User login
Discuss this article at http://currentpsychiatry.blogspot.com/2011/01/bariatric-procedures-managing-patients.html#comments
Bariatric surgery is the most effective treatment for obesity (defined as a body mass index [BMI] >30 kg/m2) and is recommended for extremely obese individuals (BMI >40 kg/m2) age >18.1,2 Most patients experience significant weight loss accompanied by improvements in mood, physical comorbidities, and quality of life (Box).3-8 Despite these favorable outcomes, several aspects of postoperative care—such as management of mental health issues—remain unclear. Bariatric surgery candidates show high rates of preoperative psychopathology, particularly depression and dysphoria. Little is known about how bariatric surgery affects absorption of psychiatric medications, leaving prescribing clinicians with minimal guidance when a postoperative patient reports changes in mood symptoms.
This article discusses the psychosocial status of bariatric surgery candidates and presents a rationale for increased medication monitoring after surgery.
Weight loss after bariatric surgery is associated with significant improvements in obesity-related comorbidities, including diabetes and cardiovascular disease, and decreased mortality.3,4
Many patients are able to reduce or discontinue many of their nonpsychiatric preoperative medications as their comorbid conditions improve.5 Symptoms of depression and anxiety, health-related quality of life, self-esteem, and body image often improve dramatically in the first year after surgery and endure for several years.6,7
Psychosocial improvements, however, may not translate into changes in psychotropic use. In a sample of 114 bariatric surgery patients, 43% were taking a selective serotonin reuptake inhibitor before surgery, 40% at 12 months postsurgery, and 31% at 24 months.8 These percentages do not account for patients who were taking other types of antidepressants.
Surgical treatment of obesity
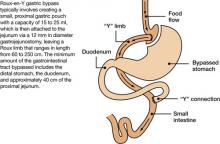
The most common surgical procedures for weight loss are adjustable gastric banding (AGB) and Roux-en-Y gastric bypass (RYGB); each can be performed laparoscopically. With both procedures, food intake is restricted by creating a gastric pouch at the base of the esophagus. RYGB (Figure)9,10 also is thought to induce weight loss through selective malabsorption and favorable effects on gut peptides11,12 and currently is the procedure of choice in the United States.13
Figure Roux-en-Y: Bariatric procedure of choice
Source: References 9,10Bariatric surgery patients typically lose 25% to 35% of their initial body weight within 12 to 18 months of surgery.3,4 However, 20% to 30% of patients fail to achieve typical postoperative weight loss or regain large amounts of weight within a few years.14-16 Suboptimal results have been attributed to multiple factors, including problematic dietary intake, disordered eating, low levels of physical activity, preoperative psychopathology, and poor follow-up.6,17,18
Preop psychopathology
Twenty percent to 60% of extremely obese persons who pursue bariatric surgery have a psychiatric illness.6,7 In a study of 288 bariatric surgery candidates assessed with the Structured Clinical Interview for DSMIV, 38% received a current axis I diagnosis and 66% were given a lifetime diagnosis.19 In a separate study of 174 individuals seeking bariatric surgery, 24% met criteria for a current axis I or axis II disorder and 37% were found to have ≥1 lifetime diagnoses.20 The most common lifetime diagnoses were affective disorders (22%), anxiety disorders (16%), and eating disorders (14%).20
Psychopathology could negatively impact postoperative outcome. In an observational study, patients with a lifetime diagnosis of any axis I disorder—particularly mood and anxiety disorders—experienced less weight loss 6 months after RYGB compared with those who never received an axis I diagnosis.21 Bariatric surgery patients with ≥2 psychiatric diagnoses were more likely to stop losing weight or regain weight after 1 year compared with those with 1 or no diagnosis.22 Psychiatric illness also appears to impact longer term weight loss.23
Most bariatric surgery programs in the United States require a mental health evaluation as part of the patient selection process.24 These assessments may include evaluating a patient’s behavior patterns, motivation, expectations, and cognitive and emotional functioning, and performing psychological testing (see Related Resources). Psychiatric problems such as substance abuse, active psychosis, bulimia nervosa, and severe, uncontrolled depression1,9,25 are widely considered contraindications to bariatric surgery.24,26
Postsurgery considerations
At the time of bariatric surgery 16% to 40% of patients are receiving mental health treatment, which often includes antidepressants.27-29 Unfortunately, little is known about how medications interact with these surgical procedures. Dramatic changes in medication absorption may occur because of reduced gastrointestinal (GI) surface area. Rapid reduction in body weight and fat mass and postoperative complications also may impact the efficacy and tolerability of antidepressants.
