and 77% (three or more) (FIGURE). Overall agreement increased with increasing age, which is consistent with the likelihood that CIN 3 lesions expand with age and become increasingly detectable by colposcopy.
Colposcopy does work, but the era of biopsying only the most abnormal-appearing area is over. Take more biopsies.
We want to hear from you! Tell us what you think.
1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533.
2. Centers for Disease Control and Prevention. Frequently asked questions about HPV vaccine safety. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html. Accessed February 1, 2011.
3. Cox JT, Moriarty AT, Castle PE. Commentary on: Statement on HPV DNA test utilization. Diagn Cytopathol. 2009;37(7):471-474.
4. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008;111(6):1266-1267.
5. Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of CIN 3 lesions in ALTS: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372-379.
6. Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol. 1992;99(7):583-586.
Over the past year, we have gained further insight into the efficacy and safety of the human papillomavirus (HPV) vaccines; received new, practical cervical screening guidance from the Centers for Disease Control and Prevention (CDC); and gathered further evidence that colposcopy is not as sensitive at detecting high-grade cervical disease as we once thought.
In this article, I describe each of these developments in depth.
Both HPV vaccines are safe and effective—
and both offer cross-protection
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13.
HPV 16 accounts for about 55% of all cases of cervical cancer and HPV 18 for another 15%—and both HPV vaccines on the market provide coverage against these two types. Because vaccination stands to reduce the burden of cervical disease so dramatically, it behooves us to achieve the highest possible vaccination rate for girls and young women.
Regrettably, fewer than 40% of the eligible female population of the United States has received one or more injections of either the bivalent (HPV 16, 18) (Cervarix) or quadrivalent (HPV 6, 11, 16, 18) (Gardasil) vaccine—with the vaccination rate varying considerably by geographic location and socioeconomic status.1 Clearly, we have much work ahead of us to improve this rate.
What’s the big picture?
Each trial of the HPV vaccine to date has demonstrated high efficacy and safety. Drawing from the individual findings of these trials to develop a snapshot of overall efficacy
and safety has been difficult, however, owing to multiple clinical endpoints, differences in both the number of virus-like particle types and in the adjuvant used in each vaccine, variability of the populations, and different definitions of efficacy. These limitations have made it difficult for clinicians and patients to make an informed decision about which vaccine to choose.
To address these concerns, Lu and colleagues conducted a comprehensive systematic review and meta-analysis of seven unique randomized, controlled trials with a total enrollment of 44,141 females. Their goal: to assess the safety and efficacy of both vaccines against multiple virologic and clinical endpoints, including efficacy not only against the primary HPV vaccine types, but closely related types as well.
They focused on two groups of girls and women:
- The per protocol population (PPP) included females who were both DNA- and sero-negative to the HPV types contained in the vaccine at the start and end of the vaccination period. The PPP group received all three injections of the vaccine, with no protocol violations.
- The intention-to-treat cohort (ITT) included women and girls who had received one or more doses of the vaccine or placebo and who had follow-up data available, regardless of HPV status at enrollment.
The PPP more closely resembles the sexually naïve population that stands to benefit most from the full vaccination series, whereas the ITT is more similar to girls and women 18 to 26 years old who are seeking “catch-up” vaccination, most of them having initiated sexual activity or had less than perfect compliance with vaccination, or both.
In the ITT cohort, the pooled relative risk (RR) for HPV 16-related cervical intraepithelial neoplasia (CIN) grade 2 or worse was 0.47, corresponding to a pooled efficacy of 53%, a statistically significant benefit. In the PPP, the RR was 0.04, corresponding to a pooled efficacy of 96% for HPV 16-related CIN 2+. The RR was similar for HPV 18 (TABLE). The reduction in CIN 1 for women not previously infected with either of these high-risk HPV types was also high—95% for HPV 16 and 97% for HPV 18.
Effect of HPV vaccination on high-grade cervical disease
| Group | Relative risk of CIN 2+ | Reduction in CIN 2+ | ||
|---|---|---|---|---|
| HpV 16 | HpV 18 | HpV 16 | HpV 18 | |
| Intention to treat | 0.47 | 0.16 | 53% | 84% |
| Per protocol | 0.04 | 0.10 | 96% | 90% |
| CIN=cervical intraepithelial neoplasia SOURCE: Lu B, et al. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13. | ||||
Vaccines offer cross-protection against 3 additional HPV types
The possibility that the HPV vaccines provide cross-protection against closely related HPV types has generated considerable interest. Lu and colleagues assessed cross-protection against 6-month persistent infection related to five HPV types:
- HPV 31—relative risk (RR) of 0.47 and 0.30 in the ITT and PPP cohorts, respectively
- HPV 45—RR of 0.50 and 0.42 in the ITT and PPP cohorts, respectively. There was significant heterogeneity between the trials in efficacy against persistent HPV 45 infection.
- HPV 33—RR of 0.65 and 0.57 in the ITT and PPP cohorts, respectively
- HPV 52 and 58—no statistically significant cross-protection.
Adverse events are minimal
The most common systemic vaccine-related adverse events reported in all the trials were headache and fatigue, which were noted in 50% to 60% of participants. The most common serious adverse events were abnormal pregnancy outcomes, such as birth defects and spontaneous abortion, but the RR of 1.0 for all serious adverse events suggests a statistically insignificant difference in the risk of serious adverse events between vaccine and control groups. These findings are consistent with the most recent review by the CDC and FDA (October 2010), which concluded that Gardasil is safe and effective for the prevention of the four types covered in the vaccine.2 CDC updates on safety do not yet include the bivalent vaccine because of its more recent release to the US market.
At every opportunity, encourage HPV vaccination for girls and women who are 9 to 26 years old.
New STD guidelines from the CDC include tips
on cervical cancer screening
Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
The CDC’s most recent sexually transmitted disease (STD) guidelines, released at the end of 2010, cover all sexually transmitted infections, including genital HPV infection. In general, the recommendations on cervical cancer screening are consistent with ACOG’s 2009 guidelines, which I discussed in the March 2010 Update on Cervical Disease. The CDC also offers concrete, useful suggestions on how to counsel patients who have genital warts or who test positive for an oncogenic strain of HPV. Although the guidelines are aimed at STD and public health clinics, they include many recommendations useful to all health care providers. For that reason, discussion of the highlights seems appropriate.
Like ACOG, CDC says screening should start at 21 years
Screening should begin when the patient is 21 years old and continue at 2-year intervals until she is 30 years old, at which time it should switch to every 3 years—provided she has had three consecutive normal Pap tests or one normal cotest (Pap and HPV test combined).
Because a woman may sometimes assume that she has undergone a Pap test by virtue of having had a pelvic examination, inaccuracies in self-reported screening intervals may arise. Therefore, it is imperative to devise a protocol for cervical cancer screening among women who do not have documentation, in their medical record, of a normal Pap test within the preceding 12 months. Although some women will undoubtedly undergo screening sooner than necessary, this approach will protect women lacking adequate documentation from being underscreened.
When to use the HPV test (and when to avoid it)
The guidelines confirm that the HPV test is an appropriate tool in the management of atypical squamous cells of undetermined significance (ASC–US) among women 21 years and older and as a cotest with the Pap for women who are 30 years and older.
The CDC recommends against the HPV test in the following situations:
- when deciding whether to vaccinate against HPV
- as part of a screen for STD
- in the triage of low-grade squamous intraepithelial lesion (LSIL) Pap results, although 2006 guidelines from the American Society for Colposcopy and Cervical Pathology and 2007 guidelines from ACOG recommend, as an option, the use of the HPV test in the triage of postmenopausal women who have LSIL
- in women younger than 21 years
- as a stand-alone primary cervical cancer screen (without the Pap test).
These recommendations are consistent with earlier conclusions.3
Perhaps the most important insights offered in the CDC’s 2010 STD guidelines are the counseling messages for women who undergo cotesting with both the HPV and Pap tests. It often is a challenge to communicate the indications for and findings of this screening approach. Here is guidance offered by the CDC:
- HPV is very common. It can infect the genital areas of both men and women. It usually has no signs or symptoms.
- Most sexually active persons get HPV at some time in their life, although few will ever know it. Even a person who has had only one lifetime sex partner can get HPV if the partner was infected.
- Although the immune system clears HPV infection most of the time, the infection fails to resolve in some people
- No clinically validated test exists for men to determine whether they have HPV infection. The most common manifestation of HPV infection in men is genital warts. High-risk HPV types seldom cause genital warts.
- Partners who are in a long-term relationship tend to share HPV. Sexual partners of HPV-infected people also likely have HPV, even though they may have no signs or symptoms of infection.
- Detection of high-risk HPV infection in a woman does not mean that she or her partner is engaging in sexual activity outside of a relationship. HPV infection can be present for many years before it is detected, and no method can accurately confirm when HPV infection was acquired.
The pap test is not a screening test for Std
Other findings that may be useful for all clinicians, as well as for those who practice in an STD clinic:
- The Pap test is not a screening test for STD
- All eligible women should undergo cervical cancer screening, regardless of sexual orientation (i.e., heterosexual, lesbian, or bisexual)
- Conventional cytology should be delayed if the patient is menstruating, and she should be advised to undergo a Pap test at the earliest opportunity
- If specific infections other than HPV are identified, the patient may need to undergo a repeat Pap test after appropriate treatment for those infections. However, in most instances, the Pap test will be reported as satisfactory for evaluation, and a reliable final report can be produced without the need to repeat the Pap test after treatment.
- The presence of a mucopurulent discharge should not delay the Pap test. The test can be performed after careful removal of the discharge with a saline-soaked cotton swab.
- When the Pap test is repeated because the previous test was interpreted as unsatisfactory, the patient should not be returned to regular screening intervals until the Pap test is reported as satisfactory and negative
- Cervical screening should not be accelerated for women who have genital warts and no other indication.
CDC recommendations on cervical cancer prevention and screening are consistent with those of other organizations, including ACOG. the counseling messages should be adopted universally.
When colposcopic biopsy is indicated, take more than one sample
Stoler MH, Vichnin MD, Ferenczy A, et al; the FUTURE I, II and III Investigators. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–1362.
The progress we have made in cervical cancer prevention is largely due to our ability to detect and treat precancer, particularly CIN 3, before it gains the capacity to invade. Until recently, few experts would have questioned the value of the partnership between cervical cytology screening and treatment of lesions detected on colposcopically directed biopsy.4 However, over the past decade, the accuracy of colposcopy for detection of high-grade lesions has been widely questioned, first by studies assessing static digitized cervigrams or colposcopy photo images, and more recently by studies comparing “real-time” colposcopy to histology obtained during colposcopy or excisional biopsy, or both.
The largest of these studies was conducted by Stoler and colleagues to compare the results of colposcopically directed biopsy and subsequent cervical excision among 737 women (16 to 45 years old) in the placebo arm of the quadrivalent HPV vaccine (FUTURE) randomized, controlled trials. In these trials, all women were referred for colposcopy according to a Pap triage algorithm, and one or more biopsies was taken from the area with the greatest apparent abnormality, as viewed by colposcopy. When excisional treatment was indicated, a biopsy of the worst-appearing area was taken again just before the excision.
Each patient’s most severe pathology-panel diagnosis for the excisional specimen was compared with:
- the most severe biopsy result from the preceding 6 months (excluding the biopsy taken on the same day as the excisional procedure) (Analysis 1)
- the biopsy taken on the same day as the definitive excisional procedure (Analysis 2).
When CIN 2 and CIN 3 are managed similarly, a discrepancy of one degree between colposcopically directed biopsy and the excisional specimen is considered sufficient agreement. Therefore, in this study, a difference of one degree in histologic diagnosis was considered agreement.
High-grade disease was more likely to be underestimated
on the same-day biopsy
Colposcopically directed biopsies obtained within 6 months before definitive treatment (Analysis 1) had lower overall agreement with the excisional specimen than biopsies collected on the same day as definitive treatment (Analysis 2). However, underestimation of high-grade disease was lower (26% overall underestimation of CIN 2 or 3 or adenocarcinoma in situ [AIS]) on earlier biopsy specimens than on those collected on the same day as definitive treatment (57% overall underestimation of CIN 2 or 3 or AIS).
Conversely, overestimation, or removal, of disease was higher (36%) in biopsies collected within 6 months before the excisional treatment, compared with biopsies collected on the same day as definitive treatment (5%).
The investigators suggested that any discrepancy in accuracy between the biopsy obtained at treatment and the biopsy obtained earlier might be the result of less diligent colposcopic evaluation and biopsy placement when the colposcopist knew that definitive therapy would immediately follow. Another possibility, they noted, is that lesions biopsied as early as 6 months before definitive treatment may have regressed in the process of tissue repair or were completely removed by the biopsy.
When all biopsies were compared with the final diagnosis of the excisional specimen, the colposcopically directed biopsy was less severe 42% to 66% of the time when the excisional histology was read as CIN 3 or AIS. However, when one degree of discrepancy was allowed, as it is in clinical practice, agreement was 92%. This suggests that women in the FUTURE trials, as well as those in real clinical practice, are typically managed appropriately under current protocols that combine cytology and colposcopy results to properly identify women who have cervical lesions that require surgical intervention.
Most CIN 3 lesions were small
Many of the CIN 3 lesions in this trial were small, as they were in the ASCUS LSIL Triage Study (ALTS), in which the median length of CIN 3 lesions was only 6.5 mm. Also in ALTS, lesions in one third of patients were so small that colposcopically directed biopsy did not leave any residual disease to be detected in the loop electrosurgical excision specimen.5 The size of a CIN 3 lesion that has associated invasion is, on average, seven times larger than without invasion.6 Although colposcopy is much less likely to miss large lesions, it is important to miss as little high-grade disease as possible because the risk of invasion is cumulative over time and unpredictable in a given patient.

Multiple biopsies boost detection
Sampling more than one area improves the accuracy of colposcopically directed biopsy, even when one area looks most abnormal. This colposcopy photo shows potential biopsy sites (within the ovals), although other choices may also be reasonable. Several studies have shown that colposcopically directed biopsy of even normal-appearing areas at the squamocolumnar junction or within large ectopies can improve detection of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.Studies have shown that it is possible to increase the accuracy of detection of CIN 2+ by increasing the number of biopsies. In this study by Stoler and colleagues, the sensitivity of initial colposcopy improved from 47% (for one biopsy) to 65% (two biopsies) and 77% (three or more) (FIGURE). Overall agreement increased with increasing age, which is consistent with the likelihood that CIN 3 lesions expand with age and become increasingly detectable by colposcopy.
Colposcopy does work, but the era of biopsying only the most abnormal-appearing area is over. Take more biopsies.
We want to hear from you! Tell us what you think.
Over the past year, we have gained further insight into the efficacy and safety of the human papillomavirus (HPV) vaccines; received new, practical cervical screening guidance from the Centers for Disease Control and Prevention (CDC); and gathered further evidence that colposcopy is not as sensitive at detecting high-grade cervical disease as we once thought.
In this article, I describe each of these developments in depth.
Both HPV vaccines are safe and effective—
and both offer cross-protection
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13.
HPV 16 accounts for about 55% of all cases of cervical cancer and HPV 18 for another 15%—and both HPV vaccines on the market provide coverage against these two types. Because vaccination stands to reduce the burden of cervical disease so dramatically, it behooves us to achieve the highest possible vaccination rate for girls and young women.
Regrettably, fewer than 40% of the eligible female population of the United States has received one or more injections of either the bivalent (HPV 16, 18) (Cervarix) or quadrivalent (HPV 6, 11, 16, 18) (Gardasil) vaccine—with the vaccination rate varying considerably by geographic location and socioeconomic status.1 Clearly, we have much work ahead of us to improve this rate.
What’s the big picture?
Each trial of the HPV vaccine to date has demonstrated high efficacy and safety. Drawing from the individual findings of these trials to develop a snapshot of overall efficacy
and safety has been difficult, however, owing to multiple clinical endpoints, differences in both the number of virus-like particle types and in the adjuvant used in each vaccine, variability of the populations, and different definitions of efficacy. These limitations have made it difficult for clinicians and patients to make an informed decision about which vaccine to choose.
To address these concerns, Lu and colleagues conducted a comprehensive systematic review and meta-analysis of seven unique randomized, controlled trials with a total enrollment of 44,141 females. Their goal: to assess the safety and efficacy of both vaccines against multiple virologic and clinical endpoints, including efficacy not only against the primary HPV vaccine types, but closely related types as well.
They focused on two groups of girls and women:
- The per protocol population (PPP) included females who were both DNA- and sero-negative to the HPV types contained in the vaccine at the start and end of the vaccination period. The PPP group received all three injections of the vaccine, with no protocol violations.
- The intention-to-treat cohort (ITT) included women and girls who had received one or more doses of the vaccine or placebo and who had follow-up data available, regardless of HPV status at enrollment.
The PPP more closely resembles the sexually naïve population that stands to benefit most from the full vaccination series, whereas the ITT is more similar to girls and women 18 to 26 years old who are seeking “catch-up” vaccination, most of them having initiated sexual activity or had less than perfect compliance with vaccination, or both.
In the ITT cohort, the pooled relative risk (RR) for HPV 16-related cervical intraepithelial neoplasia (CIN) grade 2 or worse was 0.47, corresponding to a pooled efficacy of 53%, a statistically significant benefit. In the PPP, the RR was 0.04, corresponding to a pooled efficacy of 96% for HPV 16-related CIN 2+. The RR was similar for HPV 18 (TABLE). The reduction in CIN 1 for women not previously infected with either of these high-risk HPV types was also high—95% for HPV 16 and 97% for HPV 18.
Effect of HPV vaccination on high-grade cervical disease
| Group | Relative risk of CIN 2+ | Reduction in CIN 2+ | ||
|---|---|---|---|---|
| HpV 16 | HpV 18 | HpV 16 | HpV 18 | |
| Intention to treat | 0.47 | 0.16 | 53% | 84% |
| Per protocol | 0.04 | 0.10 | 96% | 90% |
| CIN=cervical intraepithelial neoplasia SOURCE: Lu B, et al. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13. | ||||
Vaccines offer cross-protection against 3 additional HPV types
The possibility that the HPV vaccines provide cross-protection against closely related HPV types has generated considerable interest. Lu and colleagues assessed cross-protection against 6-month persistent infection related to five HPV types:
- HPV 31—relative risk (RR) of 0.47 and 0.30 in the ITT and PPP cohorts, respectively
- HPV 45—RR of 0.50 and 0.42 in the ITT and PPP cohorts, respectively. There was significant heterogeneity between the trials in efficacy against persistent HPV 45 infection.
- HPV 33—RR of 0.65 and 0.57 in the ITT and PPP cohorts, respectively
- HPV 52 and 58—no statistically significant cross-protection.
Adverse events are minimal
The most common systemic vaccine-related adverse events reported in all the trials were headache and fatigue, which were noted in 50% to 60% of participants. The most common serious adverse events were abnormal pregnancy outcomes, such as birth defects and spontaneous abortion, but the RR of 1.0 for all serious adverse events suggests a statistically insignificant difference in the risk of serious adverse events between vaccine and control groups. These findings are consistent with the most recent review by the CDC and FDA (October 2010), which concluded that Gardasil is safe and effective for the prevention of the four types covered in the vaccine.2 CDC updates on safety do not yet include the bivalent vaccine because of its more recent release to the US market.
At every opportunity, encourage HPV vaccination for girls and women who are 9 to 26 years old.
New STD guidelines from the CDC include tips
on cervical cancer screening
Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
The CDC’s most recent sexually transmitted disease (STD) guidelines, released at the end of 2010, cover all sexually transmitted infections, including genital HPV infection. In general, the recommendations on cervical cancer screening are consistent with ACOG’s 2009 guidelines, which I discussed in the March 2010 Update on Cervical Disease. The CDC also offers concrete, useful suggestions on how to counsel patients who have genital warts or who test positive for an oncogenic strain of HPV. Although the guidelines are aimed at STD and public health clinics, they include many recommendations useful to all health care providers. For that reason, discussion of the highlights seems appropriate.
Like ACOG, CDC says screening should start at 21 years
Screening should begin when the patient is 21 years old and continue at 2-year intervals until she is 30 years old, at which time it should switch to every 3 years—provided she has had three consecutive normal Pap tests or one normal cotest (Pap and HPV test combined).
Because a woman may sometimes assume that she has undergone a Pap test by virtue of having had a pelvic examination, inaccuracies in self-reported screening intervals may arise. Therefore, it is imperative to devise a protocol for cervical cancer screening among women who do not have documentation, in their medical record, of a normal Pap test within the preceding 12 months. Although some women will undoubtedly undergo screening sooner than necessary, this approach will protect women lacking adequate documentation from being underscreened.
When to use the HPV test (and when to avoid it)
The guidelines confirm that the HPV test is an appropriate tool in the management of atypical squamous cells of undetermined significance (ASC–US) among women 21 years and older and as a cotest with the Pap for women who are 30 years and older.
The CDC recommends against the HPV test in the following situations:
- when deciding whether to vaccinate against HPV
- as part of a screen for STD
- in the triage of low-grade squamous intraepithelial lesion (LSIL) Pap results, although 2006 guidelines from the American Society for Colposcopy and Cervical Pathology and 2007 guidelines from ACOG recommend, as an option, the use of the HPV test in the triage of postmenopausal women who have LSIL
- in women younger than 21 years
- as a stand-alone primary cervical cancer screen (without the Pap test).
These recommendations are consistent with earlier conclusions.3
Perhaps the most important insights offered in the CDC’s 2010 STD guidelines are the counseling messages for women who undergo cotesting with both the HPV and Pap tests. It often is a challenge to communicate the indications for and findings of this screening approach. Here is guidance offered by the CDC:
- HPV is very common. It can infect the genital areas of both men and women. It usually has no signs or symptoms.
- Most sexually active persons get HPV at some time in their life, although few will ever know it. Even a person who has had only one lifetime sex partner can get HPV if the partner was infected.
- Although the immune system clears HPV infection most of the time, the infection fails to resolve in some people
- No clinically validated test exists for men to determine whether they have HPV infection. The most common manifestation of HPV infection in men is genital warts. High-risk HPV types seldom cause genital warts.
- Partners who are in a long-term relationship tend to share HPV. Sexual partners of HPV-infected people also likely have HPV, even though they may have no signs or symptoms of infection.
- Detection of high-risk HPV infection in a woman does not mean that she or her partner is engaging in sexual activity outside of a relationship. HPV infection can be present for many years before it is detected, and no method can accurately confirm when HPV infection was acquired.
The pap test is not a screening test for Std
Other findings that may be useful for all clinicians, as well as for those who practice in an STD clinic:
- The Pap test is not a screening test for STD
- All eligible women should undergo cervical cancer screening, regardless of sexual orientation (i.e., heterosexual, lesbian, or bisexual)
- Conventional cytology should be delayed if the patient is menstruating, and she should be advised to undergo a Pap test at the earliest opportunity
- If specific infections other than HPV are identified, the patient may need to undergo a repeat Pap test after appropriate treatment for those infections. However, in most instances, the Pap test will be reported as satisfactory for evaluation, and a reliable final report can be produced without the need to repeat the Pap test after treatment.
- The presence of a mucopurulent discharge should not delay the Pap test. The test can be performed after careful removal of the discharge with a saline-soaked cotton swab.
- When the Pap test is repeated because the previous test was interpreted as unsatisfactory, the patient should not be returned to regular screening intervals until the Pap test is reported as satisfactory and negative
- Cervical screening should not be accelerated for women who have genital warts and no other indication.
CDC recommendations on cervical cancer prevention and screening are consistent with those of other organizations, including ACOG. the counseling messages should be adopted universally.
When colposcopic biopsy is indicated, take more than one sample
Stoler MH, Vichnin MD, Ferenczy A, et al; the FUTURE I, II and III Investigators. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–1362.
The progress we have made in cervical cancer prevention is largely due to our ability to detect and treat precancer, particularly CIN 3, before it gains the capacity to invade. Until recently, few experts would have questioned the value of the partnership between cervical cytology screening and treatment of lesions detected on colposcopically directed biopsy.4 However, over the past decade, the accuracy of colposcopy for detection of high-grade lesions has been widely questioned, first by studies assessing static digitized cervigrams or colposcopy photo images, and more recently by studies comparing “real-time” colposcopy to histology obtained during colposcopy or excisional biopsy, or both.
The largest of these studies was conducted by Stoler and colleagues to compare the results of colposcopically directed biopsy and subsequent cervical excision among 737 women (16 to 45 years old) in the placebo arm of the quadrivalent HPV vaccine (FUTURE) randomized, controlled trials. In these trials, all women were referred for colposcopy according to a Pap triage algorithm, and one or more biopsies was taken from the area with the greatest apparent abnormality, as viewed by colposcopy. When excisional treatment was indicated, a biopsy of the worst-appearing area was taken again just before the excision.
Each patient’s most severe pathology-panel diagnosis for the excisional specimen was compared with:
- the most severe biopsy result from the preceding 6 months (excluding the biopsy taken on the same day as the excisional procedure) (Analysis 1)
- the biopsy taken on the same day as the definitive excisional procedure (Analysis 2).
When CIN 2 and CIN 3 are managed similarly, a discrepancy of one degree between colposcopically directed biopsy and the excisional specimen is considered sufficient agreement. Therefore, in this study, a difference of one degree in histologic diagnosis was considered agreement.
High-grade disease was more likely to be underestimated
on the same-day biopsy
Colposcopically directed biopsies obtained within 6 months before definitive treatment (Analysis 1) had lower overall agreement with the excisional specimen than biopsies collected on the same day as definitive treatment (Analysis 2). However, underestimation of high-grade disease was lower (26% overall underestimation of CIN 2 or 3 or adenocarcinoma in situ [AIS]) on earlier biopsy specimens than on those collected on the same day as definitive treatment (57% overall underestimation of CIN 2 or 3 or AIS).
Conversely, overestimation, or removal, of disease was higher (36%) in biopsies collected within 6 months before the excisional treatment, compared with biopsies collected on the same day as definitive treatment (5%).
The investigators suggested that any discrepancy in accuracy between the biopsy obtained at treatment and the biopsy obtained earlier might be the result of less diligent colposcopic evaluation and biopsy placement when the colposcopist knew that definitive therapy would immediately follow. Another possibility, they noted, is that lesions biopsied as early as 6 months before definitive treatment may have regressed in the process of tissue repair or were completely removed by the biopsy.
When all biopsies were compared with the final diagnosis of the excisional specimen, the colposcopically directed biopsy was less severe 42% to 66% of the time when the excisional histology was read as CIN 3 or AIS. However, when one degree of discrepancy was allowed, as it is in clinical practice, agreement was 92%. This suggests that women in the FUTURE trials, as well as those in real clinical practice, are typically managed appropriately under current protocols that combine cytology and colposcopy results to properly identify women who have cervical lesions that require surgical intervention.
Most CIN 3 lesions were small
Many of the CIN 3 lesions in this trial were small, as they were in the ASCUS LSIL Triage Study (ALTS), in which the median length of CIN 3 lesions was only 6.5 mm. Also in ALTS, lesions in one third of patients were so small that colposcopically directed biopsy did not leave any residual disease to be detected in the loop electrosurgical excision specimen.5 The size of a CIN 3 lesion that has associated invasion is, on average, seven times larger than without invasion.6 Although colposcopy is much less likely to miss large lesions, it is important to miss as little high-grade disease as possible because the risk of invasion is cumulative over time and unpredictable in a given patient.

Multiple biopsies boost detection
Sampling more than one area improves the accuracy of colposcopically directed biopsy, even when one area looks most abnormal. This colposcopy photo shows potential biopsy sites (within the ovals), although other choices may also be reasonable. Several studies have shown that colposcopically directed biopsy of even normal-appearing areas at the squamocolumnar junction or within large ectopies can improve detection of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.Studies have shown that it is possible to increase the accuracy of detection of CIN 2+ by increasing the number of biopsies. In this study by Stoler and colleagues, the sensitivity of initial colposcopy improved from 47% (for one biopsy) to 65% (two biopsies) and 77% (three or more) (FIGURE). Overall agreement increased with increasing age, which is consistent with the likelihood that CIN 3 lesions expand with age and become increasingly detectable by colposcopy.
Colposcopy does work, but the era of biopsying only the most abnormal-appearing area is over. Take more biopsies.
We want to hear from you! Tell us what you think.
1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533.
2. Centers for Disease Control and Prevention. Frequently asked questions about HPV vaccine safety. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html. Accessed February 1, 2011.
3. Cox JT, Moriarty AT, Castle PE. Commentary on: Statement on HPV DNA test utilization. Diagn Cytopathol. 2009;37(7):471-474.
4. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008;111(6):1266-1267.
5. Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of CIN 3 lesions in ALTS: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372-379.
6. Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol. 1992;99(7):583-586.
1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533.
2. Centers for Disease Control and Prevention. Frequently asked questions about HPV vaccine safety. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html. Accessed February 1, 2011.
3. Cox JT, Moriarty AT, Castle PE. Commentary on: Statement on HPV DNA test utilization. Diagn Cytopathol. 2009;37(7):471-474.
4. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008;111(6):1266-1267.
5. Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of CIN 3 lesions in ALTS: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372-379.
6. Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol. 1992;99(7):583-586.
Is the HPV test effective as the primary screen for cervical cancer?
Until now, the HPV test has been evaluated primarily as an adjunct to the Pap test and not as the primary screen for cervical cancer. In this randomized trial from Finland, 58,076 women 30 to 60 years old were invited to participate in a routine, population-based screening program for cervical cancer. Participants were randomized to primary screening with the HPV DNA test (hybrid capture 2) or to conventional cytology. In the group undergoing HPV testing, women who had a positive result were triaged to conventional cytology.
The HPV and conventional-cytology arms involved 95,600 and 95,700 woman-years of follow-up, respectively, and detected 76 and 53 cases of CIN 3 or higher. Six and eight cases, respectively, involved cancer.
The relative risk (RR) of CIN 3 or higher in the HPV arm versus conventional cytology was 1.44 (95% confidence interval [CI], 1.01–2.05) among all women invited for screening and 1.77 (95% CI, 1.16–2.74) among those who attended. Among women who had a normal or negative HPV test, the RR of subsequent CIN 3 or greater was 0.28 (95% CI, 0.04–1.17).
The greatest strengths of this study are the 1:1 randomization of just over 58,000 women and the ability to link study participants to outcomes, over a 5-year period, using the comprehensive Finnish population database and cancer registry.
One concern that clinicians may have is whether the findings are applicable to a US population that is now rarely screened using conventional cytology (liquid-based cytology is the norm). That concern should be allayed by a large meta-analysis that found no difference in the sensitivity of liquid-based cytology versus conventional Pap testing.1
Although nearly one third of women invited to participate in screening did not do so, the two groups had comparable numbers of women deciding not to participate (9,588 in the HPV arm versus 9,818 in the conventional-cytology arm).
One variable limiting applicability to a US population is the lack of an organized screening program like the one in Finland.
Despite its large size, the study had limited statistical power to show the impact of the two screening modalities on the rate of cervical cancer, primarily because that rate is so low in the population screened. To determine that impact, the screening options need to be repeated for another round, with follow-up extended to 10 years.
I recommend that you follow current US guidelines and screen women 30 years and older with both the Pap and HPV tests and extend the screening interval to 3 years for women who have a negative result on both tests. Numerous studies support the overwhelming conclusion that HPV testing in primary cervical cancer screening significantly increases detection of CIN 3 or higher and should reduce the woman’s subsequent risk of developing cervical cancer.—J. THOMAS COX, MD
Co-testing is the standard
US guidelines from the American Cancer Society (2002) and ACOG (2003, 2009) offer clinicians the option of screening women 30 years and older using both cytology and HPV testing—an approach known as “co-testing.” However, even though about 90% of the women who have a negative response to both tests can safely forgo further screening for at least 3 years, many clinicians screen them more frequently with co-testing, decreasing the cost-effectiveness of this option.2
The findings of Antilla and coworkers are in line with those of other authors. For example, Naucler and colleagues found that using the most sensitive test first (the HPV test), followed by reflex testing of positive HPV findings using the most specific test (the Pap), increased the sensitivity of screening for CIN 3 or greater by 30%, compared with screening with the Pap test alone.3 Other authors, including Ronco and coworkers and Sankaranarayanan and colleagues, have pointed to the superiority of either co-testing or HPV testing to use of the Pap test alone.4,5
1. Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers Ag, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111(1):167-177.
2. Saraiya M, Berkotwitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med. 2010;170(11):977-985.
3. Naucler P, Ryd W, Törnberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101(2):88-99.
4. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249-257.
5. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385-1394.
Until now, the HPV test has been evaluated primarily as an adjunct to the Pap test and not as the primary screen for cervical cancer. In this randomized trial from Finland, 58,076 women 30 to 60 years old were invited to participate in a routine, population-based screening program for cervical cancer. Participants were randomized to primary screening with the HPV DNA test (hybrid capture 2) or to conventional cytology. In the group undergoing HPV testing, women who had a positive result were triaged to conventional cytology.
The HPV and conventional-cytology arms involved 95,600 and 95,700 woman-years of follow-up, respectively, and detected 76 and 53 cases of CIN 3 or higher. Six and eight cases, respectively, involved cancer.
The relative risk (RR) of CIN 3 or higher in the HPV arm versus conventional cytology was 1.44 (95% confidence interval [CI], 1.01–2.05) among all women invited for screening and 1.77 (95% CI, 1.16–2.74) among those who attended. Among women who had a normal or negative HPV test, the RR of subsequent CIN 3 or greater was 0.28 (95% CI, 0.04–1.17).
The greatest strengths of this study are the 1:1 randomization of just over 58,000 women and the ability to link study participants to outcomes, over a 5-year period, using the comprehensive Finnish population database and cancer registry.
One concern that clinicians may have is whether the findings are applicable to a US population that is now rarely screened using conventional cytology (liquid-based cytology is the norm). That concern should be allayed by a large meta-analysis that found no difference in the sensitivity of liquid-based cytology versus conventional Pap testing.1
Although nearly one third of women invited to participate in screening did not do so, the two groups had comparable numbers of women deciding not to participate (9,588 in the HPV arm versus 9,818 in the conventional-cytology arm).
One variable limiting applicability to a US population is the lack of an organized screening program like the one in Finland.
Despite its large size, the study had limited statistical power to show the impact of the two screening modalities on the rate of cervical cancer, primarily because that rate is so low in the population screened. To determine that impact, the screening options need to be repeated for another round, with follow-up extended to 10 years.
I recommend that you follow current US guidelines and screen women 30 years and older with both the Pap and HPV tests and extend the screening interval to 3 years for women who have a negative result on both tests. Numerous studies support the overwhelming conclusion that HPV testing in primary cervical cancer screening significantly increases detection of CIN 3 or higher and should reduce the woman’s subsequent risk of developing cervical cancer.—J. THOMAS COX, MD
Co-testing is the standard
US guidelines from the American Cancer Society (2002) and ACOG (2003, 2009) offer clinicians the option of screening women 30 years and older using both cytology and HPV testing—an approach known as “co-testing.” However, even though about 90% of the women who have a negative response to both tests can safely forgo further screening for at least 3 years, many clinicians screen them more frequently with co-testing, decreasing the cost-effectiveness of this option.2
The findings of Antilla and coworkers are in line with those of other authors. For example, Naucler and colleagues found that using the most sensitive test first (the HPV test), followed by reflex testing of positive HPV findings using the most specific test (the Pap), increased the sensitivity of screening for CIN 3 or greater by 30%, compared with screening with the Pap test alone.3 Other authors, including Ronco and coworkers and Sankaranarayanan and colleagues, have pointed to the superiority of either co-testing or HPV testing to use of the Pap test alone.4,5
Until now, the HPV test has been evaluated primarily as an adjunct to the Pap test and not as the primary screen for cervical cancer. In this randomized trial from Finland, 58,076 women 30 to 60 years old were invited to participate in a routine, population-based screening program for cervical cancer. Participants were randomized to primary screening with the HPV DNA test (hybrid capture 2) or to conventional cytology. In the group undergoing HPV testing, women who had a positive result were triaged to conventional cytology.
The HPV and conventional-cytology arms involved 95,600 and 95,700 woman-years of follow-up, respectively, and detected 76 and 53 cases of CIN 3 or higher. Six and eight cases, respectively, involved cancer.
The relative risk (RR) of CIN 3 or higher in the HPV arm versus conventional cytology was 1.44 (95% confidence interval [CI], 1.01–2.05) among all women invited for screening and 1.77 (95% CI, 1.16–2.74) among those who attended. Among women who had a normal or negative HPV test, the RR of subsequent CIN 3 or greater was 0.28 (95% CI, 0.04–1.17).
The greatest strengths of this study are the 1:1 randomization of just over 58,000 women and the ability to link study participants to outcomes, over a 5-year period, using the comprehensive Finnish population database and cancer registry.
One concern that clinicians may have is whether the findings are applicable to a US population that is now rarely screened using conventional cytology (liquid-based cytology is the norm). That concern should be allayed by a large meta-analysis that found no difference in the sensitivity of liquid-based cytology versus conventional Pap testing.1
Although nearly one third of women invited to participate in screening did not do so, the two groups had comparable numbers of women deciding not to participate (9,588 in the HPV arm versus 9,818 in the conventional-cytology arm).
One variable limiting applicability to a US population is the lack of an organized screening program like the one in Finland.
Despite its large size, the study had limited statistical power to show the impact of the two screening modalities on the rate of cervical cancer, primarily because that rate is so low in the population screened. To determine that impact, the screening options need to be repeated for another round, with follow-up extended to 10 years.
I recommend that you follow current US guidelines and screen women 30 years and older with both the Pap and HPV tests and extend the screening interval to 3 years for women who have a negative result on both tests. Numerous studies support the overwhelming conclusion that HPV testing in primary cervical cancer screening significantly increases detection of CIN 3 or higher and should reduce the woman’s subsequent risk of developing cervical cancer.—J. THOMAS COX, MD
Co-testing is the standard
US guidelines from the American Cancer Society (2002) and ACOG (2003, 2009) offer clinicians the option of screening women 30 years and older using both cytology and HPV testing—an approach known as “co-testing.” However, even though about 90% of the women who have a negative response to both tests can safely forgo further screening for at least 3 years, many clinicians screen them more frequently with co-testing, decreasing the cost-effectiveness of this option.2
The findings of Antilla and coworkers are in line with those of other authors. For example, Naucler and colleagues found that using the most sensitive test first (the HPV test), followed by reflex testing of positive HPV findings using the most specific test (the Pap), increased the sensitivity of screening for CIN 3 or greater by 30%, compared with screening with the Pap test alone.3 Other authors, including Ronco and coworkers and Sankaranarayanan and colleagues, have pointed to the superiority of either co-testing or HPV testing to use of the Pap test alone.4,5
1. Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers Ag, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111(1):167-177.