Pharmacokinetics. Anatomic and physiologic changes with bariatric surgery may lead to changes in the pharmacokinetic (PK) parameters of certain medications, particularly after RYGB. PK studies typically are conducted by collecting a series of plasma samples at predetermined intervals after a patient takes a medication. The blood levels of the medication and its active metabolites are used to compute multiple PK parameters that illustrate drug absorption, distribution, and metabolism. Theoretically, a bariatric surgery patient may experience changes in the rate and/or extent of:
- medication absorption from the GI tract into systemic circulation
- distribution throughout the body as fat mass and total body water change after surgery
- drug metabolism.
The effects of bariatric surgery on medication PK appears to be drug-specific.30-33
The bypassed portion of the GI tract is the primary absorption site for most medications; therefore, the length of the Roux limb may affect the extent of drug absorption impairment. However, the duodenum wall is one of many locations of the cytochrome P450 (CYP) isoenzymes CYP3A4 and CYP3A5,34 which are the primary metabolic enzymes for drugs such as atorvastatin. Eliminating this portion of the bowel could increase rather than decrease bioavailability.35 Alterations in drug absorption also may result from changes in gastric emptying rate, reduced exposure to absorptive mucosal surfaces, and alterations in gastric pH that can impair drug dissolution and solubility.30 These changes could reduce medication bioavailability.33 The impact of such changes may differ according to the characteristics of the specific drug. It has been theorized that drugs that are intrinsically poorly absorbed, are highly lipophilic, and undergo enterohepatic circulation carry the highest risk of malabsorption.30 Antidepressants vary in the extent to which they demonstrate these characteristics. Progressive changes in the volume of distribution as weight is lost also could affect the blood levels of some antidepressants.
A series of small studies and case reports of PK changes in medications such as digoxin, oral contraceptives, cyclosporine, sulfisoxazole, and tacrolimus after jejunoileal bypass—an older, obsolete bariatric procedure—reveal variability in the surgery’s effect on PK parameters, although most reported reduced absorption. Data specific to RYGB consist of small studies and case series that show reduced absorption after surgery with significant variability among agents (see our bibliography of studies describing PK changes in nonpsychiatric medications after bariatric surgery). In a systematic literature review, Padwal et al found evidence for a decreased magnitude of absorption in 15 of 22 studies of jejunoileal bypass patients, 1 of 3 studies of gastric bypass/gastroplasty, and no studies examining biliopancreatic diversion.30
It is unclear if antidepressant absorption is impaired after RYGB because currently only 1 case report presents in-vivo data. Hamad et al describe an obese patient (BMI 46 kg/m2) taking sertraline, 100 mg/d, for depression.36 Researchers measured sertraline levels before and 1 month after RYGB, at which time the patient’s depression worsened. After surgery, sertraline maximum concentration was lower (14.4 ng/ml vs 41.6 ng/ml), trough concentration was lower (11.1 ng/ml vs 17.5 ng/ml), and time to maximum concentration was shorter (240 vs 300 minutes). This suggests that a shift in sertraline absorption after surgery may have contributed to the patient’s worsened mood symptoms.
An in-vitro study that simulated preand post-RYGB GI environments found that 12 of 22 psychotropic drugs tested dissolved differently between the models.37 Whereas the dissolved fractions of amitriptyline, fluoxetine, paroxetine, and sertraline were significantly less in the post-RYGB environment, bupropion dissolved to a greater extent in the pre-RYGB environment, and venlafaxine and citalopram were not different between the 2 conditions (Table).37 Although several limitations prevent translating these data into clinical recommendations, this study suggests that there may be significant variability among medications with regard to the implications of an altered GI environment.
Blouin RA, Bauer LA, Miller DD, et al. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575-580.
Blouin RA, Brouwer KL, Record KE, et al. Amikacin pharmacokinetics in morbidly obese patients undergoing gastric-bypass surgery. Clin Pharm. 1985;4:70-72.
Chenhsu RY, Wu Y, Katz D, et al. Dose-adjusted cyclosporine c2 in a patient with jejunoileal bypass as compared to seven other liver transplant recipients. Ther Drug Monit. 2003;25(6):665-670.
Fuller AK, Tingle D, DeVane CL, et al. Haloperidol pharmacokinetics following gastric bypass surgery. J Clin Psychopharmacol. 1986;6:376-378.
Garrett ER, Süverkrup RS, Eberst K, et al. Surgically affected sulfisoxazole pharmacokinetics in the morbidly obese. Biopharm Drug Dispos. 1981;2:329-365.
Gerson CD, Lowe EH, Lindenbaum J. Bioavailability of digoxin tablets in patients with gastrointestinal dysfunction. Am J Med. 1980;69:43-49.
Hamad GG, Kozak GM, Wisner KL, et al. The effect of gastric bypass on SSRI pharmacokinetics and pharmacodynamics. Abstract presented at: American Society for Metabolic and Bariatric Surgery 25th Annual Meeting; June 15-20, 2008; Washington, DC.
Kelley M, Jain A, Kashyap R, et al. Change in oral absorption of tacrolimus in a liver transplant recipient after reversal of jejunoileal bypass: case report. Transplant Proc. 2005; 37:3165-3167.
Knight GC, Macris MP, Peric M, et al. Cyclosporine A pharmacokinetics in a cardiac allograft recipient with a jejunoileal bypass. Transplant Proc. 1988;20:351-355.
Marcus FI, Quinn EJ, Horton H, et al. The effect of jejunoileal bypass on the pharmacokinetics of digoxin in man. Circulation. 1977;55:537-541.
Magee SR, Shih G, Hume A. Malabsorption of oral antibiotics in pregnancy after gastric bypass surgery. J Am Board Fam Med. 2007;20:310-313.
Marterre WF, Hariharan S, First MR, et al. Gastric bypass in morbidly obese kidney transplant recipients. Clin Transplant. 1996;10:414-419.
Prince RA, Pincheira JC, Mason EE, et al. Influence of bariatric surgery on erythromycin absorption. J Clin Pharmacol. 1984;24:523-527.
Rogers CC, Alloway RR, Alexander JW, et al. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281-291.
Shepherd MF, Rosborough TK, Schwartz ML. Heparin thrombophylaxis in gastric bypass surgery. Obes Surg. 2003;13(2):249-253.
Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311-318.
Victor A, Odlind V, Kral JG. Oral contraceptive absorption and sex hormone binding globulins in obese women: effects of jejunoileal bypass. Gastroenterol Clin North Am. 1987;16(3):483-491.
PK: pharmacokinetic
Table
Weights of dissolved portions of antidepressants before and after simulated RYGB
| Simulated pre-RYGB environment | Simulated post-RYGB environment | ||||
|---|---|---|---|---|---|
| Drug | Median weight of dissolved portion (mg) | Percentage* | Median weight of dissolved portion (mg) | Percentage* | P† |
| Amitriptyline, 75 mg/d | 80 | 28% | 60 | 21% | <.04 |
| Fluoxetine, 20 mg/d | 110 | 30% | 40 | 11% | <.04 |
| Paroxetine, 20 mg/d | 30 | 9% | 10 | 3% | <.04 |
| Sertraline, 100 mg/d | 50 | 16% | 30 | 10% | <.04 |
| Bupropion, 100 mg/d | 320 | 52% | 450 | 73% | <.05 |
| Venlafaxine, 75 mg/d | 180 | 59% | 180 | 59% | Not significant |
| Citalopram, 20 mg/d | 70 | 27% | 80 | 31% | Not significant |
| *Relative to original pill weight †Mann-Whitney U test RYGB: Roux-en-Y gastric bypass Source: Adapted from reference 37 | |||||
Altering antidepressant doses
Current PK data are insufficient to make clinical recommendations regarding appropriate postsurgical adjustment of dose or alternate dosage formulations (liquid, extended-release, etc.). However, based on theoretical considerations, Miller and Smith suggest that patients avoid extended-release preparations whenever possible after bariatric surgery, citing the rationale that decreased intestinal length and surface area leads to reduced absorption.33 No data are available to advise clinicians regarding the appropriateness of switching patients from extended-release products to immediate-release or liquid preparations following surgery.