2. Saraiya M, Berkotwitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med. 2010;170(11):977-985.
3. Naucler P, Ryd W, Törnberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101(2):88-99.
4. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249-257.
5. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385-1394.
1. Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers Ag, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111(1):167-177.
2. Saraiya M, Berkotwitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med. 2010;170(11):977-985.
3. Naucler P, Ryd W, Törnberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101(2):88-99.
4. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249-257.
5. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385-1394.
UPDATE: CERVICAL DISEASE
“Astonishment fatigue.” That phenomenon may be responsible for clinicians’ muted reaction to new ACOG guidelines on cervical cancer screening, which were released late last year. 1 Through a coincidence of timing, the new guidelines hit the airwaves just after the U. S. Preventive Services Task Force announced controversial changes to its recommendations on mammography. As a result, the cervical cytology guidelines seemed to dissolve into the stratosphere.
Or, perhaps, the cervical cancer screening guidelines slipped by with little fanfare because they were soundly based in evidence and, therefore, widely accepted among ObGyns. Even if that is the case, the medical community may not be familiar with the specific data behind the guideline changes. In this article, I discuss the evidence driving all major changes to the guidelines based on Level-A evidence. Changes based on Level-B or -C evidence are listed in TABLE 1 .
Hold off on cervical cancer screening until the patient is 21 years old
Screening before age 21 should be avoided because it may lead to unnecessary and harmful evaluation and treatment in women at very low risk of cancer.1
How different is this from the 2003 ACOG recommendation to begin screening within 3 years of first intercourse or at age 21, whichever comes first?
Very, very different. In fact, it is the most dramatic change in the 2009 screening recommendations.
It is even more striking in comparison with ACOG’s earlier recommendation—which prevailed from the late 1970s through 2002—to begin cervical screening at age 18 or at the onset of intercourse, whichever comes first.
The median age of first intercourse in the United States is 16 years. Until this latest change in guidelines, most young women began cervical screening during adolescence.
What’s wrong with screening adolescents? Don’t they acquire human papillomavirus (HPV)? (Yes.) And once they do, aren’t they at risk of cervical cancer? (Yes.)
Several variables support the delay of screening to age 21:
- the transience of most HPV infections
- the typically long natural history of carcinogenesis in the few young women in whom HPV might persist
- the adverse consequences of over-screening and over-management of adolescents who have cervical intraepithelial neoplasia (CIN).
Let’s look more closely at these variables.
TABLE 1
Other ACOG cervical disease guidelines are based on Level-B and Level-C evidence*
| Recommendation | Level of evidence | Comment |
|---|---|---|
| Test sexually active adolescents (i.e., females 21 years or younger) for sexually transmitted infection, and counsel them about safe sexual practices and contraception | B | These measures can be carried out without cervical cytology and, in the asymptomatic patient, without the introduction of a speculum |
| It is reasonable to discontinue cervical cancer screening in any woman 65 to 70 years old who has had three or more consecutive negative Pap tests and no abnormal tests in the past 10 years | B | |
| Continue annual screening for at least 20 years in any woman who has been treated for CIN 2, CIN 3, or cancer. This population remains at risk of persistent or recurrent disease for at least 20 years after treatment and after initial posttreatment surveillance | B | |
| Continue to screen any woman who has had a total hysterectomy if she has a history of CIN 2 or CIN 3 or if a negative history cannot be documented. This screening should continue even after initial post-treatment surveillance | B | Although the screening interval may ultimately be extended, we lack reliable data to support or refute the discontinuation of screening in this population |
| Inform the patient that annual gynecologic examination may still be appropriate even if cervical cytology is not assessed at each visit | C | |
| Screen any woman who has been immunized against HPV 16 and 18 as though she has not been immunized | C | |
| * Level-B recommendations are based on limited and inconsistent scientific evidence. Level-C recommendations are based primarily on consensus and expert opinion. | ||
HPV is common but usually resolves on its own
It’s common for young women to acquire HPV shortly after they become sexually active, but their immune system clears most infections within 1 or 2 years without the virus producing neoplastic changes.1
HPV detection peaks in the late teens and early 20s, when approximately 25% of women test positive for the virus, resulting in high rates of low-grade squamous intraepithelial lesions (LSIL) and HPV-positive, atypical squamous cells of undetermined significance (ASC-US).2 These findings are mostly transient.2
Detection of CIN 3 does not peak until a woman reaches her late 20s, and the median detection of microinvasive cancer does not peak until she reaches her early 40s. These facts indicate that adolescents have the lowest risk of incipient cervical cancer but the highest risk of undergoing unnecessary procedures for HPV-related events—events that are highly likely to resolve without treatment.
From 1998 to 2006, an average of 14 cervical cancers occurred annually in women 15 to 19 years old, an incidence of only 1 or 2 cases of cervical cancer for every 1 million women in that age group ( TABLE 2 ).
TABLE 2
Incidence of invasive cervical carcinoma: United States, 1998-2003
| Age (y) | Average annual count | Incidence (95% CI) | Incidence as a percentage | Median age at diagnosis |
|---|---|---|---|---|
| All ages | 10,846 | 8.9 (8.8–9.0) | 100 | 47 |
| 0–14 | 0 | 0 | 0 | Not applicable (NA) |
| 15–19 | 14 | 0.2 (0.1–0.2) | 0.1 | NA |
| 20–24 | 123 | 1.6 (1.5–1.7) | 1.1 | NA |
| 25–29 | 543 | 6.9 (6.7–7.2) | 5.0 | NA |
| 30–34 | 1,045 | 12.3 (12.0–12.6) | 9.6 | NA |
| 35–39 | 1,350 | 14.6 (14.3–14.9) | 12.5 | NA |
| 40–44 | 1,534 | 16.3 (15.9–16.6) | 14.1 | NA |
| 45–49 | 1,323 | 15.4 (15.0–15.7) | 12.2 | NA |
| 50–59 | 1,958 | 14.5 (14.2–14.7) | 18.0 | NA |
| 60–69 | 1,352 | 14.8 (14.5–15.1) | 12.5 | NA |
| 70–79 | 1,008 | 12.9 (12.6–13.3) | 9.3 | NA |
| ≥80 | 595 | 11.2 (10.9–11.6) | 5.5 | NA |
| Source: Watson et al5 | ||||
In teens, screening does not reduce mortality
Even this low rate of cervical cancer might justify the screening of adolescents, provided such screening was shown to reduce the incidence of and mortality from cervical cancer in that age group. However, all data point to the opposite conclusion:
- The incidence of cervical cancer in this age group has not changed since the years between 1973 and 1977, a period that preceded the recommendation to begin screening at age 18 or first intercourse
- No data demonstrate a benefit of screening in women younger than 21 years in regard to future rates of CIN 2 and 3—or even that screening women 20 to 24 years old reduces the rate of cervical cancer in women 30 years or younger3
- CIN 2 and 3 do occur in adolescents, and the fear of delaying their diagnosis has driven much of the opposition to the guideline change—specifically, the omission of the option to begin screening within 3 years after first intercourse; however, even when high-grade CIN develops, spontaneous regression is common in this age group (e.g., 65% rate of regression of CIN 2 after 18 months; 75% after 36 months)
- When CIN 3 develops and persists, more than 10 years are typically required for the lesion to acquire the capacity to become invasive.1,2
In addition, extensive data suggest that screening adolescents may be harmful. Adverse psychological effects related to cervical cancer screening, evaluation of abnormal results, and treatment of CIN have been reported, including negative effects on sexual function and a higher risk of preterm and low-birth-weight infants.1
Virtually all studies of pregnancy outcomes following loop electrosurgical excision procedure (LEEP) have demonstrated a doubling or tripling of the rate of preterm birth.
Screening women 21 years or younger for cervical cancer may be harmful and lacks proven benefit. Screening should not begin until the patient is 21, regardless of the age of first intercourse.
Extend the screening interval to 2 years for women 21 to 29 years old
Both liquid-based and conventional methods of cervical cytology are acceptable for screening; hence, screening frequency should not vary based on the method used.1
The 2003 ACOG guidelines recommended annual cervical screening of women in their 20s using either conventional or liquid-based cytology. In contrast, in 2002, the American Cancer Society (ACS) recommended annual screening when the conventional Pap test was used, and a 2-year interval when screening involved liquid-based cytology. With ACOG’s latest recommendation—a 2-year interval for women 21 to 29 years old, regardless of test method—the College moves in line with the ACS in regard to liquid-based cytology. It also acknowledges more recent evidence that liquid-based cytology is no more sensitive than conventional cytology.1
Liquid-based cytology does have a number of other unquestionable advantages, however:
- It offers the convenience of being able to test for HPV, Neisseria gonorrhoeae, and Chlamydia trachomatis directly from the residual sample
- It produces fewer unsatisfactory cytology results than conventional cytology
- Cytotechnologists find liquid-based cytology easier to read.
More than 90% of Pap tests in the United States utilize liquid-based cytology, and that percentage is not likely to diminish.
Women 21 to 29 years old should have a Pap test every 2 years, regardless of the method used.
Some women 30 years and older can be screened every 3 years
Cervical cytology screening is recommended every 3 years for women age 30 years and older if:
- they have had three consecutive negative cervical cytology screening test results and have no history of CIN 2 or CIN 3, are not HIV-infected, are not immunocompromised, and were not exposed to diethylstilbestrol in utero or
- they have received negative test cotest results on both cervical cytology screening and HPV DNA testing and are considered low risk.1
A study of the detection of squamous cell cervical cancer (SCC) within 3.5 years of one, two, or three consecutive normal Pap tests demonstrated that the incidence of cervical cancer increases to 3 to 5 cases for every 100,000 woman-years in each of the subsequent 2 years. Some experts argue that this relatively low increase—the equivalent of the incidence of breast cancer in men—supports extension of the screening interval to 3 years after three consecutive normal Pap results.
Clinicians have generally been hesitant to widen the screening interval, despite ACS and ACOG recommendations for 2- or 3-year screening among women who have had three consecutive normal results. Many of these clinicians may find it difficult to dismiss even this low number of excess cancers (3 to 5 cases for every 100,000 woman-years) when more frequent or better screening would likely prevent them. As a result, the extension of screening intervals on the basis of negative cytology alone may continue to meet resistance from clinicians and their patients.
Wider intervals reduce the risk of unnecessary treatment
The extension of screening intervals, whether it is based on cytology alone or cytology combined with HPV testing, benefits most women by reducing the likelihood that transient, HPV-induced events will be detected and treated even though they are not destined to become CIN 3, adenocarcinoma in situ, or cervical cancer.
At the same time, however, extending the screening interval to 3 years in the setting of “opportunistic” screening—the screening approach used in the United States—may lead to irregular screening for many women at intervals beyond the recommended 3 years, thereby reducing the protective effect of a program based on cytology alone. Approximately 10% of cervical cancers occur in women who have not had a Pap test in the preceding 5 years.
Cotesting may be the solution
There is no question that extending cytology-only screening beyond 3 years significantly increases the risk of cervical cancer. However, among women tested for HPV, the risk of CIN 3 or greater does not begin to rise until at least 6 years following a negative test result, providing a margin of safety that would protect most women who miss the recommended 3-year screening interval.
Earlier this year, Ronco and colleagues published the results of a large primary cervical screening trial involving more than 94,000 women who were randomly assigned to screening with cytology alone or cotesting (i.e., cytology plus HPV testing).4 In the cytology-only group, women were referred to colposcopy for a Pap result of ASC-US or higher-grade findings. In the cotesting group, they were referred to colposcopy if the HPV or Pap test (or both) was positive. A second screening was performed an average of 3 years later, and the incidences of CIN 2, CIN 3, and cancer at each screening were compared between groups.
The number of cancers detected in the initial round of screening did not differ between groups. In the second round of screening, no cancers were found in the cotesting group, compared with nine cancers in the cytology-only group. The authors attributed this difference to the detection and treatment of twice as many cases of CIN 3 in the initial round of screening among women undergoing cotesting, compared with those tested with cytology alone.4
In addition, women in the cotesting group had an extremely low rate of CIN 3 in the second round of screening (2 cases for every 10,000 women). Investigators also noted that a high proportion of invasive cancers detected in the cytology group during the second round of screening were adenocarcinomas, consistent with reports from earlier studies that found cytology to be less effective in detecting adenocarcinomas than in detecting SCC.4
Although HPV testing was previously shown to outperform cytology in reducing the risk of cervical cancer in a low-resource country (India), this is the first study to do so in a developed country with a well-screened population and a low incidence of cervical cancer.4
Although ACOG guidelines encourage the extension of screening intervals to 3 years for women 30 years or older who have had three consecutive normal Pap tests, many clinicians have been reluctant to take this step. Cotesting with HPV and Pap tests should provide the reassurance necessary for these clinicians to adopt the wider screening intervals.
Keep annual screening for women who have a history of CIN, HIV, or certain chronic conditions
The recommendations to screen women every 2 years until age 30 and to extend the screening interval to 3 years thereafter, provided three consecutive Pap tests are normal or cotesting is negative, apply only to women at average risk of cervical cancer. Conditions that indicate elevated risk include:
- HIV infection
- immunosuppression for other reasons, e.g., organ transplant
- in utero exposure to diethylstilbestrol
- history of CIN 2, CIN 3, or cancer.
Two Pap tests are recommended in the first year after diagnosis of HIV infection, followed by annual screening. It can be presumed that women who have chronic immunosuppression should be managed similarly.
As for women known to have been exposed to diethylstilbestrol in utero, no specific recommendation is given other than “more frequent screening.”1
The relatively recent documentation that women with a history of CIN 2 or 3 (and probably adenocarcinoma in situ) remain at risk of developing cervical cancer for at least 20 years after treatment warrants annual screening for at least 20 years. The increased reassurance that no CIN 3 or greater is missed when cotesting is negative for both cytology and HPV testing might argue for extension of the screening interval for women who have negative cotest results and who have completed recommended posttreatment follow-up. However, at this time, we lack data on long-term follow-up of women who have been treated for cervical neoplasia and who have negative cotest results. Therefore, such a recommendation cannot be made at this time.
Do not increase the screening interval beyond annual testing for women who are HIV-positive, who are immunosuppressed, who were exposed in utero to diethylstilbestrol, or who have been treated for CIN 2 or 3 or adenocarcinoma in situ.
Routine cytology testing should be discontinued after total hysterectomy for benign indications, provided the woman has no history of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.1 This recommendation has not changed since the 2003 ACOG guidelines on cervical cancer screening were published, and it is consistent with guidelines from the U.S. Preventive Services Task Force and the American Cancer Society.
1. Cervical cytology screening. ACOG Practice Bulletin #109. Obstet Gynecol. 2009;114(6):1409-1420.
2. Moscicki AB, Cox JT. Practice Improvement in Cervical Screening and Management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Gen Tract Dis. 2010;14(1):73-80.
3. Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population-based case-control study of prospectively recorded data. BMJ. 2009;339:b2968.-
4. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. http://www.thelancet.com/oncology. Published online January 19, 2010. DOI:10.1016/S1470–2045(09)70360-2.
5. Watson M, Saraiya M, Bernard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(suppl 10):2855-2864.
6. Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353(20):2101-2104.
“Astonishment fatigue.” That phenomenon may be responsible for clinicians’ muted reaction to new ACOG guidelines on cervical cancer screening, which were released late last year. 1 Through a coincidence of timing, the new guidelines hit the airwaves just after the U. S. Preventive Services Task Force announced controversial changes to its recommendations on mammography. As a result, the cervical cytology guidelines seemed to dissolve into the stratosphere.
Or, perhaps, the cervical cancer screening guidelines slipped by with little fanfare because they were soundly based in evidence and, therefore, widely accepted among ObGyns. Even if that is the case, the medical community may not be familiar with the specific data behind the guideline changes. In this article, I discuss the evidence driving all major changes to the guidelines based on Level-A evidence. Changes based on Level-B or -C evidence are listed in TABLE 1 .
Hold off on cervical cancer screening until the patient is 21 years old
Screening before age 21 should be avoided because it may lead to unnecessary and harmful evaluation and treatment in women at very low risk of cancer.1
How different is this from the 2003 ACOG recommendation to begin screening within 3 years of first intercourse or at age 21, whichever comes first?
Very, very different. In fact, it is the most dramatic change in the 2009 screening recommendations.
It is even more striking in comparison with ACOG’s earlier recommendation—which prevailed from the late 1970s through 2002—to begin cervical screening at age 18 or at the onset of intercourse, whichever comes first.
The median age of first intercourse in the United States is 16 years. Until this latest change in guidelines, most young women began cervical screening during adolescence.
What’s wrong with screening adolescents? Don’t they acquire human papillomavirus (HPV)? (Yes.) And once they do, aren’t they at risk of cervical cancer? (Yes.)
Several variables support the delay of screening to age 21:
- the transience of most HPV infections
- the typically long natural history of carcinogenesis in the few young women in whom HPV might persist
- the adverse consequences of over-screening and over-management of adolescents who have cervical intraepithelial neoplasia (CIN).
Let’s look more closely at these variables.
TABLE 1
Other ACOG cervical disease guidelines are based on Level-B and Level-C evidence*
| Recommendation | Level of evidence | Comment |
|---|---|---|
| Test sexually active adolescents (i.e., females 21 years or younger) for sexually transmitted infection, and counsel them about safe sexual practices and contraception | B | These measures can be carried out without cervical cytology and, in the asymptomatic patient, without the introduction of a speculum |
| It is reasonable to discontinue cervical cancer screening in any woman 65 to 70 years old who has had three or more consecutive negative Pap tests and no abnormal tests in the past 10 years | B | |
| Continue annual screening for at least 20 years in any woman who has been treated for CIN 2, CIN 3, or cancer. This population remains at risk of persistent or recurrent disease for at least 20 years after treatment and after initial posttreatment surveillance | B | |
| Continue to screen any woman who has had a total hysterectomy if she has a history of CIN 2 or CIN 3 or if a negative history cannot be documented. This screening should continue even after initial post-treatment surveillance | B | Although the screening interval may ultimately be extended, we lack reliable data to support or refute the discontinuation of screening in this population |
| Inform the patient that annual gynecologic examination may still be appropriate even if cervical cytology is not assessed at each visit | C | |
| Screen any woman who has been immunized against HPV 16 and 18 as though she has not been immunized | C | |
| * Level-B recommendations are based on limited and inconsistent scientific evidence. Level-C recommendations are based primarily on consensus and expert opinion. | ||
HPV is common but usually resolves on its own
It’s common for young women to acquire HPV shortly after they become sexually active, but their immune system clears most infections within 1 or 2 years without the virus producing neoplastic changes.1
HPV detection peaks in the late teens and early 20s, when approximately 25% of women test positive for the virus, resulting in high rates of low-grade squamous intraepithelial lesions (LSIL) and HPV-positive, atypical squamous cells of undetermined significance (ASC-US).2 These findings are mostly transient.2
Detection of CIN 3 does not peak until a woman reaches her late 20s, and the median detection of microinvasive cancer does not peak until she reaches her early 40s. These facts indicate that adolescents have the lowest risk of incipient cervical cancer but the highest risk of undergoing unnecessary procedures for HPV-related events—events that are highly likely to resolve without treatment.
From 1998 to 2006, an average of 14 cervical cancers occurred annually in women 15 to 19 years old, an incidence of only 1 or 2 cases of cervical cancer for every 1 million women in that age group ( TABLE 2 ).