Presently, increased medication monitoring may be the most appropriate clinical approach. If appropriate doses have little or no effect, consider the possibility of decreased medication absorption.33 Monitoring plasma levels of medications that have therapeutic ranges also is advisable.
Areas for future research
Before specific clinical recommendations for managing antidepressants following RYGB can be proposed, the extent to which the absorption, volume of distribution, drug metabolism, and other measures change after surgery need to be quantified. It is also unclear whether changes in medication absorption are subject to inter-patient variability, whether predictive characteristics can be identified, and whether any observed changes remain stable over time. Similarly, the extent to which variability in surgical procedures (eg, surgeon preference regarding remnant intestinal length) affects medication absorption is unknown. Data regarding medication absorption following AGB and other bariatric procedures also will be needed.
- American Society for Metabolic and Bariatric Surgery. Fact sheet: Metabolic and bariatric surgery. www.asbs.org/Newsite07/media/asmbs_fs_surgery.pdf.
- American Society for Metabolic and Bariatric Surgery. Suggestions for the pre-surgical psychological assessment of bariatric surgery candidates. www.asmbs.org/html/pdf/PsychPreSurgicalAssessment.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atorvastatin • Lipitor
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Cyclosporine • Sandimmune
- Digoxin • Lanoxin
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sulfisoxazole • Truxazole
- Tacrolimus • Prograf
- Venlafaxine • Effexor
Disclosures
Dr. Sarwer receives grant/research support from the National Institutes of Health, the American Society for Metabolic and Bariatric Surgery, and Ethicon Endo-Surgery, Inc., is consultant to Allergan, BAROnova, Inc., EnteroMedics, and Ethocon Endo-Surgery, Inc., and is on the board of directors of Surgical Review Corporation.
Dr. Roerig receives grant/research support from Eli Lilly and Company.
Drs. Faulconbridge, Steffen, and Mitchell report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic and Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318-336.
2. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956-961.
3. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
4. Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559.
5. Hodo DM, Waller JL, Martindale RG, et al. Medication use after bariatric surgery in a managed care cohort. Surg Obes Rel Dis. 2008;4:601-607.
6. Sarwer DB, Wadden TA, Fabricatore AN. Psychosocial and behavioral aspects of bariatric surgery. Obes Res. 2005;13:639-648.
7. Mitchell JE, de Zwaan M. Bariatric surgery: a guide for mental health professionals. New York, NY: Routledge; 2005.
8. Malone M, Alger-Mayer SA. Medication use patterns after gastric bypass surgery for weight management. Ann Pharmacopher. 2005;39:637-642.
9. Buchwald H. and the Consensus Conference Panel. Consensus conference statement: bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Rel Dis. 2005;1:371-381.
10. Needleman BJ, Happel LC. Bariatric surgery: choosing the optimal procedure. Surg Clin North Am. 2008;88:991-1007.
11. Albrecht RJ, Pories WJ. Surgical intervention for the severely obese. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:149-172.
12. Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793-2796.
13. Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909-1917.
14. Brolin RE, Kenler HA, Gorman RC, et al. The dilemma of outcome assessment after operations for morbid obesity. Surgery. 1989;105:337-346.
15. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693.
16. Sjöström L, Narbro K, Sjöström CD, et al. and the Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752.
17. Bocchieri LE, Meana M, Fisher BL. A review of psychosocial outcomes of surgery for morbid obesity. J Psychosom Res. 2002;52:155-165.
18. Herpertz S, Kielmann R, Wolf AM, et al. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obes Res. 2004;12:1554-1569.
19. Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164:328-334.
20. Rosenberger PH, Henderson KE, Grilo CM. Psychiatric disorder comorbidity and association with eating disorders in bariatric surgery patients: a cross-sectional study using structured interview based diagnosis. J Clin Psychiatry. 2006;67:1080-1085.
21. Kalarchian MA, Marcus MD, Levine MD, et al. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surg Obes Relat Dis. 2008;4:544-549.
22. Rutledge T, Groesz LM, Savu M. Psychiatric factors and weight loss patterns following gastric bypass surgery in a veteran population. Obes Surg. 2009;[Epub ahead of print].
23. Kinzl JF, Schrattenecker M, Traweger C, et al. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg. 2006;16:1609-1614.