TABLE 2
Incidence of invasive cervical carcinoma: United States, 1998-2003
| Age (y) | Average annual count | Incidence (95% CI) | Incidence as a percentage | Median age at diagnosis |
|---|---|---|---|---|
| All ages | 10,846 | 8.9 (8.8–9.0) | 100 | 47 |
| 0–14 | 0 | 0 | 0 | Not applicable (NA) |
| 15–19 | 14 | 0.2 (0.1–0.2) | 0.1 | NA |
| 20–24 | 123 | 1.6 (1.5–1.7) | 1.1 | NA |
| 25–29 | 543 | 6.9 (6.7–7.2) | 5.0 | NA |
| 30–34 | 1,045 | 12.3 (12.0–12.6) | 9.6 | NA |
| 35–39 | 1,350 | 14.6 (14.3–14.9) | 12.5 | NA |
| 40–44 | 1,534 | 16.3 (15.9–16.6) | 14.1 | NA |
| 45–49 | 1,323 | 15.4 (15.0–15.7) | 12.2 | NA |
| 50–59 | 1,958 | 14.5 (14.2–14.7) | 18.0 | NA |
| 60–69 | 1,352 | 14.8 (14.5–15.1) | 12.5 | NA |
| 70–79 | 1,008 | 12.9 (12.6–13.3) | 9.3 | NA |
| ≥80 | 595 | 11.2 (10.9–11.6) | 5.5 | NA |
| Source: Watson et al5 | ||||
In teens, screening does not reduce mortality
Even this low rate of cervical cancer might justify the screening of adolescents, provided such screening was shown to reduce the incidence of and mortality from cervical cancer in that age group. However, all data point to the opposite conclusion:
- The incidence of cervical cancer in this age group has not changed since the years between 1973 and 1977, a period that preceded the recommendation to begin screening at age 18 or first intercourse
- No data demonstrate a benefit of screening in women younger than 21 years in regard to future rates of CIN 2 and 3—or even that screening women 20 to 24 years old reduces the rate of cervical cancer in women 30 years or younger3
- CIN 2 and 3 do occur in adolescents, and the fear of delaying their diagnosis has driven much of the opposition to the guideline change—specifically, the omission of the option to begin screening within 3 years after first intercourse; however, even when high-grade CIN develops, spontaneous regression is common in this age group (e.g., 65% rate of regression of CIN 2 after 18 months; 75% after 36 months)
- When CIN 3 develops and persists, more than 10 years are typically required for the lesion to acquire the capacity to become invasive.1,2
In addition, extensive data suggest that screening adolescents may be harmful. Adverse psychological effects related to cervical cancer screening, evaluation of abnormal results, and treatment of CIN have been reported, including negative effects on sexual function and a higher risk of preterm and low-birth-weight infants.1
Virtually all studies of pregnancy outcomes following loop electrosurgical excision procedure (LEEP) have demonstrated a doubling or tripling of the rate of preterm birth.
Screening women 21 years or younger for cervical cancer may be harmful and lacks proven benefit. Screening should not begin until the patient is 21, regardless of the age of first intercourse.
Extend the screening interval to 2 years for women 21 to 29 years old
Both liquid-based and conventional methods of cervical cytology are acceptable for screening; hence, screening frequency should not vary based on the method used.1
The 2003 ACOG guidelines recommended annual cervical screening of women in their 20s using either conventional or liquid-based cytology. In contrast, in 2002, the American Cancer Society (ACS) recommended annual screening when the conventional Pap test was used, and a 2-year interval when screening involved liquid-based cytology. With ACOG’s latest recommendation—a 2-year interval for women 21 to 29 years old, regardless of test method—the College moves in line with the ACS in regard to liquid-based cytology. It also acknowledges more recent evidence that liquid-based cytology is no more sensitive than conventional cytology.1
Liquid-based cytology does have a number of other unquestionable advantages, however:
- It offers the convenience of being able to test for HPV, Neisseria gonorrhoeae, and Chlamydia trachomatis directly from the residual sample
- It produces fewer unsatisfactory cytology results than conventional cytology
- Cytotechnologists find liquid-based cytology easier to read.
More than 90% of Pap tests in the United States utilize liquid-based cytology, and that percentage is not likely to diminish.
Women 21 to 29 years old should have a Pap test every 2 years, regardless of the method used.
Some women 30 years and older can be screened every 3 years
Cervical cytology screening is recommended every 3 years for women age 30 years and older if:
- they have had three consecutive negative cervical cytology screening test results and have no history of CIN 2 or CIN 3, are not HIV-infected, are not immunocompromised, and were not exposed to diethylstilbestrol in utero or
- they have received negative test cotest results on both cervical cytology screening and HPV DNA testing and are considered low risk.1
A study of the detection of squamous cell cervical cancer (SCC) within 3.5 years of one, two, or three consecutive normal Pap tests demonstrated that the incidence of cervical cancer increases to 3 to 5 cases for every 100,000 woman-years in each of the subsequent 2 years. Some experts argue that this relatively low increase—the equivalent of the incidence of breast cancer in men—supports extension of the screening interval to 3 years after three consecutive normal Pap results.
Clinicians have generally been hesitant to widen the screening interval, despite ACS and ACOG recommendations for 2- or 3-year screening among women who have had three consecutive normal results. Many of these clinicians may find it difficult to dismiss even this low number of excess cancers (3 to 5 cases for every 100,000 woman-years) when more frequent or better screening would likely prevent them. As a result, the extension of screening intervals on the basis of negative cytology alone may continue to meet resistance from clinicians and their patients.
Wider intervals reduce the risk of unnecessary treatment
The extension of screening intervals, whether it is based on cytology alone or cytology combined with HPV testing, benefits most women by reducing the likelihood that transient, HPV-induced events will be detected and treated even though they are not destined to become CIN 3, adenocarcinoma in situ, or cervical cancer.
At the same time, however, extending the screening interval to 3 years in the setting of “opportunistic” screening—the screening approach used in the United States—may lead to irregular screening for many women at intervals beyond the recommended 3 years, thereby reducing the protective effect of a program based on cytology alone. Approximately 10% of cervical cancers occur in women who have not had a Pap test in the preceding 5 years.
Cotesting may be the solution
There is no question that extending cytology-only screening beyond 3 years significantly increases the risk of cervical cancer. However, among women tested for HPV, the risk of CIN 3 or greater does not begin to rise until at least 6 years following a negative test result, providing a margin of safety that would protect most women who miss the recommended 3-year screening interval.
Earlier this year, Ronco and colleagues published the results of a large primary cervical screening trial involving more than 94,000 women who were randomly assigned to screening with cytology alone or cotesting (i.e., cytology plus HPV testing).4 In the cytology-only group, women were referred to colposcopy for a Pap result of ASC-US or higher-grade findings. In the cotesting group, they were referred to colposcopy if the HPV or Pap test (or both) was positive. A second screening was performed an average of 3 years later, and the incidences of CIN 2, CIN 3, and cancer at each screening were compared between groups.
The number of cancers detected in the initial round of screening did not differ between groups. In the second round of screening, no cancers were found in the cotesting group, compared with nine cancers in the cytology-only group. The authors attributed this difference to the detection and treatment of twice as many cases of CIN 3 in the initial round of screening among women undergoing cotesting, compared with those tested with cytology alone.4
In addition, women in the cotesting group had an extremely low rate of CIN 3 in the second round of screening (2 cases for every 10,000 women). Investigators also noted that a high proportion of invasive cancers detected in the cytology group during the second round of screening were adenocarcinomas, consistent with reports from earlier studies that found cytology to be less effective in detecting adenocarcinomas than in detecting SCC.4
Although HPV testing was previously shown to outperform cytology in reducing the risk of cervical cancer in a low-resource country (India), this is the first study to do so in a developed country with a well-screened population and a low incidence of cervical cancer.4
Although ACOG guidelines encourage the extension of screening intervals to 3 years for women 30 years or older who have had three consecutive normal Pap tests, many clinicians have been reluctant to take this step. Cotesting with HPV and Pap tests should provide the reassurance necessary for these clinicians to adopt the wider screening intervals.
Keep annual screening for women who have a history of CIN, HIV, or certain chronic conditions
The recommendations to screen women every 2 years until age 30 and to extend the screening interval to 3 years thereafter, provided three consecutive Pap tests are normal or cotesting is negative, apply only to women at average risk of cervical cancer. Conditions that indicate elevated risk include:
- HIV infection
- immunosuppression for other reasons, e.g., organ transplant
- in utero exposure to diethylstilbestrol
- history of CIN 2, CIN 3, or cancer.
Two Pap tests are recommended in the first year after diagnosis of HIV infection, followed by annual screening. It can be presumed that women who have chronic immunosuppression should be managed similarly.
As for women known to have been exposed to diethylstilbestrol in utero, no specific recommendation is given other than “more frequent screening.”1
The relatively recent documentation that women with a history of CIN 2 or 3 (and probably adenocarcinoma in situ) remain at risk of developing cervical cancer for at least 20 years after treatment warrants annual screening for at least 20 years. The increased reassurance that no CIN 3 or greater is missed when cotesting is negative for both cytology and HPV testing might argue for extension of the screening interval for women who have negative cotest results and who have completed recommended posttreatment follow-up. However, at this time, we lack data on long-term follow-up of women who have been treated for cervical neoplasia and who have negative cotest results. Therefore, such a recommendation cannot be made at this time.
Do not increase the screening interval beyond annual testing for women who are HIV-positive, who are immunosuppressed, who were exposed in utero to diethylstilbestrol, or who have been treated for CIN 2 or 3 or adenocarcinoma in situ.
Routine cytology testing should be discontinued after total hysterectomy for benign indications, provided the woman has no history of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.1 This recommendation has not changed since the 2003 ACOG guidelines on cervical cancer screening were published, and it is consistent with guidelines from the U.S. Preventive Services Task Force and the American Cancer Society.
“Astonishment fatigue.” That phenomenon may be responsible for clinicians’ muted reaction to new ACOG guidelines on cervical cancer screening, which were released late last year. 1 Through a coincidence of timing, the new guidelines hit the airwaves just after the U. S. Preventive Services Task Force announced controversial changes to its recommendations on mammography. As a result, the cervical cytology guidelines seemed to dissolve into the stratosphere.
Or, perhaps, the cervical cancer screening guidelines slipped by with little fanfare because they were soundly based in evidence and, therefore, widely accepted among ObGyns. Even if that is the case, the medical community may not be familiar with the specific data behind the guideline changes. In this article, I discuss the evidence driving all major changes to the guidelines based on Level-A evidence. Changes based on Level-B or -C evidence are listed in TABLE 1 .
Hold off on cervical cancer screening until the patient is 21 years old
Screening before age 21 should be avoided because it may lead to unnecessary and harmful evaluation and treatment in women at very low risk of cancer.1
How different is this from the 2003 ACOG recommendation to begin screening within 3 years of first intercourse or at age 21, whichever comes first?
Very, very different. In fact, it is the most dramatic change in the 2009 screening recommendations.
It is even more striking in comparison with ACOG’s earlier recommendation—which prevailed from the late 1970s through 2002—to begin cervical screening at age 18 or at the onset of intercourse, whichever comes first.
The median age of first intercourse in the United States is 16 years. Until this latest change in guidelines, most young women began cervical screening during adolescence.
What’s wrong with screening adolescents? Don’t they acquire human papillomavirus (HPV)? (Yes.) And once they do, aren’t they at risk of cervical cancer? (Yes.)
Several variables support the delay of screening to age 21:
- the transience of most HPV infections
- the typically long natural history of carcinogenesis in the few young women in whom HPV might persist
- the adverse consequences of over-screening and over-management of adolescents who have cervical intraepithelial neoplasia (CIN).
Let’s look more closely at these variables.
TABLE 1
Other ACOG cervical disease guidelines are based on Level-B and Level-C evidence*
| Recommendation | Level of evidence | Comment |
|---|---|---|
| Test sexually active adolescents (i.e., females 21 years or younger) for sexually transmitted infection, and counsel them about safe sexual practices and contraception | B | These measures can be carried out without cervical cytology and, in the asymptomatic patient, without the introduction of a speculum |
| It is reasonable to discontinue cervical cancer screening in any woman 65 to 70 years old who has had three or more consecutive negative Pap tests and no abnormal tests in the past 10 years | B | |
| Continue annual screening for at least 20 years in any woman who has been treated for CIN 2, CIN 3, or cancer. This population remains at risk of persistent or recurrent disease for at least 20 years after treatment and after initial posttreatment surveillance | B | |
| Continue to screen any woman who has had a total hysterectomy if she has a history of CIN 2 or CIN 3 or if a negative history cannot be documented. This screening should continue even after initial post-treatment surveillance | B | Although the screening interval may ultimately be extended, we lack reliable data to support or refute the discontinuation of screening in this population |
| Inform the patient that annual gynecologic examination may still be appropriate even if cervical cytology is not assessed at each visit | C | |
| Screen any woman who has been immunized against HPV 16 and 18 as though she has not been immunized | C | |
| * Level-B recommendations are based on limited and inconsistent scientific evidence. Level-C recommendations are based primarily on consensus and expert opinion. | ||
HPV is common but usually resolves on its own
It’s common for young women to acquire HPV shortly after they become sexually active, but their immune system clears most infections within 1 or 2 years without the virus producing neoplastic changes.1
HPV detection peaks in the late teens and early 20s, when approximately 25% of women test positive for the virus, resulting in high rates of low-grade squamous intraepithelial lesions (LSIL) and HPV-positive, atypical squamous cells of undetermined significance (ASC-US).2 These findings are mostly transient.2
Detection of CIN 3 does not peak until a woman reaches her late 20s, and the median detection of microinvasive cancer does not peak until she reaches her early 40s. These facts indicate that adolescents have the lowest risk of incipient cervical cancer but the highest risk of undergoing unnecessary procedures for HPV-related events—events that are highly likely to resolve without treatment.
From 1998 to 2006, an average of 14 cervical cancers occurred annually in women 15 to 19 years old, an incidence of only 1 or 2 cases of cervical cancer for every 1 million women in that age group ( TABLE 2 ).
TABLE 2
Incidence of invasive cervical carcinoma: United States, 1998-2003
| Age (y) | Average annual count | Incidence (95% CI) | Incidence as a percentage | Median age at diagnosis |
|---|---|---|---|---|
| All ages | 10,846 | 8.9 (8.8–9.0) | 100 | 47 |
| 0–14 | 0 | 0 | 0 | Not applicable (NA) |
| 15–19 | 14 | 0.2 (0.1–0.2) | 0.1 | NA |
| 20–24 | 123 | 1.6 (1.5–1.7) | 1.1 | NA |
| 25–29 | 543 | 6.9 (6.7–7.2) | 5.0 | NA |
| 30–34 | 1,045 | 12.3 (12.0–12.6) | 9.6 | NA |
| 35–39 | 1,350 | 14.6 (14.3–14.9) | 12.5 | NA |
| 40–44 | 1,534 | 16.3 (15.9–16.6) | 14.1 | NA |
| 45–49 | 1,323 | 15.4 (15.0–15.7) | 12.2 | NA |
| 50–59 | 1,958 | 14.5 (14.2–14.7) | 18.0 | NA |
| 60–69 | 1,352 | 14.8 (14.5–15.1) | 12.5 | NA |
| 70–79 | 1,008 | 12.9 (12.6–13.3) | 9.3 | NA |
| ≥80 | 595 | 11.2 (10.9–11.6) | 5.5 | NA |
| Source: Watson et al5 | ||||
In teens, screening does not reduce mortality
Even this low rate of cervical cancer might justify the screening of adolescents, provided such screening was shown to reduce the incidence of and mortality from cervical cancer in that age group. However, all data point to the opposite conclusion:
- The incidence of cervical cancer in this age group has not changed since the years between 1973 and 1977, a period that preceded the recommendation to begin screening at age 18 or first intercourse
- No data demonstrate a benefit of screening in women younger than 21 years in regard to future rates of CIN 2 and 3—or even that screening women 20 to 24 years old reduces the rate of cervical cancer in women 30 years or younger3
- CIN 2 and 3 do occur in adolescents, and the fear of delaying their diagnosis has driven much of the opposition to the guideline change—specifically, the omission of the option to begin screening within 3 years after first intercourse; however, even when high-grade CIN develops, spontaneous regression is common in this age group (e.g., 65% rate of regression of CIN 2 after 18 months; 75% after 36 months)
- When CIN 3 develops and persists, more than 10 years are typically required for the lesion to acquire the capacity to become invasive.1,2
In addition, extensive data suggest that screening adolescents may be harmful. Adverse psychological effects related to cervical cancer screening, evaluation of abnormal results, and treatment of CIN have been reported, including negative effects on sexual function and a higher risk of preterm and low-birth-weight infants.1
Virtually all studies of pregnancy outcomes following loop electrosurgical excision procedure (LEEP) have demonstrated a doubling or tripling of the rate of preterm birth.
Screening women 21 years or younger for cervical cancer may be harmful and lacks proven benefit. Screening should not begin until the patient is 21, regardless of the age of first intercourse.
Extend the screening interval to 2 years for women 21 to 29 years old
Both liquid-based and conventional methods of cervical cytology are acceptable for screening; hence, screening frequency should not vary based on the method used.1
The 2003 ACOG guidelines recommended annual cervical screening of women in their 20s using either conventional or liquid-based cytology. In contrast, in 2002, the American Cancer Society (ACS) recommended annual screening when the conventional Pap test was used, and a 2-year interval when screening involved liquid-based cytology. With ACOG’s latest recommendation—a 2-year interval for women 21 to 29 years old, regardless of test method—the College moves in line with the ACS in regard to liquid-based cytology. It also acknowledges more recent evidence that liquid-based cytology is no more sensitive than conventional cytology.1
Liquid-based cytology does have a number of other unquestionable advantages, however:
- It offers the convenience of being able to test for HPV, Neisseria gonorrhoeae, and Chlamydia trachomatis directly from the residual sample
- It produces fewer unsatisfactory cytology results than conventional cytology
- Cytotechnologists find liquid-based cytology easier to read.
More than 90% of Pap tests in the United States utilize liquid-based cytology, and that percentage is not likely to diminish.
Women 21 to 29 years old should have a Pap test every 2 years, regardless of the method used.
Some women 30 years and older can be screened every 3 years
Cervical cytology screening is recommended every 3 years for women age 30 years and older if:
- they have had three consecutive negative cervical cytology screening test results and have no history of CIN 2 or CIN 3, are not HIV-infected, are not immunocompromised, and were not exposed to diethylstilbestrol in utero or
- they have received negative test cotest results on both cervical cytology screening and HPV DNA testing and are considered low risk.1
A study of the detection of squamous cell cervical cancer (SCC) within 3.5 years of one, two, or three consecutive normal Pap tests demonstrated that the incidence of cervical cancer increases to 3 to 5 cases for every 100,000 woman-years in each of the subsequent 2 years. Some experts argue that this relatively low increase—the equivalent of the incidence of breast cancer in men—supports extension of the screening interval to 3 years after three consecutive normal Pap results.
Clinicians have generally been hesitant to widen the screening interval, despite ACS and ACOG recommendations for 2- or 3-year screening among women who have had three consecutive normal results. Many of these clinicians may find it difficult to dismiss even this low number of excess cancers (3 to 5 cases for every 100,000 woman-years) when more frequent or better screening would likely prevent them. As a result, the extension of screening intervals on the basis of negative cytology alone may continue to meet resistance from clinicians and their patients.
Wider intervals reduce the risk of unnecessary treatment
The extension of screening intervals, whether it is based on cytology alone or cytology combined with HPV testing, benefits most women by reducing the likelihood that transient, HPV-induced events will be detected and treated even though they are not destined to become CIN 3, adenocarcinoma in situ, or cervical cancer.