24. Bauchowitz AU, Gonder-Frederick LA, Olbrisch ME, et al. Psychosocial evaluation of bariatric surgery candidates: a survey of present practices. Psychosom Med. 2005;67(5):825-832.
25. Wadden TA, Sarwer DB, Womble LG, et al. Psychosocial aspects of obesity and obesity surgery. Surg Clin North Am. 2001;81:1001-1024.
26. Fabricatore AN, Crerand CE, Wadden TA, et al. How do mental health professionals evaluate candidates for bariatric surgery? Survey results. Obes Surg. 2006;16:567-573.
27. Sarwer DB, Cohn NI, Gibbons LM, et al. Psychiatric diagnoses and psychiatric treatment among bariatric surgery candidates. Obes Surg. 2004;14(9):1148-1156.
28. Larsen JK, Greenen R, van Ramshorst B, et al. Psychosocial functioning before and after laparoscopic adjustable gastric banding: a cross-sectional study. Obes Surg. 2003;13(4):629-636.
29. Clark MM, Balsiger BM, Sletten CD, et al. Psychosocial factors and 2-year outcome following bariatric surgery for weight loss. Obes Surg. 2003;13(5):739-745.
30. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
31. Macgreggor AMC, Boggs L. Drug distribution in obesity and following bariatric surgery: a literature review. Obes Surg. 1996;6:17-27.
32. Malone M. Altered drug disposition in obesity and after bariatric surgery. Nutr Clin Pract. 2003;18:131-135.
33. Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm. 2006;63:1852-1857.
34. Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552-1562.
35. Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311-318.
36. Hamad GG, Kozak GM, Wisner KL, et al. The effect of gastric bypass on SSRI pharmacokinetics and pharmacodynamics. Abstract presented at: American Society for Metabolic and Bariatric Surgery 25 Annual Meeting; June 15-20, 2008; Washington, DC.
37. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46:250-253.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/01/bariatric-procedures-managing-patients.html#comments
Bariatric surgery is the most effective treatment for obesity (defined as a body mass index [BMI] >30 kg/m2) and is recommended for extremely obese individuals (BMI >40 kg/m2) age >18.1,2 Most patients experience significant weight loss accompanied by improvements in mood, physical comorbidities, and quality of life (Box).3-8 Despite these favorable outcomes, several aspects of postoperative care—such as management of mental health issues—remain unclear. Bariatric surgery candidates show high rates of preoperative psychopathology, particularly depression and dysphoria. Little is known about how bariatric surgery affects absorption of psychiatric medications, leaving prescribing clinicians with minimal guidance when a postoperative patient reports changes in mood symptoms.
This article discusses the psychosocial status of bariatric surgery candidates and presents a rationale for increased medication monitoring after surgery.
Weight loss after bariatric surgery is associated with significant improvements in obesity-related comorbidities, including diabetes and cardiovascular disease, and decreased mortality.3,4
Many patients are able to reduce or discontinue many of their nonpsychiatric preoperative medications as their comorbid conditions improve.5 Symptoms of depression and anxiety, health-related quality of life, self-esteem, and body image often improve dramatically in the first year after surgery and endure for several years.6,7
Psychosocial improvements, however, may not translate into changes in psychotropic use. In a sample of 114 bariatric surgery patients, 43% were taking a selective serotonin reuptake inhibitor before surgery, 40% at 12 months postsurgery, and 31% at 24 months.8 These percentages do not account for patients who were taking other types of antidepressants.