At the same time, however, extending the screening interval to 3 years in the setting of “opportunistic” screening—the screening approach used in the United States—may lead to irregular screening for many women at intervals beyond the recommended 3 years, thereby reducing the protective effect of a program based on cytology alone. Approximately 10% of cervical cancers occur in women who have not had a Pap test in the preceding 5 years.
Cotesting may be the solution
There is no question that extending cytology-only screening beyond 3 years significantly increases the risk of cervical cancer. However, among women tested for HPV, the risk of CIN 3 or greater does not begin to rise until at least 6 years following a negative test result, providing a margin of safety that would protect most women who miss the recommended 3-year screening interval.
Earlier this year, Ronco and colleagues published the results of a large primary cervical screening trial involving more than 94,000 women who were randomly assigned to screening with cytology alone or cotesting (i.e., cytology plus HPV testing).4 In the cytology-only group, women were referred to colposcopy for a Pap result of ASC-US or higher-grade findings. In the cotesting group, they were referred to colposcopy if the HPV or Pap test (or both) was positive. A second screening was performed an average of 3 years later, and the incidences of CIN 2, CIN 3, and cancer at each screening were compared between groups.
The number of cancers detected in the initial round of screening did not differ between groups. In the second round of screening, no cancers were found in the cotesting group, compared with nine cancers in the cytology-only group. The authors attributed this difference to the detection and treatment of twice as many cases of CIN 3 in the initial round of screening among women undergoing cotesting, compared with those tested with cytology alone.4
In addition, women in the cotesting group had an extremely low rate of CIN 3 in the second round of screening (2 cases for every 10,000 women). Investigators also noted that a high proportion of invasive cancers detected in the cytology group during the second round of screening were adenocarcinomas, consistent with reports from earlier studies that found cytology to be less effective in detecting adenocarcinomas than in detecting SCC.4
Although HPV testing was previously shown to outperform cytology in reducing the risk of cervical cancer in a low-resource country (India), this is the first study to do so in a developed country with a well-screened population and a low incidence of cervical cancer.4
Although ACOG guidelines encourage the extension of screening intervals to 3 years for women 30 years or older who have had three consecutive normal Pap tests, many clinicians have been reluctant to take this step. Cotesting with HPV and Pap tests should provide the reassurance necessary for these clinicians to adopt the wider screening intervals.
Keep annual screening for women who have a history of CIN, HIV, or certain chronic conditions
The recommendations to screen women every 2 years until age 30 and to extend the screening interval to 3 years thereafter, provided three consecutive Pap tests are normal or cotesting is negative, apply only to women at average risk of cervical cancer. Conditions that indicate elevated risk include:
- HIV infection
- immunosuppression for other reasons, e.g., organ transplant
- in utero exposure to diethylstilbestrol
- history of CIN 2, CIN 3, or cancer.
Two Pap tests are recommended in the first year after diagnosis of HIV infection, followed by annual screening. It can be presumed that women who have chronic immunosuppression should be managed similarly.
As for women known to have been exposed to diethylstilbestrol in utero, no specific recommendation is given other than “more frequent screening.”1
The relatively recent documentation that women with a history of CIN 2 or 3 (and probably adenocarcinoma in situ) remain at risk of developing cervical cancer for at least 20 years after treatment warrants annual screening for at least 20 years. The increased reassurance that no CIN 3 or greater is missed when cotesting is negative for both cytology and HPV testing might argue for extension of the screening interval for women who have negative cotest results and who have completed recommended posttreatment follow-up. However, at this time, we lack data on long-term follow-up of women who have been treated for cervical neoplasia and who have negative cotest results. Therefore, such a recommendation cannot be made at this time.
Do not increase the screening interval beyond annual testing for women who are HIV-positive, who are immunosuppressed, who were exposed in utero to diethylstilbestrol, or who have been treated for CIN 2 or 3 or adenocarcinoma in situ.
Routine cytology testing should be discontinued after total hysterectomy for benign indications, provided the woman has no history of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.1 This recommendation has not changed since the 2003 ACOG guidelines on cervical cancer screening were published, and it is consistent with guidelines from the U.S. Preventive Services Task Force and the American Cancer Society.
1. Cervical cytology screening. ACOG Practice Bulletin #109. Obstet Gynecol. 2009;114(6):1409-1420.
2. Moscicki AB, Cox JT. Practice Improvement in Cervical Screening and Management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Gen Tract Dis. 2010;14(1):73-80.
3. Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population-based case-control study of prospectively recorded data. BMJ. 2009;339:b2968.-
4. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. http://www.thelancet.com/oncology. Published online January 19, 2010. DOI:10.1016/S1470–2045(09)70360-2.
5. Watson M, Saraiya M, Bernard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(suppl 10):2855-2864.
6. Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353(20):2101-2104.
1. Cervical cytology screening. ACOG Practice Bulletin #109. Obstet Gynecol. 2009;114(6):1409-1420.
2. Moscicki AB, Cox JT. Practice Improvement in Cervical Screening and Management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Gen Tract Dis. 2010;14(1):73-80.
3. Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population-based case-control study of prospectively recorded data. BMJ. 2009;339:b2968.-
4. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. http://www.thelancet.com/oncology. Published online January 19, 2010. DOI:10.1016/S1470–2045(09)70360-2.
5. Watson M, Saraiya M, Bernard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(suppl 10):2855-2864.
6. Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353(20):2101-2104.
Should primary cervical cancer screening of women 30 years and older include HPV testing?
- conventional Pap test
- HPV test (Hybrid Capture 2)
- visual inspection of the cervix with acetic acid (VIA)
- no screen (the current standard in India).
Within 8 years of a single HPV test, the incidence of both advanced cervical cancer and cervical cancer mortality declined significantly. In contrast, neither a single Pap test nor a single VIA had a substantial impact on either the incidence of advanced cervical cancer or mortality. Not a single cancer occurred among the 90% of women who tested negative for HPV at enrollment.
Properly timed, the HPV test can avert more advanced cervical cancers and deaths in developing countries than other screening methods
The burden of cervical cancer is shouldered largely by the developing world, where an estimated 80% of the nearly 500,000 cervical cancers occur annually, and where screening has been too costly and complicated to instigate. This study provides the first evidence that a single screen with an HPV test, best performed 15 to 20 years after the median age of first intercourse, would save more lives than other options and may be affordable.
A low-cost, simple, and highly sensitive HPV test (careHPV test) has been developed, with financial backing from the Bill and Melinda Gates Foundation, through the Alliance for Cervical Cancer Prevention. This test provides results within 3 hours and is now being used in demonstration projects in several countries. This appears to offer a way to prevent thousands of deaths from cervical cancer worldwide.
In the United States, cotesting is more effective—and affordable—than the Pap test alone
Castle and associates provide in-depth information on cotesting in women 30 years and older using both the Pap and HPV tests in primary screening in a US setting.
A major concern about cotesting has been that the addition of the HPV test might burden the system with too many positive results, but this study demonstrates otherwise. In the general population of women of this age, the number of HPV-positive results is not burdensome.
The authors point out that, for the 90% of women who tested negative on both the Pap and HPV tests, the risk of incipient precancer or cancer is likely to be very low for the next 10 years or so. Therefore, most women who undergo cotesting could be safely screened at 3-year intervals, and those screened more irregularly would likely be better protected than women screened by cytology alone. Extending the interval makes cotesting more cost-effective and has other benefits, as well.
Rate of HPV-positive findings varied by age
Among women 30 years of age and older, there is some variation in the rate of HPV-positive results. In this study, HPV-positive results were found in:
- 10.8% of women 30 to 34 years old
- 8.0% of women 35 to 39
- 6.3% of women 40 to 44
- 4.9% of women 45 to 49
- 4.3% of women 50 to 54
- 3.9% of women 55 to 59
- 3.7% of women 60 to 69
- 5.3% of women 80 years and older.
Findings are in line with other studies
The study by Castle and colleagues is the largest general-population screening investigation of cotesting published so far. The rate of HPV-positive and Pap-negative findings was 3.99% for the group of women 30 years and older, which is right on target with the 3.7% rate for cotesting demonstrated in the Netherlands and the 4% rate reported in a US survey by the College of American Pathologists. All women of this age in the Kaiser system were given the option of continuing to get an annual Pap or switching to cotesting every 3 years; 91.6% chose the cotesting option.
More and more, data point to cotesting as the screen of choice for women 30 years and older, with the screening interval extended to 3 years for any patient who tests negative on both HPV and Pap tests.—J. THOMAS COX, MD
- conventional Pap test
- HPV test (Hybrid Capture 2)
- visual inspection of the cervix with acetic acid (VIA)
- no screen (the current standard in India).
Within 8 years of a single HPV test, the incidence of both advanced cervical cancer and cervical cancer mortality declined significantly. In contrast, neither a single Pap test nor a single VIA had a substantial impact on either the incidence of advanced cervical cancer or mortality. Not a single cancer occurred among the 90% of women who tested negative for HPV at enrollment.
Properly timed, the HPV test can avert more advanced cervical cancers and deaths in developing countries than other screening methods
The burden of cervical cancer is shouldered largely by the developing world, where an estimated 80% of the nearly 500,000 cervical cancers occur annually, and where screening has been too costly and complicated to instigate. This study provides the first evidence that a single screen with an HPV test, best performed 15 to 20 years after the median age of first intercourse, would save more lives than other options and may be affordable.
A low-cost, simple, and highly sensitive HPV test (careHPV test) has been developed, with financial backing from the Bill and Melinda Gates Foundation, through the Alliance for Cervical Cancer Prevention. This test provides results within 3 hours and is now being used in demonstration projects in several countries. This appears to offer a way to prevent thousands of deaths from cervical cancer worldwide.
In the United States, cotesting is more effective—and affordable—than the Pap test alone
Castle and associates provide in-depth information on cotesting in women 30 years and older using both the Pap and HPV tests in primary screening in a US setting.
A major concern about cotesting has been that the addition of the HPV test might burden the system with too many positive results, but this study demonstrates otherwise. In the general population of women of this age, the number of HPV-positive results is not burdensome.
The authors point out that, for the 90% of women who tested negative on both the Pap and HPV tests, the risk of incipient precancer or cancer is likely to be very low for the next 10 years or so. Therefore, most women who undergo cotesting could be safely screened at 3-year intervals, and those screened more irregularly would likely be better protected than women screened by cytology alone. Extending the interval makes cotesting more cost-effective and has other benefits, as well.
Rate of HPV-positive findings varied by age
Among women 30 years of age and older, there is some variation in the rate of HPV-positive results. In this study, HPV-positive results were found in:
- 10.8% of women 30 to 34 years old
- 8.0% of women 35 to 39
- 6.3% of women 40 to 44
- 4.9% of women 45 to 49
- 4.3% of women 50 to 54
- 3.9% of women 55 to 59
- 3.7% of women 60 to 69
- 5.3% of women 80 years and older.
Findings are in line with other studies
The study by Castle and colleagues is the largest general-population screening investigation of cotesting published so far. The rate of HPV-positive and Pap-negative findings was 3.99% for the group of women 30 years and older, which is right on target with the 3.7% rate for cotesting demonstrated in the Netherlands and the 4% rate reported in a US survey by the College of American Pathologists. All women of this age in the Kaiser system were given the option of continuing to get an annual Pap or switching to cotesting every 3 years; 91.6% chose the cotesting option.
More and more, data point to cotesting as the screen of choice for women 30 years and older, with the screening interval extended to 3 years for any patient who tests negative on both HPV and Pap tests.—J. THOMAS COX, MD
- conventional Pap test
- HPV test (Hybrid Capture 2)
- visual inspection of the cervix with acetic acid (VIA)
- no screen (the current standard in India).
Within 8 years of a single HPV test, the incidence of both advanced cervical cancer and cervical cancer mortality declined significantly. In contrast, neither a single Pap test nor a single VIA had a substantial impact on either the incidence of advanced cervical cancer or mortality. Not a single cancer occurred among the 90% of women who tested negative for HPV at enrollment.
Properly timed, the HPV test can avert more advanced cervical cancers and deaths in developing countries than other screening methods
The burden of cervical cancer is shouldered largely by the developing world, where an estimated 80% of the nearly 500,000 cervical cancers occur annually, and where screening has been too costly and complicated to instigate. This study provides the first evidence that a single screen with an HPV test, best performed 15 to 20 years after the median age of first intercourse, would save more lives than other options and may be affordable.
A low-cost, simple, and highly sensitive HPV test (careHPV test) has been developed, with financial backing from the Bill and Melinda Gates Foundation, through the Alliance for Cervical Cancer Prevention. This test provides results within 3 hours and is now being used in demonstration projects in several countries. This appears to offer a way to prevent thousands of deaths from cervical cancer worldwide.
In the United States, cotesting is more effective—and affordable—than the Pap test alone
Castle and associates provide in-depth information on cotesting in women 30 years and older using both the Pap and HPV tests in primary screening in a US setting.
A major concern about cotesting has been that the addition of the HPV test might burden the system with too many positive results, but this study demonstrates otherwise. In the general population of women of this age, the number of HPV-positive results is not burdensome.
The authors point out that, for the 90% of women who tested negative on both the Pap and HPV tests, the risk of incipient precancer or cancer is likely to be very low for the next 10 years or so. Therefore, most women who undergo cotesting could be safely screened at 3-year intervals, and those screened more irregularly would likely be better protected than women screened by cytology alone. Extending the interval makes cotesting more cost-effective and has other benefits, as well.
Rate of HPV-positive findings varied by age
Among women 30 years of age and older, there is some variation in the rate of HPV-positive results. In this study, HPV-positive results were found in:
- 10.8% of women 30 to 34 years old
- 8.0% of women 35 to 39
- 6.3% of women 40 to 44
- 4.9% of women 45 to 49
- 4.3% of women 50 to 54
- 3.9% of women 55 to 59
- 3.7% of women 60 to 69
- 5.3% of women 80 years and older.
Findings are in line with other studies
The study by Castle and colleagues is the largest general-population screening investigation of cotesting published so far. The rate of HPV-positive and Pap-negative findings was 3.99% for the group of women 30 years and older, which is right on target with the 3.7% rate for cotesting demonstrated in the Netherlands and the 4% rate reported in a US survey by the College of American Pathologists. All women of this age in the Kaiser system were given the option of continuing to get an annual Pap or switching to cotesting every 3 years; 91.6% chose the cotesting option.
More and more, data point to cotesting as the screen of choice for women 30 years and older, with the screening interval extended to 3 years for any patient who tests negative on both HPV and Pap tests.—J. THOMAS COX, MD
What you need to know about cervical cancer, genital warts, and HPV
Atypical squamous cells: The case for HPV testing
- Management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASC-US, but testing for HPV is preferred when the Pap test is liquid-based.
- The sensitivity of HPV triage for high-grade CIN is essentially equivalent to colposcopy, and reduces the need for colposcopy by half.
- HPV testing is a good option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization.
What’s the best management strategy for the roughly 2 to 3 million women each yearUpdate on Cervical Disease, for commentary by Thomas C. Wright, Jr, MD, Department of Pathology, College of Physicians and Surgeons of Columbia University.The difference is even more pronounced when the cumulative 2-year detection rate for CIN 2,3 is added in for women referred for HPV-positive ASCUS but not found to have CIN 2,3 at initial colposcopy. That rate rises from 20.1% at initial colposcopy to 26.9% at 2 years.8 Although many experts consider even HPV-positive ASCUS of minimal risk, few would consider a risk of high-grade disease exceeding 1 in 4 to be minimal. In fact, 39% of the total CIN 2,3 cases reported from a routine screening population were detected following triage of ASCUS, and fully 69% were from all equivocal and low-grade Pap diagnoses.9
TABLE 1
Risk of cervical intraepithelial neoplasia grade 2 or greater at initial colposcopy
| ASCUS | ||||
|---|---|---|---|---|
| STUDY | HPV TEST | HPV–POSITIVE | HPV–NEGATIVE | TOTAL RISK FOR ALL ASCUS |
| Cox6 | Hybrid capture 1 (expanded first–generation test) | 17% (14/81) | 0.74% (1/136) | 6.9% (15/217) |
| Manos7 | Hybrid capture 2 | 15% (45/300) | 1.2% (6/498) | 6.4% (51/798) |
| Solomon4 (ALTS) | Hybrid capture 2 | 18% (195/1,087) | 1.1% (13/1,175) | 9.2% (208/2,262) |
| ALTS = ASCUS/LSIL Triage Study; ASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus | ||||
Bethesda 3 redefines ASCUS
The third Bethesda System workshop took place in May 2001 with the aim of evaluating and updating earlier terminology.10 It began by eliminating the words “of undetermined significance” from the overall ASCUS category, which is now called simply “atypical squamous cells,” or ASC. Most subcategories of the former ASCUS were eliminated as well. (Note: Within this article, the acronyms ASCUS and ASC-US are both used to describe atypical squamous cells of undetermined significance. The latter acronym reflects usage and guidelines developed after the third Bethesda workshop.)
Now the ASC classification is broken down into 2 distinct groups:
Atypical squamous cells–undetermined significance, or ASC-US. This new subcategory includes cells previously termed “favor reactive” but not relegated by the pathologist to normal, as well as cells previously in the “unqualified” and “favor HPV” or “favor low-grade squamous intraepithelial lesion (LSIL)” subcategories.
Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesions, or ASC-H. This category includes atypical cells difficult to distinguish from high-grade cells but not definitive for that classification. Women with such Pap tests are at greater risk for high-risk HPV and histologic CIN 2,3 (TABLE 2).
Evidence-based guidelines reflect Bethesda 3 changes. By the time of Bethesda 3, extensive new data on the management of abnormal cytology was available, including but not limited to data from ALTS, making it possible to create evidence-based guidelines on management of abnormal cervical cytology and CIN. These guidelines were developed in 2001 at a consensus conference hosted by the American Society for Colposcopy and Cervical Pathology (ASCCP),11 with input from 29 professional organizations, federal agencies, and national and international health organizations.
The entire set recommendations for all types of abnormal Pap tests were published in the April 24, 2002 issue of the Journal of the American Medical Association, and management recommendations for histologically proven CIN were published in the July 2003 American Journal of Obstetrics and Gynecology and the July 2003 Journal of Lower Genital Tract Disease. The management algorithms for both cytology and histology can be downloaded from http://ASCCP.org.
TABLE 2
Comparison of risk for high-risk HPV and CIN grade 2,3, by Pap results
| HISTOLOGY | |||||
|---|---|---|---|---|---|
| PAP TEST | HIGH-RISK HPV | CIN 2 OR GREATER | CIN 3 | ||
| ASC-US | 63% | 12% | 5% | ||
| ASC-H | 86% | 40% | 24% | ||
| HSIL | 99% | 59% | 38% | ||
| Data from Sherman et al29 | |||||
| ASC-US = atypical squamous cells–undetermined significance; | |||||
| ASC-H = Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesion; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion | |||||
All 3 triage options safe, effective
An evidence-based review found all 3 options safe and effective.11 Therefore, management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASCUS, but testing for HPV is preferred when the Pap test is liquid-based (FIGURE 1).
Liquid-based cytology (ThinPrep; Cytyc, Boxborough, Mass and SurePath, Raleigh-Durham, NC) has several advantages. For example, residual cells in the fluid can be tested for HPV, eliminating a return visit.