Surgical treatment of obesity
The most common surgical procedures for weight loss are adjustable gastric banding (AGB) and Roux-en-Y gastric bypass (RYGB); each can be performed laparoscopically. With both procedures, food intake is restricted by creating a gastric pouch at the base of the esophagus. RYGB (Figure)9,10 also is thought to induce weight loss through selective malabsorption and favorable effects on gut peptides11,12 and currently is the procedure of choice in the United States.13
Figure Roux-en-Y: Bariatric procedure of choice
Source: References 9,10Bariatric surgery patients typically lose 25% to 35% of their initial body weight within 12 to 18 months of surgery.3,4 However, 20% to 30% of patients fail to achieve typical postoperative weight loss or regain large amounts of weight within a few years.14-16 Suboptimal results have been attributed to multiple factors, including problematic dietary intake, disordered eating, low levels of physical activity, preoperative psychopathology, and poor follow-up.6,17,18
Preop psychopathology
Twenty percent to 60% of extremely obese persons who pursue bariatric surgery have a psychiatric illness.6,7 In a study of 288 bariatric surgery candidates assessed with the Structured Clinical Interview for DSMIV, 38% received a current axis I diagnosis and 66% were given a lifetime diagnosis.19 In a separate study of 174 individuals seeking bariatric surgery, 24% met criteria for a current axis I or axis II disorder and 37% were found to have ≥1 lifetime diagnoses.20 The most common lifetime diagnoses were affective disorders (22%), anxiety disorders (16%), and eating disorders (14%).20
Psychopathology could negatively impact postoperative outcome. In an observational study, patients with a lifetime diagnosis of any axis I disorder—particularly mood and anxiety disorders—experienced less weight loss 6 months after RYGB compared with those who never received an axis I diagnosis.21 Bariatric surgery patients with ≥2 psychiatric diagnoses were more likely to stop losing weight or regain weight after 1 year compared with those with 1 or no diagnosis.22 Psychiatric illness also appears to impact longer term weight loss.23
Most bariatric surgery programs in the United States require a mental health evaluation as part of the patient selection process.24 These assessments may include evaluating a patient’s behavior patterns, motivation, expectations, and cognitive and emotional functioning, and performing psychological testing (see Related Resources). Psychiatric problems such as substance abuse, active psychosis, bulimia nervosa, and severe, uncontrolled depression1,9,25 are widely considered contraindications to bariatric surgery.24,26
Postsurgery considerations
At the time of bariatric surgery 16% to 40% of patients are receiving mental health treatment, which often includes antidepressants.27-29 Unfortunately, little is known about how medications interact with these surgical procedures. Dramatic changes in medication absorption may occur because of reduced gastrointestinal (GI) surface area. Rapid reduction in body weight and fat mass and postoperative complications also may impact the efficacy and tolerability of antidepressants.
Pharmacokinetics. Anatomic and physiologic changes with bariatric surgery may lead to changes in the pharmacokinetic (PK) parameters of certain medications, particularly after RYGB. PK studies typically are conducted by collecting a series of plasma samples at predetermined intervals after a patient takes a medication. The blood levels of the medication and its active metabolites are used to compute multiple PK parameters that illustrate drug absorption, distribution, and metabolism. Theoretically, a bariatric surgery patient may experience changes in the rate and/or extent of:
- medication absorption from the GI tract into systemic circulation
- distribution throughout the body as fat mass and total body water change after surgery
- drug metabolism.
The effects of bariatric surgery on medication PK appears to be drug-specific.30-33
The bypassed portion of the GI tract is the primary absorption site for most medications; therefore, the length of the Roux limb may affect the extent of drug absorption impairment. However, the duodenum wall is one of many locations of the cytochrome P450 (CYP) isoenzymes CYP3A4 and CYP3A5,34 which are the primary metabolic enzymes for drugs such as atorvastatin. Eliminating this portion of the bowel could increase rather than decrease bioavailability.35 Alterations in drug absorption also may result from changes in gastric emptying rate, reduced exposure to absorptive mucosal surfaces, and alterations in gastric pH that can impair drug dissolution and solubility.30 These changes could reduce medication bioavailability.33 The impact of such changes may differ according to the characteristics of the specific drug. It has been theorized that drugs that are intrinsically poorly absorbed, are highly lipophilic, and undergo enterohepatic circulation carry the highest risk of malabsorption.30 Antidepressants vary in the extent to which they demonstrate these characteristics. Progressive changes in the volume of distribution as weight is lost also could affect the blood levels of some antidepressants.