Immediate colposcopy: Low predictive value, high anxiety and expense. Proponents of immediate colposcopy for all women with ASC-US argue that this would theoretically detect all CIN 2,3 and cancer. However, the positive predictive value of this approach will always be extremely low due to the low rate (6.4% to 11.9%) of CIN 2,3 in women with ASCUS.4,6,7 What’s more, the cost and anxiety generated by immediate colposcopy are high.12
2 repeat cytologies: Sensitivity, cost issues. This approach requires at least 2 repeat, optimized (liquid-based) Pap tests to equal the sensitivity of a single HPV test. This, compounded with the high rate of repeat abnormal cytology requiring colposcopic evaluation, means repeat cytology is unlikely to be cost-competitive with HPV testing.4,13
- Cervical cytology as a triage option. Cytology has been a good screening test, but its comparatively low sensitivity (51% to 83%) and poor reproducibility reduces its value as a triage test.13-17 For example, in ALTS, of 1,473 repeat Paps originally read as ASCUS by good clinical pathologists, only 633 were reread as ASCUS when 2-of-3 agreement was obtained in a blinded review by an expert panel of pathologists.16 In other words, 840 (57%) were reread as something other than ASCUS. Most were downgraded to normal.
- The sensitivity of the HPV test in detecting CIN 2,3 was 92.4%. This rate was matched only by 2 repeat Pap tests, provided the threshold for referral to colposcopy was ASCUS or greater.17 At this threshold, 95% of the CIN 2,3 was detected with repeat Pap testing, but only after an average of 8 to 12 months. This contrasts with the immediate reassurance provided by the initial HPV test.
- ALTS did not evaluate repeat conventional Pap smears. Nor do the guidelines differentiate between conventional and liquid-based methods in the number of follow-up Pap tests required for reassurance, despite consensus that the sensitivity of liquid-based cytology is better than that of the conventional “dry slide.”
- Any woman with a repeat Pap result of ASC-US or greater should be referred to colposcopy. Referral at a threshold of LSIL or greater would result in far fewer colposcopies, but has not been shown to be sufficiently sensitive for CIN 2,3.17
HPV testing identifies clear risk. Any objective test that initially indicates which women with ASC-US are at risk for CIN 2,3 and which are not—either now or in the future—should confer a major advantage.
HPV-positive women are clearly at risk, justifying the anxiety and cost of colposcopic referral, while HPV-negative women may be reassured (FIGURE 2). Also, ALTS data showed HPV triage is essentially equivalent to immediate colposcopy in sensitivity for high-grade CIN, while halving colposcopic referrals.17,18
Because low-risk HPV types do not cause CIN 3 or cancer, the HPV test should document only high-risk types.11 The only HPV test approved by the US Food and Drug Administration (Hybrid Capture 2, Digene, Gaithersburg, Md) includes both low- and high-risk HPV panels. For cost savings, the laboratory can be asked to use only the high-risk panel. All positive high-risk HPV cases should be referred to colposcopy.
FIGURE 1 3 triage options for management of ASC-US
FIGURE 2 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
Some high-grade lesions are still overlooked
A single HPV test or 2 repeat liquid-based Pap tests with a colposcopy-referral threshold of any findings of ASC-US or greater have similar sensitivity for CIN 2,3.17,18
The guidelines state that women who undergo immediate colposcopy with negative results or who have a negative initial HPV test should undergo a follow-up Pap test in 12 months. Note that the guidelines do not state that these women can return directly to routine screening. The reason: In some settings, “routine” screening is at 2- or 3-year intervals, and some risk still exists—albeit minimal—for missed CIN 2,3.
For example, 1 of 83 cases of CIN 2,3 were missed by HPV testing in the study by Manos et al.7 In ALTS, that number was 1 in 90,4 and in the study by Cox et al6 it was 1 in 136. Further, colposcopy did not initially detect 25% of the cumulative high-grade lesions detected over 2 years of follow-up in ALTS.17
In contrast, the recommendation for women with 2 repeat normal Pap tests is to return to “routine screening.” This inexplicably departs from the 12-month repeat Pap testing urged for women with negative results on the other 2 triage options, despite a similar risk of missed high-grade disease.
- In my opinion, all 3 scenarios should be managed by repeat Pap testing in 12 months.
Reducing referrals to colposcopy
If all women returned as directed for repeat cytology, more of them would be referred to colposcopy by repeat abnormal Pap tests at the ASC-US threshold than by testing positive for high-risk HPV types. In ALTS, 53% tested positive for high-risk HPV and were referred to colposcopy, compared with 67% who had an abnormal Pap test on the first or second repeat (these women also had 1 or 2 more office visits prior to referral to colposcopy.).
No difference for conventional smears. All the advantages of HPV testing in the triage of women with ASC-US persist when the initial referral Pap test is a conventional smear. The only exception is that HPV testing would require the patient to return for a repeat office visit. An alternative would be co-collecting an HPV-test sample at the time of the primary screening Pap test.
One major health-maintenance organization collects a separate sample from all women when the routine conventional Pap test is obtained using a standard Hybrid Capture 2 HPV test kit. The HPV-testing samples are then held until the results of the Pap smear are reported. For women reported to have ASCUS, the samples are sent to the lab for HPV testing; the remaining samples (approximately 95% in most practices) are discarded as medical waste. The cost of each discarded kit is approximately $1. Modeling has found this approach to be cost-effective.19
Postcolposcopy management
Many clinicians are concerned that women referred for the evaluation of HPV-positive ASC-US and found not to have CIN or other manifestations of HPV at colposcopy have a “false-positive” HPV test. However, although there are occasional HPV tests that misclassify a low-risk HPV type as high-risk, actual false-positive tests are very rare.
The 2-year ALTS longitudinal data provide the best information on what to expect when a woman with HPV-positive ASC-US or LSIL is found at colposcopy to have no CIN or to have only CIN 1 that is subsequently managed expectantly.8
The cumulative risk of CIN 2,3 over the 2 years was nearly equivalent for women referred initially for LSIL (27.6%) and for women referred for HPV-positive ASCUS (26.7%), further verifying that management should be similar. Two thirds of the CIN 2,3 was detected at initial colposcopy, and the remaining one third during the postcolposcopy 2-year follow-up.
The risk for subsequent detection of high-grade CIN was nearly identical for all women initially found not to have CIN 2,3 regardless of whether CIN 1 was detected at initial colposcopy, whether the colposcopy was initially completely normal, or whether there were changes that were biopsied and found not to have CIN (risk for CIN 2,3 was 13%, 11.3%, and 11.7% respectively).
Hence, all women referred for evaluation of HPV-positive ASC-US or LSIL and not treated for CIN 2,3 require similar diligent follow-up.
A single HPV test at 12 months detected 92% of all CIN 2,3 found over the 24-month follow-up; 55% tested HPV-positive and were referred to colposcopy.20 Repeat liquid-based cytology at 6 and 12 months referred to colposcopy 63% of women (using a threshold of a repeat Pap test of ASCUS or greater). Cumulative sensitivity of 2 repeat cytologies for CIN 2,3 was slightly less (88%). Combining a repeat Pap test with an HPV test did not increase sensitivity, but did significantly increase referral to colposcopy.
An HPV test alone at 12 months might be the most efficient test for identifying women with CIN 2,3 after colposcopy.20 Further support for this approach can be found in the substantial body of evidence showing that only persistent HPV progresses to CIN 321 and that testing for high-risk HPV detects most CIN 3.4,17,20
The ASCCP guidelines for women referred for either HPV-positive ASC-US or LSIL and found not to have CIN 2,3 or greater at initial colposcopy recommend either HPV testing at 12 months or repeat cytology at 6 and 12 months (FIGURE 1).11,22
Posttreatment follow-up. The ASCCP treatment guidelines also list HPV testing as an acceptable option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization,22 since there is substantial evidence that women successfully treated for CIN become HPV-negative, whereas women with persistent disease remain HPV-positive.23-25
A posttreatment HPV test should be performed no sooner than 6 months following the procedure, as it takes time for the patient to return to HPV-negative status. A positive HPV test is an indication for colposcopy. However, the guidelines advise against basing repeat treatment on a positive HPV test alone without documentation of persistent CIN.22
Other options for posttreatment surveillance include either repeat cytology or a combination of Pap testing and colposcopy at 4- to 6-month intervals until at least 3 cytologic results are “negative for squamous intraepithelial lesion or malignancy.”22
Annual cytologic follow-up is recommended thereafter. During that follow-up, any abnormal Pap test (ASC-US or greater) should be referred to colposcopy.
Managing ASC-US in special populations
Management of ASC-US may differ from the general recommendations when the patient is postmenopausal or immunosuppressed. However, there are no differences in the management guidelines during pregnancy.
HPV-negative postmenopausal patients. All 3 management options—immediate colposcopy, repeat cytology, and HPV DNA testing—are acceptable for postmenopausal women with ASC-US.11 However, estrogen deficiency is a common cause of ASC-US and is responsible for increasing rates of HPV-negative ASC-US in this age group despite high sensitivity of HPV testing for CIN 2 and 3.5
- Treatment with vaginal estrogen cream followed by repeat cytology approximately 1 week after completing the regimen is an option for postmenopausal women with ASC-US. This approach also may be helpful for perimenopausal women and for women of any age on progestin-only contraception who have clinical or cytologic evidence of atrophy.
- Women with ASC-US or greater on repeat cytology should be referred for colposcopy, whereas women with normal repeat cytology should have a second Pap test in 4 to 6 months. Repeating the course of vaginal estrogen prior to each Pap test may be helpful when atrophy is likely to persist. After 2 normal repeat Pap tests, the patient can return to routine screening.
Refer all immunosuppressed women for colposcopy. The management of ASC-US in HIV-infected women is particularly problematic because the rates of ASC-US and HPV detection are 2 to 3 times greater than in HIV-negative women. In addition, the risk of CIN 2 and 3 is much higher.26 HPV testing as a triage for ASC-US is not efficient in immunosuppressed women because the majority of ASC-US Pap tests in these women are HPV-positive.
ASCCP recommends colposcopy referral of all immunosuppressed women with ASCUS Pap test, regardless of their CD4 count, HIV viral load, or anti-retroviral therapy.26
Managing ASC-H: First, colposcopy
Clearly, women with ASC-H test results face a greater risk for CIN 2,3 and should be referred for immediate colposcopy.
The ASC-H designation is uncommon, reported in 0.27% to 0.6% of all Pap tests,27,28 or approximately 1 in 10 Pap smears read as ASC.
In ALTS, HPV testing and histology results were compared for women with Pap tests categorized as equivocal LSIL (ASCUSL), ASCUS-H, and high-grade squamous intraepithelial lesion (HSIL) (TABLE 2).29 High-risk HPV DNA was detected in 86% of ASCUS-H liquid-based Pap tests and 69.8% of ASCUS-H conventional smears. CIN 2,3 was found in 40% of liquid-based ASCUS-H smears and in 27.2% of conventional ASCUSH smears. A 3-year retrospective review of ASC-H with follow-up at Johns Hopkins Medical Institutions determined that 49% of patients had no CIN or glandular lesions.28 Of the 51% with CIN, approximately half the lesions were CIN 1 and half were CIN 2,3.
Further management depends on whether CIN is detected (FIGURE 3). If no CIN is found, the ASCCP guidelines recommend that cytology, colposcopy, and histology be reviewed. If there is a change in the diagnosis—eg, if the Pap interpretation is revised to HSIL—the patient should be managed accordingly.11
If there is no change, the patient should be followed with repeat cytology at 6-and 12-month intervals or HPV testing at 12 months. Women having any repeat abnormal Pap test at a threshold of ASC-US or greater or a positive HPV test should undergo repeat colposcopy.
ASC-H is of greater risk than ASC-US, but it is not as risky as HSIL. Therefore, a surgical excision procedure in the absence of documented CIN 2,3 would not normally be indicated.11
FIGURE 3 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
HPV test as triage option would mean retooling the system
Cytologic management systems have traditionally involved follow-up by repeat cytology, colposcopy, and, when necessary, treatment. Adding another triage option—HPV testing—requires that this system be retooled.
The labs. This is not difficult when the laboratory interpreting the liquid-based Pap test is the same lab that performs the “reflex” HPV test, as this allows the ASC-US Pap test to be reported as HPV-negative or HPV-positive. However, if the HPV test must be performed in a separate reference laboratory, the results of the Pap and HPV tests will arrive separately, and the clinician must collate the 2 reports before relaying the result to the patient.
The patients. Remember than an HPV test is a test for a sexually transmitted disease. (So is the Pap test, although it has not traditionally been considered as such.) For that reason, I give all patients a written explanation of the rationale behind testing ASC-US Pap tests for HPV. This explanation includes 2 check-off options at the bottom of the sheet where patients can indicate whether they would prefer HPV testing or one of the other follow-up options.
Most patients elect the HPV option. Our Pap test requisitions also have a check-off portion that allows us to notify the lab of patients who wants an HPV test if the Pap is interpreted as ASC-US.
The office staff. Whenever a new test or procedure is introduced, it is of primary importance that the office staff responsible for completing critical information on the requisition form is adequately trained. This involves knowing when and how to order the test and how to complete insurance information and clinical history on the Pap requisition—including the correct International Classification of Diseases, Ninth Revision code—to ensure that the HPV test is covered by the patient’s insurer.
Clinicians must understand the usually benign nature of HPV infection. Reporting a positive HPV test in a manner that is not unduly concerning requires reassuring and nonjudgmental communication of the results based on a broad understanding of the usually low-risk natural history of the virus, yet fosters responsible follow-up.
Why all HPV-positive ASC-US requires diligent follow-up
The recently released ASCCP guidelines recognize HPV testing as an option in the management of ASC results, including:
- initial management of ASC-US,
- postcolposcopy management of ASC-H or HPV-positive ASC Pap tests found to be normal or to have CIN 1, and
- posttreatment follow-up.
For each indication, the HPV test identifies women most likely to have CIN (HPV-positive) and those likely to have benign processes not related to HPV (HPV-negative).
New longitudinal data verify that women with HPV-positive ASC-US continue at risk for detection of CIN 2,3 (about 12% overall), whether the original colposcopic finding was normal or CIN 1.8 Therefore, they need continued diligent follow-up.
Dr. Cox serves on the Speaker’s Bureaus of Cytyc Corporation and Digene Corporation.
1. Kurman RJ, Henson DE, Berbst AL, Noller KL, Schiffman MH. Interim guidelines for the management of abnormal cervical cytology. JAMA. 1994;271:1866-1869.
2. Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials. Int J Gynecol Cancer. 1995;5:321-328.
3. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726-742.
4. Solomon D, Schiffman M, Tarone R, et al. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293-299.
5. Sherman ME, Schiffman M, Cox JT, et al. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-107.
6. Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172:946-954.
7. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
8. Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406-1412.
9. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of the minimally abnormal Papanicolaou diagnosis. Obstet Gynecol. 1998;91:973-976.
10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119.
11. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
12. Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient awareness and choice of management. J Royal Soc Med. 1996;89:257.-
13. Wright TC, Lorincz AT, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Pap smears. Am J Obstet Gynecol. 1998;178:926-966.
14. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
15. Agency for Health Care Policy and Research. Evidence Report/Technology Assessment #5: Evaluation of Cervical Cytology. Rockville, Md: AHCPR; January 1999.
16. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance—Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505.
17. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383-1392.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003;127:946-949.
19. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382-2390.
20. Guido R, Schiffman M, Solomon D, et al. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401-1405.
21. Nobbenhuis M, Walboomers JM, Helmerhorst TI, Rozendaal L. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20.-
22. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
23. Paraskevaidis E, Koliopoulos G, Alamanos Y, Malamou-Mitsi V, Lolis ED, Kitchener HC. Human papillomavirus testing and the outcome of treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2001;98:833-836.
24. Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84:796-801.
25. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001;82:177-180.
26. Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432-442.
27. Selvaggi SM. Reporting of atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H) on cervical samples: is it significant? Diagn Cytopathol. 2003;29:38-41.
28. Alli PM, Ali SZ. Atypical squamous cells of undetermined significance—rule out high-grade squamous intraepithelial lesion: cytopathologic characteristics and clinical correlates. Diagn Cytopathol. 2003;28:308-312.
29. Sherman ME, Solomon D, Schiffman M, et al. A comparison of equivocal LSIL and equivocal HSIL cervical cytology in the ASCUS LSIL Triage Study. Am J Clin Pathol. 2001;116:386-394.
- Management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASC-US, but testing for HPV is preferred when the Pap test is liquid-based.
- The sensitivity of HPV triage for high-grade CIN is essentially equivalent to colposcopy, and reduces the need for colposcopy by half.
- HPV testing is a good option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization.
What’s the best management strategy for the roughly 2 to 3 million women each yearUpdate on Cervical Disease, for commentary by Thomas C. Wright, Jr, MD, Department of Pathology, College of Physicians and Surgeons of Columbia University.The difference is even more pronounced when the cumulative 2-year detection rate for CIN 2,3 is added in for women referred for HPV-positive ASCUS but not found to have CIN 2,3 at initial colposcopy. That rate rises from 20.1% at initial colposcopy to 26.9% at 2 years.8 Although many experts consider even HPV-positive ASCUS of minimal risk, few would consider a risk of high-grade disease exceeding 1 in 4 to be minimal. In fact, 39% of the total CIN 2,3 cases reported from a routine screening population were detected following triage of ASCUS, and fully 69% were from all equivocal and low-grade Pap diagnoses.9
TABLE 1
Risk of cervical intraepithelial neoplasia grade 2 or greater at initial colposcopy
| ASCUS | ||||
|---|---|---|---|---|
| STUDY | HPV TEST | HPV–POSITIVE | HPV–NEGATIVE | TOTAL RISK FOR ALL ASCUS |
| Cox6 | Hybrid capture 1 (expanded first–generation test) | 17% (14/81) | 0.74% (1/136) | 6.9% (15/217) |
| Manos7 | Hybrid capture 2 | 15% (45/300) | 1.2% (6/498) | 6.4% (51/798) |
| Solomon4 (ALTS) | Hybrid capture 2 | 18% (195/1,087) | 1.1% (13/1,175) | 9.2% (208/2,262) |
| ALTS = ASCUS/LSIL Triage Study; ASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus | ||||
Bethesda 3 redefines ASCUS
The third Bethesda System workshop took place in May 2001 with the aim of evaluating and updating earlier terminology.10 It began by eliminating the words “of undetermined significance” from the overall ASCUS category, which is now called simply “atypical squamous cells,” or ASC. Most subcategories of the former ASCUS were eliminated as well. (Note: Within this article, the acronyms ASCUS and ASC-US are both used to describe atypical squamous cells of undetermined significance. The latter acronym reflects usage and guidelines developed after the third Bethesda workshop.)
Now the ASC classification is broken down into 2 distinct groups:
Atypical squamous cells–undetermined significance, or ASC-US. This new subcategory includes cells previously termed “favor reactive” but not relegated by the pathologist to normal, as well as cells previously in the “unqualified” and “favor HPV” or “favor low-grade squamous intraepithelial lesion (LSIL)” subcategories.
Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesions, or ASC-H. This category includes atypical cells difficult to distinguish from high-grade cells but not definitive for that classification. Women with such Pap tests are at greater risk for high-risk HPV and histologic CIN 2,3 (TABLE 2).
Evidence-based guidelines reflect Bethesda 3 changes. By the time of Bethesda 3, extensive new data on the management of abnormal cytology was available, including but not limited to data from ALTS, making it possible to create evidence-based guidelines on management of abnormal cervical cytology and CIN. These guidelines were developed in 2001 at a consensus conference hosted by the American Society for Colposcopy and Cervical Pathology (ASCCP),11 with input from 29 professional organizations, federal agencies, and national and international health organizations.
The entire set recommendations for all types of abnormal Pap tests were published in the April 24, 2002 issue of the Journal of the American Medical Association, and management recommendations for histologically proven CIN were published in the July 2003 American Journal of Obstetrics and Gynecology and the July 2003 Journal of Lower Genital Tract Disease. The management algorithms for both cytology and histology can be downloaded from http://ASCCP.org.
TABLE 2
Comparison of risk for high-risk HPV and CIN grade 2,3, by Pap results
| HISTOLOGY | |||||
|---|---|---|---|---|---|
| PAP TEST | HIGH-RISK HPV | CIN 2 OR GREATER | CIN 3 | ||
| ASC-US | 63% | 12% | 5% | ||
| ASC-H | 86% | 40% | 24% | ||
| HSIL | 99% | 59% | 38% | ||
| Data from Sherman et al29 | |||||
| ASC-US = atypical squamous cells–undetermined significance; | |||||
| ASC-H = Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesion; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion | |||||
All 3 triage options safe, effective
An evidence-based review found all 3 options safe and effective.11 Therefore, management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASCUS, but testing for HPV is preferred when the Pap test is liquid-based (FIGURE 1).
Liquid-based cytology (ThinPrep; Cytyc, Boxborough, Mass and SurePath, Raleigh-Durham, NC) has several advantages. For example, residual cells in the fluid can be tested for HPV, eliminating a return visit.
Immediate colposcopy: Low predictive value, high anxiety and expense. Proponents of immediate colposcopy for all women with ASC-US argue that this would theoretically detect all CIN 2,3 and cancer. However, the positive predictive value of this approach will always be extremely low due to the low rate (6.4% to 11.9%) of CIN 2,3 in women with ASCUS.4,6,7 What’s more, the cost and anxiety generated by immediate colposcopy are high.12
2 repeat cytologies: Sensitivity, cost issues. This approach requires at least 2 repeat, optimized (liquid-based) Pap tests to equal the sensitivity of a single HPV test. This, compounded with the high rate of repeat abnormal cytology requiring colposcopic evaluation, means repeat cytology is unlikely to be cost-competitive with HPV testing.4,13
- Cervical cytology as a triage option. Cytology has been a good screening test, but its comparatively low sensitivity (51% to 83%) and poor reproducibility reduces its value as a triage test.13-17 For example, in ALTS, of 1,473 repeat Paps originally read as ASCUS by good clinical pathologists, only 633 were reread as ASCUS when 2-of-3 agreement was obtained in a blinded review by an expert panel of pathologists.16 In other words, 840 (57%) were reread as something other than ASCUS. Most were downgraded to normal.
- The sensitivity of the HPV test in detecting CIN 2,3 was 92.4%. This rate was matched only by 2 repeat Pap tests, provided the threshold for referral to colposcopy was ASCUS or greater.17 At this threshold, 95% of the CIN 2,3 was detected with repeat Pap testing, but only after an average of 8 to 12 months. This contrasts with the immediate reassurance provided by the initial HPV test.
- ALTS did not evaluate repeat conventional Pap smears. Nor do the guidelines differentiate between conventional and liquid-based methods in the number of follow-up Pap tests required for reassurance, despite consensus that the sensitivity of liquid-based cytology is better than that of the conventional “dry slide.”
- Any woman with a repeat Pap result of ASC-US or greater should be referred to colposcopy. Referral at a threshold of LSIL or greater would result in far fewer colposcopies, but has not been shown to be sufficiently sensitive for CIN 2,3.17
HPV testing identifies clear risk. Any objective test that initially indicates which women with ASC-US are at risk for CIN 2,3 and which are not—either now or in the future—should confer a major advantage.
HPV-positive women are clearly at risk, justifying the anxiety and cost of colposcopic referral, while HPV-negative women may be reassured (FIGURE 2). Also, ALTS data showed HPV triage is essentially equivalent to immediate colposcopy in sensitivity for high-grade CIN, while halving colposcopic referrals.17,18
Because low-risk HPV types do not cause CIN 3 or cancer, the HPV test should document only high-risk types.11 The only HPV test approved by the US Food and Drug Administration (Hybrid Capture 2, Digene, Gaithersburg, Md) includes both low- and high-risk HPV panels. For cost savings, the laboratory can be asked to use only the high-risk panel. All positive high-risk HPV cases should be referred to colposcopy.
FIGURE 1 3 triage options for management of ASC-US
FIGURE 2 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
Some high-grade lesions are still overlooked
A single HPV test or 2 repeat liquid-based Pap tests with a colposcopy-referral threshold of any findings of ASC-US or greater have similar sensitivity for CIN 2,3.17,18
The guidelines state that women who undergo immediate colposcopy with negative results or who have a negative initial HPV test should undergo a follow-up Pap test in 12 months. Note that the guidelines do not state that these women can return directly to routine screening. The reason: In some settings, “routine” screening is at 2- or 3-year intervals, and some risk still exists—albeit minimal—for missed CIN 2,3.
For example, 1 of 83 cases of CIN 2,3 were missed by HPV testing in the study by Manos et al.7 In ALTS, that number was 1 in 90,4 and in the study by Cox et al6 it was 1 in 136. Further, colposcopy did not initially detect 25% of the cumulative high-grade lesions detected over 2 years of follow-up in ALTS.17
In contrast, the recommendation for women with 2 repeat normal Pap tests is to return to “routine screening.” This inexplicably departs from the 12-month repeat Pap testing urged for women with negative results on the other 2 triage options, despite a similar risk of missed high-grade disease.
- In my opinion, all 3 scenarios should be managed by repeat Pap testing in 12 months.
Reducing referrals to colposcopy
If all women returned as directed for repeat cytology, more of them would be referred to colposcopy by repeat abnormal Pap tests at the ASC-US threshold than by testing positive for high-risk HPV types. In ALTS, 53% tested positive for high-risk HPV and were referred to colposcopy, compared with 67% who had an abnormal Pap test on the first or second repeat (these women also had 1 or 2 more office visits prior to referral to colposcopy.).
No difference for conventional smears. All the advantages of HPV testing in the triage of women with ASC-US persist when the initial referral Pap test is a conventional smear. The only exception is that HPV testing would require the patient to return for a repeat office visit. An alternative would be co-collecting an HPV-test sample at the time of the primary screening Pap test.
One major health-maintenance organization collects a separate sample from all women when the routine conventional Pap test is obtained using a standard Hybrid Capture 2 HPV test kit. The HPV-testing samples are then held until the results of the Pap smear are reported. For women reported to have ASCUS, the samples are sent to the lab for HPV testing; the remaining samples (approximately 95% in most practices) are discarded as medical waste. The cost of each discarded kit is approximately $1. Modeling has found this approach to be cost-effective.19
Postcolposcopy management
Many clinicians are concerned that women referred for the evaluation of HPV-positive ASC-US and found not to have CIN or other manifestations of HPV at colposcopy have a “false-positive” HPV test. However, although there are occasional HPV tests that misclassify a low-risk HPV type as high-risk, actual false-positive tests are very rare.
The 2-year ALTS longitudinal data provide the best information on what to expect when a woman with HPV-positive ASC-US or LSIL is found at colposcopy to have no CIN or to have only CIN 1 that is subsequently managed expectantly.8
The cumulative risk of CIN 2,3 over the 2 years was nearly equivalent for women referred initially for LSIL (27.6%) and for women referred for HPV-positive ASCUS (26.7%), further verifying that management should be similar. Two thirds of the CIN 2,3 was detected at initial colposcopy, and the remaining one third during the postcolposcopy 2-year follow-up.
The risk for subsequent detection of high-grade CIN was nearly identical for all women initially found not to have CIN 2,3 regardless of whether CIN 1 was detected at initial colposcopy, whether the colposcopy was initially completely normal, or whether there were changes that were biopsied and found not to have CIN (risk for CIN 2,3 was 13%, 11.3%, and 11.7% respectively).
Hence, all women referred for evaluation of HPV-positive ASC-US or LSIL and not treated for CIN 2,3 require similar diligent follow-up.
A single HPV test at 12 months detected 92% of all CIN 2,3 found over the 24-month follow-up; 55% tested HPV-positive and were referred to colposcopy.20 Repeat liquid-based cytology at 6 and 12 months referred to colposcopy 63% of women (using a threshold of a repeat Pap test of ASCUS or greater). Cumulative sensitivity of 2 repeat cytologies for CIN 2,3 was slightly less (88%). Combining a repeat Pap test with an HPV test did not increase sensitivity, but did significantly increase referral to colposcopy.
An HPV test alone at 12 months might be the most efficient test for identifying women with CIN 2,3 after colposcopy.20 Further support for this approach can be found in the substantial body of evidence showing that only persistent HPV progresses to CIN 321 and that testing for high-risk HPV detects most CIN 3.4,17,20
The ASCCP guidelines for women referred for either HPV-positive ASC-US or LSIL and found not to have CIN 2,3 or greater at initial colposcopy recommend either HPV testing at 12 months or repeat cytology at 6 and 12 months (FIGURE 1).11,22
Posttreatment follow-up. The ASCCP treatment guidelines also list HPV testing as an acceptable option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization,22 since there is substantial evidence that women successfully treated for CIN become HPV-negative, whereas women with persistent disease remain HPV-positive.23-25
A posttreatment HPV test should be performed no sooner than 6 months following the procedure, as it takes time for the patient to return to HPV-negative status. A positive HPV test is an indication for colposcopy. However, the guidelines advise against basing repeat treatment on a positive HPV test alone without documentation of persistent CIN.22
Other options for posttreatment surveillance include either repeat cytology or a combination of Pap testing and colposcopy at 4- to 6-month intervals until at least 3 cytologic results are “negative for squamous intraepithelial lesion or malignancy.”22
Annual cytologic follow-up is recommended thereafter. During that follow-up, any abnormal Pap test (ASC-US or greater) should be referred to colposcopy.
Managing ASC-US in special populations
Management of ASC-US may differ from the general recommendations when the patient is postmenopausal or immunosuppressed. However, there are no differences in the management guidelines during pregnancy.
HPV-negative postmenopausal patients. All 3 management options—immediate colposcopy, repeat cytology, and HPV DNA testing—are acceptable for postmenopausal women with ASC-US.11 However, estrogen deficiency is a common cause of ASC-US and is responsible for increasing rates of HPV-negative ASC-US in this age group despite high sensitivity of HPV testing for CIN 2 and 3.5
- Treatment with vaginal estrogen cream followed by repeat cytology approximately 1 week after completing the regimen is an option for postmenopausal women with ASC-US. This approach also may be helpful for perimenopausal women and for women of any age on progestin-only contraception who have clinical or cytologic evidence of atrophy.
- Women with ASC-US or greater on repeat cytology should be referred for colposcopy, whereas women with normal repeat cytology should have a second Pap test in 4 to 6 months. Repeating the course of vaginal estrogen prior to each Pap test may be helpful when atrophy is likely to persist. After 2 normal repeat Pap tests, the patient can return to routine screening.
Refer all immunosuppressed women for colposcopy. The management of ASC-US in HIV-infected women is particularly problematic because the rates of ASC-US and HPV detection are 2 to 3 times greater than in HIV-negative women. In addition, the risk of CIN 2 and 3 is much higher.26 HPV testing as a triage for ASC-US is not efficient in immunosuppressed women because the majority of ASC-US Pap tests in these women are HPV-positive.
ASCCP recommends colposcopy referral of all immunosuppressed women with ASCUS Pap test, regardless of their CD4 count, HIV viral load, or anti-retroviral therapy.26
Managing ASC-H: First, colposcopy
Clearly, women with ASC-H test results face a greater risk for CIN 2,3 and should be referred for immediate colposcopy.
The ASC-H designation is uncommon, reported in 0.27% to 0.6% of all Pap tests,27,28 or approximately 1 in 10 Pap smears read as ASC.
In ALTS, HPV testing and histology results were compared for women with Pap tests categorized as equivocal LSIL (ASCUSL), ASCUS-H, and high-grade squamous intraepithelial lesion (HSIL) (TABLE 2).29 High-risk HPV DNA was detected in 86% of ASCUS-H liquid-based Pap tests and 69.8% of ASCUS-H conventional smears. CIN 2,3 was found in 40% of liquid-based ASCUS-H smears and in 27.2% of conventional ASCUSH smears. A 3-year retrospective review of ASC-H with follow-up at Johns Hopkins Medical Institutions determined that 49% of patients had no CIN or glandular lesions.28 Of the 51% with CIN, approximately half the lesions were CIN 1 and half were CIN 2,3.
Further management depends on whether CIN is detected (FIGURE 3). If no CIN is found, the ASCCP guidelines recommend that cytology, colposcopy, and histology be reviewed. If there is a change in the diagnosis—eg, if the Pap interpretation is revised to HSIL—the patient should be managed accordingly.11
If there is no change, the patient should be followed with repeat cytology at 6-and 12-month intervals or HPV testing at 12 months. Women having any repeat abnormal Pap test at a threshold of ASC-US or greater or a positive HPV test should undergo repeat colposcopy.
ASC-H is of greater risk than ASC-US, but it is not as risky as HSIL. Therefore, a surgical excision procedure in the absence of documented CIN 2,3 would not normally be indicated.11
FIGURE 3 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
HPV test as triage option would mean retooling the system
Cytologic management systems have traditionally involved follow-up by repeat cytology, colposcopy, and, when necessary, treatment. Adding another triage option—HPV testing—requires that this system be retooled.
The labs. This is not difficult when the laboratory interpreting the liquid-based Pap test is the same lab that performs the “reflex” HPV test, as this allows the ASC-US Pap test to be reported as HPV-negative or HPV-positive. However, if the HPV test must be performed in a separate reference laboratory, the results of the Pap and HPV tests will arrive separately, and the clinician must collate the 2 reports before relaying the result to the patient.
The patients. Remember than an HPV test is a test for a sexually transmitted disease. (So is the Pap test, although it has not traditionally been considered as such.) For that reason, I give all patients a written explanation of the rationale behind testing ASC-US Pap tests for HPV. This explanation includes 2 check-off options at the bottom of the sheet where patients can indicate whether they would prefer HPV testing or one of the other follow-up options.
Most patients elect the HPV option. Our Pap test requisitions also have a check-off portion that allows us to notify the lab of patients who wants an HPV test if the Pap is interpreted as ASC-US.
The office staff. Whenever a new test or procedure is introduced, it is of primary importance that the office staff responsible for completing critical information on the requisition form is adequately trained. This involves knowing when and how to order the test and how to complete insurance information and clinical history on the Pap requisition—including the correct International Classification of Diseases, Ninth Revision code—to ensure that the HPV test is covered by the patient’s insurer.
Clinicians must understand the usually benign nature of HPV infection. Reporting a positive HPV test in a manner that is not unduly concerning requires reassuring and nonjudgmental communication of the results based on a broad understanding of the usually low-risk natural history of the virus, yet fosters responsible follow-up.
Why all HPV-positive ASC-US requires diligent follow-up
The recently released ASCCP guidelines recognize HPV testing as an option in the management of ASC results, including:
- initial management of ASC-US,
- postcolposcopy management of ASC-H or HPV-positive ASC Pap tests found to be normal or to have CIN 1, and
- posttreatment follow-up.
For each indication, the HPV test identifies women most likely to have CIN (HPV-positive) and those likely to have benign processes not related to HPV (HPV-negative).
New longitudinal data verify that women with HPV-positive ASC-US continue at risk for detection of CIN 2,3 (about 12% overall), whether the original colposcopic finding was normal or CIN 1.8 Therefore, they need continued diligent follow-up.
Dr. Cox serves on the Speaker’s Bureaus of Cytyc Corporation and Digene Corporation.
- Management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASC-US, but testing for HPV is preferred when the Pap test is liquid-based.
- The sensitivity of HPV triage for high-grade CIN is essentially equivalent to colposcopy, and reduces the need for colposcopy by half.
- HPV testing is a good option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization.
What’s the best management strategy for the roughly 2 to 3 million women each yearUpdate on Cervical Disease, for commentary by Thomas C. Wright, Jr, MD, Department of Pathology, College of Physicians and Surgeons of Columbia University.The difference is even more pronounced when the cumulative 2-year detection rate for CIN 2,3 is added in for women referred for HPV-positive ASCUS but not found to have CIN 2,3 at initial colposcopy. That rate rises from 20.1% at initial colposcopy to 26.9% at 2 years.8 Although many experts consider even HPV-positive ASCUS of minimal risk, few would consider a risk of high-grade disease exceeding 1 in 4 to be minimal. In fact, 39% of the total CIN 2,3 cases reported from a routine screening population were detected following triage of ASCUS, and fully 69% were from all equivocal and low-grade Pap diagnoses.9
TABLE 1
Risk of cervical intraepithelial neoplasia grade 2 or greater at initial colposcopy
| ASCUS | ||||
|---|---|---|---|---|
| STUDY | HPV TEST | HPV–POSITIVE | HPV–NEGATIVE | TOTAL RISK FOR ALL ASCUS |
| Cox6 | Hybrid capture 1 (expanded first–generation test) | 17% (14/81) | 0.74% (1/136) | 6.9% (15/217) |
| Manos7 | Hybrid capture 2 | 15% (45/300) | 1.2% (6/498) | 6.4% (51/798) |
| Solomon4 (ALTS) | Hybrid capture 2 | 18% (195/1,087) | 1.1% (13/1,175) | 9.2% (208/2,262) |
| ALTS = ASCUS/LSIL Triage Study; ASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus | ||||
Bethesda 3 redefines ASCUS
The third Bethesda System workshop took place in May 2001 with the aim of evaluating and updating earlier terminology.10 It began by eliminating the words “of undetermined significance” from the overall ASCUS category, which is now called simply “atypical squamous cells,” or ASC. Most subcategories of the former ASCUS were eliminated as well. (Note: Within this article, the acronyms ASCUS and ASC-US are both used to describe atypical squamous cells of undetermined significance. The latter acronym reflects usage and guidelines developed after the third Bethesda workshop.)
Now the ASC classification is broken down into 2 distinct groups:
Atypical squamous cells–undetermined significance, or ASC-US. This new subcategory includes cells previously termed “favor reactive” but not relegated by the pathologist to normal, as well as cells previously in the “unqualified” and “favor HPV” or “favor low-grade squamous intraepithelial lesion (LSIL)” subcategories.
Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesions, or ASC-H. This category includes atypical cells difficult to distinguish from high-grade cells but not definitive for that classification. Women with such Pap tests are at greater risk for high-risk HPV and histologic CIN 2,3 (TABLE 2).
Evidence-based guidelines reflect Bethesda 3 changes. By the time of Bethesda 3, extensive new data on the management of abnormal cytology was available, including but not limited to data from ALTS, making it possible to create evidence-based guidelines on management of abnormal cervical cytology and CIN. These guidelines were developed in 2001 at a consensus conference hosted by the American Society for Colposcopy and Cervical Pathology (ASCCP),11 with input from 29 professional organizations, federal agencies, and national and international health organizations.
The entire set recommendations for all types of abnormal Pap tests were published in the April 24, 2002 issue of the Journal of the American Medical Association, and management recommendations for histologically proven CIN were published in the July 2003 American Journal of Obstetrics and Gynecology and the July 2003 Journal of Lower Genital Tract Disease. The management algorithms for both cytology and histology can be downloaded from http://ASCCP.org.
TABLE 2
Comparison of risk for high-risk HPV and CIN grade 2,3, by Pap results
| HISTOLOGY | |||||
|---|---|---|---|---|---|
| PAP TEST | HIGH-RISK HPV | CIN 2 OR GREATER | CIN 3 | ||
| ASC-US | 63% | 12% | 5% | ||
| ASC-H | 86% | 40% | 24% | ||
| HSIL | 99% | 59% | 38% | ||
| Data from Sherman et al29 | |||||
| ASC-US = atypical squamous cells–undetermined significance; | |||||
| ASC-H = Atypical squamous cells–cannot rule out high-grade squamous intraepithelial lesion; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion | |||||
All 3 triage options safe, effective
An evidence-based review found all 3 options safe and effective.11 Therefore, management by immediate colposcopy, repeat cytology, or HPV testing is acceptable for ASCUS, but testing for HPV is preferred when the Pap test is liquid-based (FIGURE 1).
Liquid-based cytology (ThinPrep; Cytyc, Boxborough, Mass and SurePath, Raleigh-Durham, NC) has several advantages. For example, residual cells in the fluid can be tested for HPV, eliminating a return visit.
Immediate colposcopy: Low predictive value, high anxiety and expense. Proponents of immediate colposcopy for all women with ASC-US argue that this would theoretically detect all CIN 2,3 and cancer. However, the positive predictive value of this approach will always be extremely low due to the low rate (6.4% to 11.9%) of CIN 2,3 in women with ASCUS.4,6,7 What’s more, the cost and anxiety generated by immediate colposcopy are high.12
2 repeat cytologies: Sensitivity, cost issues. This approach requires at least 2 repeat, optimized (liquid-based) Pap tests to equal the sensitivity of a single HPV test. This, compounded with the high rate of repeat abnormal cytology requiring colposcopic evaluation, means repeat cytology is unlikely to be cost-competitive with HPV testing.4,13
- Cervical cytology as a triage option. Cytology has been a good screening test, but its comparatively low sensitivity (51% to 83%) and poor reproducibility reduces its value as a triage test.13-17 For example, in ALTS, of 1,473 repeat Paps originally read as ASCUS by good clinical pathologists, only 633 were reread as ASCUS when 2-of-3 agreement was obtained in a blinded review by an expert panel of pathologists.16 In other words, 840 (57%) were reread as something other than ASCUS. Most were downgraded to normal.
- The sensitivity of the HPV test in detecting CIN 2,3 was 92.4%. This rate was matched only by 2 repeat Pap tests, provided the threshold for referral to colposcopy was ASCUS or greater.17 At this threshold, 95% of the CIN 2,3 was detected with repeat Pap testing, but only after an average of 8 to 12 months. This contrasts with the immediate reassurance provided by the initial HPV test.
- ALTS did not evaluate repeat conventional Pap smears. Nor do the guidelines differentiate between conventional and liquid-based methods in the number of follow-up Pap tests required for reassurance, despite consensus that the sensitivity of liquid-based cytology is better than that of the conventional “dry slide.”
- Any woman with a repeat Pap result of ASC-US or greater should be referred to colposcopy. Referral at a threshold of LSIL or greater would result in far fewer colposcopies, but has not been shown to be sufficiently sensitive for CIN 2,3.17
HPV testing identifies clear risk. Any objective test that initially indicates which women with ASC-US are at risk for CIN 2,3 and which are not—either now or in the future—should confer a major advantage.
HPV-positive women are clearly at risk, justifying the anxiety and cost of colposcopic referral, while HPV-negative women may be reassured (FIGURE 2). Also, ALTS data showed HPV triage is essentially equivalent to immediate colposcopy in sensitivity for high-grade CIN, while halving colposcopic referrals.17,18
Because low-risk HPV types do not cause CIN 3 or cancer, the HPV test should document only high-risk types.11 The only HPV test approved by the US Food and Drug Administration (Hybrid Capture 2, Digene, Gaithersburg, Md) includes both low- and high-risk HPV panels. For cost savings, the laboratory can be asked to use only the high-risk panel. All positive high-risk HPV cases should be referred to colposcopy.
FIGURE 1 3 triage options for management of ASC-US
FIGURE 2 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
Some high-grade lesions are still overlooked
A single HPV test or 2 repeat liquid-based Pap tests with a colposcopy-referral threshold of any findings of ASC-US or greater have similar sensitivity for CIN 2,3.17,18
The guidelines state that women who undergo immediate colposcopy with negative results or who have a negative initial HPV test should undergo a follow-up Pap test in 12 months. Note that the guidelines do not state that these women can return directly to routine screening. The reason: In some settings, “routine” screening is at 2- or 3-year intervals, and some risk still exists—albeit minimal—for missed CIN 2,3.
For example, 1 of 83 cases of CIN 2,3 were missed by HPV testing in the study by Manos et al.7 In ALTS, that number was 1 in 90,4 and in the study by Cox et al6 it was 1 in 136. Further, colposcopy did not initially detect 25% of the cumulative high-grade lesions detected over 2 years of follow-up in ALTS.17
In contrast, the recommendation for women with 2 repeat normal Pap tests is to return to “routine screening.” This inexplicably departs from the 12-month repeat Pap testing urged for women with negative results on the other 2 triage options, despite a similar risk of missed high-grade disease.
- In my opinion, all 3 scenarios should be managed by repeat Pap testing in 12 months.
Reducing referrals to colposcopy
If all women returned as directed for repeat cytology, more of them would be referred to colposcopy by repeat abnormal Pap tests at the ASC-US threshold than by testing positive for high-risk HPV types. In ALTS, 53% tested positive for high-risk HPV and were referred to colposcopy, compared with 67% who had an abnormal Pap test on the first or second repeat (these women also had 1 or 2 more office visits prior to referral to colposcopy.).
No difference for conventional smears. All the advantages of HPV testing in the triage of women with ASC-US persist when the initial referral Pap test is a conventional smear. The only exception is that HPV testing would require the patient to return for a repeat office visit. An alternative would be co-collecting an HPV-test sample at the time of the primary screening Pap test.
One major health-maintenance organization collects a separate sample from all women when the routine conventional Pap test is obtained using a standard Hybrid Capture 2 HPV test kit. The HPV-testing samples are then held until the results of the Pap smear are reported. For women reported to have ASCUS, the samples are sent to the lab for HPV testing; the remaining samples (approximately 95% in most practices) are discarded as medical waste. The cost of each discarded kit is approximately $1. Modeling has found this approach to be cost-effective.19
Postcolposcopy management
Many clinicians are concerned that women referred for the evaluation of HPV-positive ASC-US and found not to have CIN or other manifestations of HPV at colposcopy have a “false-positive” HPV test. However, although there are occasional HPV tests that misclassify a low-risk HPV type as high-risk, actual false-positive tests are very rare.
The 2-year ALTS longitudinal data provide the best information on what to expect when a woman with HPV-positive ASC-US or LSIL is found at colposcopy to have no CIN or to have only CIN 1 that is subsequently managed expectantly.8
The cumulative risk of CIN 2,3 over the 2 years was nearly equivalent for women referred initially for LSIL (27.6%) and for women referred for HPV-positive ASCUS (26.7%), further verifying that management should be similar. Two thirds of the CIN 2,3 was detected at initial colposcopy, and the remaining one third during the postcolposcopy 2-year follow-up.
The risk for subsequent detection of high-grade CIN was nearly identical for all women initially found not to have CIN 2,3 regardless of whether CIN 1 was detected at initial colposcopy, whether the colposcopy was initially completely normal, or whether there were changes that were biopsied and found not to have CIN (risk for CIN 2,3 was 13%, 11.3%, and 11.7% respectively).
Hence, all women referred for evaluation of HPV-positive ASC-US or LSIL and not treated for CIN 2,3 require similar diligent follow-up.
A single HPV test at 12 months detected 92% of all CIN 2,3 found over the 24-month follow-up; 55% tested HPV-positive and were referred to colposcopy.20 Repeat liquid-based cytology at 6 and 12 months referred to colposcopy 63% of women (using a threshold of a repeat Pap test of ASCUS or greater). Cumulative sensitivity of 2 repeat cytologies for CIN 2,3 was slightly less (88%). Combining a repeat Pap test with an HPV test did not increase sensitivity, but did significantly increase referral to colposcopy.
An HPV test alone at 12 months might be the most efficient test for identifying women with CIN 2,3 after colposcopy.20 Further support for this approach can be found in the substantial body of evidence showing that only persistent HPV progresses to CIN 321 and that testing for high-risk HPV detects most CIN 3.4,17,20
The ASCCP guidelines for women referred for either HPV-positive ASC-US or LSIL and found not to have CIN 2,3 or greater at initial colposcopy recommend either HPV testing at 12 months or repeat cytology at 6 and 12 months (FIGURE 1).11,22
Posttreatment follow-up. The ASCCP treatment guidelines also list HPV testing as an acceptable option for follow-up after treatment with cryosurgery, loop electrosurgical excision procedure, laser, or cold-knife conization,22 since there is substantial evidence that women successfully treated for CIN become HPV-negative, whereas women with persistent disease remain HPV-positive.23-25
A posttreatment HPV test should be performed no sooner than 6 months following the procedure, as it takes time for the patient to return to HPV-negative status. A positive HPV test is an indication for colposcopy. However, the guidelines advise against basing repeat treatment on a positive HPV test alone without documentation of persistent CIN.22
Other options for posttreatment surveillance include either repeat cytology or a combination of Pap testing and colposcopy at 4- to 6-month intervals until at least 3 cytologic results are “negative for squamous intraepithelial lesion or malignancy.”22
Annual cytologic follow-up is recommended thereafter. During that follow-up, any abnormal Pap test (ASC-US or greater) should be referred to colposcopy.
Managing ASC-US in special populations
Management of ASC-US may differ from the general recommendations when the patient is postmenopausal or immunosuppressed. However, there are no differences in the management guidelines during pregnancy.
HPV-negative postmenopausal patients. All 3 management options—immediate colposcopy, repeat cytology, and HPV DNA testing—are acceptable for postmenopausal women with ASC-US.11 However, estrogen deficiency is a common cause of ASC-US and is responsible for increasing rates of HPV-negative ASC-US in this age group despite high sensitivity of HPV testing for CIN 2 and 3.5
- Treatment with vaginal estrogen cream followed by repeat cytology approximately 1 week after completing the regimen is an option for postmenopausal women with ASC-US. This approach also may be helpful for perimenopausal women and for women of any age on progestin-only contraception who have clinical or cytologic evidence of atrophy.
- Women with ASC-US or greater on repeat cytology should be referred for colposcopy, whereas women with normal repeat cytology should have a second Pap test in 4 to 6 months. Repeating the course of vaginal estrogen prior to each Pap test may be helpful when atrophy is likely to persist. After 2 normal repeat Pap tests, the patient can return to routine screening.
Refer all immunosuppressed women for colposcopy. The management of ASC-US in HIV-infected women is particularly problematic because the rates of ASC-US and HPV detection are 2 to 3 times greater than in HIV-negative women. In addition, the risk of CIN 2 and 3 is much higher.26 HPV testing as a triage for ASC-US is not efficient in immunosuppressed women because the majority of ASC-US Pap tests in these women are HPV-positive.
ASCCP recommends colposcopy referral of all immunosuppressed women with ASCUS Pap test, regardless of their CD4 count, HIV viral load, or anti-retroviral therapy.26
Managing ASC-H: First, colposcopy
Clearly, women with ASC-H test results face a greater risk for CIN 2,3 and should be referred for immediate colposcopy.
The ASC-H designation is uncommon, reported in 0.27% to 0.6% of all Pap tests,27,28 or approximately 1 in 10 Pap smears read as ASC.
In ALTS, HPV testing and histology results were compared for women with Pap tests categorized as equivocal LSIL (ASCUSL), ASCUS-H, and high-grade squamous intraepithelial lesion (HSIL) (TABLE 2).29 High-risk HPV DNA was detected in 86% of ASCUS-H liquid-based Pap tests and 69.8% of ASCUS-H conventional smears. CIN 2,3 was found in 40% of liquid-based ASCUS-H smears and in 27.2% of conventional ASCUSH smears. A 3-year retrospective review of ASC-H with follow-up at Johns Hopkins Medical Institutions determined that 49% of patients had no CIN or glandular lesions.28 Of the 51% with CIN, approximately half the lesions were CIN 1 and half were CIN 2,3.
Further management depends on whether CIN is detected (FIGURE 3). If no CIN is found, the ASCCP guidelines recommend that cytology, colposcopy, and histology be reviewed. If there is a change in the diagnosis—eg, if the Pap interpretation is revised to HSIL—the patient should be managed accordingly.11
If there is no change, the patient should be followed with repeat cytology at 6-and 12-month intervals or HPV testing at 12 months. Women having any repeat abnormal Pap test at a threshold of ASC-US or greater or a positive HPV test should undergo repeat colposcopy.
ASC-H is of greater risk than ASC-US, but it is not as risky as HSIL. Therefore, a surgical excision procedure in the absence of documented CIN 2,3 would not normally be indicated.11
FIGURE 3 Management of atypical squamous cells–cannot exclude high–grade squamous intraepithelial lesions (ASC-H)
HPV test as triage option would mean retooling the system
Cytologic management systems have traditionally involved follow-up by repeat cytology, colposcopy, and, when necessary, treatment. Adding another triage option—HPV testing—requires that this system be retooled.
The labs. This is not difficult when the laboratory interpreting the liquid-based Pap test is the same lab that performs the “reflex” HPV test, as this allows the ASC-US Pap test to be reported as HPV-negative or HPV-positive. However, if the HPV test must be performed in a separate reference laboratory, the results of the Pap and HPV tests will arrive separately, and the clinician must collate the 2 reports before relaying the result to the patient.
The patients. Remember than an HPV test is a test for a sexually transmitted disease. (So is the Pap test, although it has not traditionally been considered as such.) For that reason, I give all patients a written explanation of the rationale behind testing ASC-US Pap tests for HPV. This explanation includes 2 check-off options at the bottom of the sheet where patients can indicate whether they would prefer HPV testing or one of the other follow-up options.
Most patients elect the HPV option. Our Pap test requisitions also have a check-off portion that allows us to notify the lab of patients who wants an HPV test if the Pap is interpreted as ASC-US.
The office staff. Whenever a new test or procedure is introduced, it is of primary importance that the office staff responsible for completing critical information on the requisition form is adequately trained. This involves knowing when and how to order the test and how to complete insurance information and clinical history on the Pap requisition—including the correct International Classification of Diseases, Ninth Revision code—to ensure that the HPV test is covered by the patient’s insurer.
Clinicians must understand the usually benign nature of HPV infection. Reporting a positive HPV test in a manner that is not unduly concerning requires reassuring and nonjudgmental communication of the results based on a broad understanding of the usually low-risk natural history of the virus, yet fosters responsible follow-up.
Why all HPV-positive ASC-US requires diligent follow-up
The recently released ASCCP guidelines recognize HPV testing as an option in the management of ASC results, including:
- initial management of ASC-US,
- postcolposcopy management of ASC-H or HPV-positive ASC Pap tests found to be normal or to have CIN 1, and
- posttreatment follow-up.
For each indication, the HPV test identifies women most likely to have CIN (HPV-positive) and those likely to have benign processes not related to HPV (HPV-negative).
New longitudinal data verify that women with HPV-positive ASC-US continue at risk for detection of CIN 2,3 (about 12% overall), whether the original colposcopic finding was normal or CIN 1.8 Therefore, they need continued diligent follow-up.
Dr. Cox serves on the Speaker’s Bureaus of Cytyc Corporation and Digene Corporation.
1. Kurman RJ, Henson DE, Berbst AL, Noller KL, Schiffman MH. Interim guidelines for the management of abnormal cervical cytology. JAMA. 1994;271:1866-1869.
2. Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials. Int J Gynecol Cancer. 1995;5:321-328.
3. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726-742.
4. Solomon D, Schiffman M, Tarone R, et al. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293-299.
5. Sherman ME, Schiffman M, Cox JT, et al. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-107.
6. Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172:946-954.
7. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
8. Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406-1412.
9. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of the minimally abnormal Papanicolaou diagnosis. Obstet Gynecol. 1998;91:973-976.
10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119.
11. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
12. Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient awareness and choice of management. J Royal Soc Med. 1996;89:257.-
13. Wright TC, Lorincz AT, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Pap smears. Am J Obstet Gynecol. 1998;178:926-966.
14. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
15. Agency for Health Care Policy and Research. Evidence Report/Technology Assessment #5: Evaluation of Cervical Cytology. Rockville, Md: AHCPR; January 1999.
16. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance—Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505.
17. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383-1392.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003;127:946-949.
19. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382-2390.
20. Guido R, Schiffman M, Solomon D, et al. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401-1405.
21. Nobbenhuis M, Walboomers JM, Helmerhorst TI, Rozendaal L. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20.-
22. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
23. Paraskevaidis E, Koliopoulos G, Alamanos Y, Malamou-Mitsi V, Lolis ED, Kitchener HC. Human papillomavirus testing and the outcome of treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2001;98:833-836.
24. Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84:796-801.
25. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001;82:177-180.
26. Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432-442.
27. Selvaggi SM. Reporting of atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H) on cervical samples: is it significant? Diagn Cytopathol. 2003;29:38-41.
28. Alli PM, Ali SZ. Atypical squamous cells of undetermined significance—rule out high-grade squamous intraepithelial lesion: cytopathologic characteristics and clinical correlates. Diagn Cytopathol. 2003;28:308-312.
29. Sherman ME, Solomon D, Schiffman M, et al. A comparison of equivocal LSIL and equivocal HSIL cervical cytology in the ASCUS LSIL Triage Study. Am J Clin Pathol. 2001;116:386-394.
1. Kurman RJ, Henson DE, Berbst AL, Noller KL, Schiffman MH. Interim guidelines for the management of abnormal cervical cytology. JAMA. 1994;271:1866-1869.
2. Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials. Int J Gynecol Cancer. 1995;5:321-328.
3. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726-742.
4. Solomon D, Schiffman M, Tarone R, et al. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293-299.
5. Sherman ME, Schiffman M, Cox JT, et al. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-107.
6. Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172:946-954.
7. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
8. Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406-1412.
9. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of the minimally abnormal Papanicolaou diagnosis. Obstet Gynecol. 1998;91:973-976.
10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-2119.
11. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
12. Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient awareness and choice of management. J Royal Soc Med. 1996;89:257.-
13. Wright TC, Lorincz AT, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Pap smears. Am J Obstet Gynecol. 1998;178:926-966.
14. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
15. Agency for Health Care Policy and Research. Evidence Report/Technology Assessment #5: Evaluation of Cervical Cytology. Rockville, Md: AHCPR; January 1999.
16. Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance—Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505.
17. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383-1392.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003;127:946-949.
19. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382-2390.
20. Guido R, Schiffman M, Solomon D, et al. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401-1405.
21. Nobbenhuis M, Walboomers JM, Helmerhorst TI, Rozendaal L. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20.-
22. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
23. Paraskevaidis E, Koliopoulos G, Alamanos Y, Malamou-Mitsi V, Lolis ED, Kitchener HC. Human papillomavirus testing and the outcome of treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2001;98:833-836.
24. Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84:796-801.
25. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001;82:177-180.
26. Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432-442.
27. Selvaggi SM. Reporting of atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H) on cervical samples: is it significant? Diagn Cytopathol. 2003;29:38-41.
28. Alli PM, Ali SZ. Atypical squamous cells of undetermined significance—rule out high-grade squamous intraepithelial lesion: cytopathologic characteristics and clinical correlates. Diagn Cytopathol. 2003;28:308-312.
29. Sherman ME, Solomon D, Schiffman M, et al. A comparison of equivocal LSIL and equivocal HSIL cervical cytology in the ASCUS LSIL Triage Study. Am J Clin Pathol. 2001;116:386-394.