A series of small studies and case reports of PK changes in medications such as digoxin, oral contraceptives, cyclosporine, sulfisoxazole, and tacrolimus after jejunoileal bypass—an older, obsolete bariatric procedure—reveal variability in the surgery’s effect on PK parameters, although most reported reduced absorption. Data specific to RYGB consist of small studies and case series that show reduced absorption after surgery with significant variability among agents (see our bibliography of studies describing PK changes in nonpsychiatric medications after bariatric surgery). In a systematic literature review, Padwal et al found evidence for a decreased magnitude of absorption in 15 of 22 studies of jejunoileal bypass patients, 1 of 3 studies of gastric bypass/gastroplasty, and no studies examining biliopancreatic diversion.30
It is unclear if antidepressant absorption is impaired after RYGB because currently only 1 case report presents in-vivo data. Hamad et al describe an obese patient (BMI 46 kg/m2) taking sertraline, 100 mg/d, for depression.36 Researchers measured sertraline levels before and 1 month after RYGB, at which time the patient’s depression worsened. After surgery, sertraline maximum concentration was lower (14.4 ng/ml vs 41.6 ng/ml), trough concentration was lower (11.1 ng/ml vs 17.5 ng/ml), and time to maximum concentration was shorter (240 vs 300 minutes). This suggests that a shift in sertraline absorption after surgery may have contributed to the patient’s worsened mood symptoms.
An in-vitro study that simulated preand post-RYGB GI environments found that 12 of 22 psychotropic drugs tested dissolved differently between the models.37 Whereas the dissolved fractions of amitriptyline, fluoxetine, paroxetine, and sertraline were significantly less in the post-RYGB environment, bupropion dissolved to a greater extent in the pre-RYGB environment, and venlafaxine and citalopram were not different between the 2 conditions (Table).37 Although several limitations prevent translating these data into clinical recommendations, this study suggests that there may be significant variability among medications with regard to the implications of an altered GI environment.
Blouin RA, Bauer LA, Miller DD, et al. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575-580.
Blouin RA, Brouwer KL, Record KE, et al. Amikacin pharmacokinetics in morbidly obese patients undergoing gastric-bypass surgery. Clin Pharm. 1985;4:70-72.
Chenhsu RY, Wu Y, Katz D, et al. Dose-adjusted cyclosporine c2 in a patient with jejunoileal bypass as compared to seven other liver transplant recipients. Ther Drug Monit. 2003;25(6):665-670.
Fuller AK, Tingle D, DeVane CL, et al. Haloperidol pharmacokinetics following gastric bypass surgery. J Clin Psychopharmacol. 1986;6:376-378.
Garrett ER, Süverkrup RS, Eberst K, et al. Surgically affected sulfisoxazole pharmacokinetics in the morbidly obese. Biopharm Drug Dispos. 1981;2:329-365.
Gerson CD, Lowe EH, Lindenbaum J. Bioavailability of digoxin tablets in patients with gastrointestinal dysfunction. Am J Med. 1980;69:43-49.
Hamad GG, Kozak GM, Wisner KL, et al. The effect of gastric bypass on SSRI pharmacokinetics and pharmacodynamics. Abstract presented at: American Society for Metabolic and Bariatric Surgery 25th Annual Meeting; June 15-20, 2008; Washington, DC.
Kelley M, Jain A, Kashyap R, et al. Change in oral absorption of tacrolimus in a liver transplant recipient after reversal of jejunoileal bypass: case report. Transplant Proc. 2005; 37:3165-3167.
Knight GC, Macris MP, Peric M, et al. Cyclosporine A pharmacokinetics in a cardiac allograft recipient with a jejunoileal bypass. Transplant Proc. 1988;20:351-355.
Marcus FI, Quinn EJ, Horton H, et al. The effect of jejunoileal bypass on the pharmacokinetics of digoxin in man. Circulation. 1977;55:537-541.
Magee SR, Shih G, Hume A. Malabsorption of oral antibiotics in pregnancy after gastric bypass surgery. J Am Board Fam Med. 2007;20:310-313.
Marterre WF, Hariharan S, First MR, et al. Gastric bypass in morbidly obese kidney transplant recipients. Clin Transplant. 1996;10:414-419.
Prince RA, Pincheira JC, Mason EE, et al. Influence of bariatric surgery on erythromycin absorption. J Clin Pharmacol. 1984;24:523-527.
Rogers CC, Alloway RR, Alexander JW, et al. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281-291.
Shepherd MF, Rosborough TK, Schwartz ML. Heparin thrombophylaxis in gastric bypass surgery. Obes Surg. 2003;13(2):249-253.
Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311-318.
Victor A, Odlind V, Kral JG. Oral contraceptive absorption and sex hormone binding globulins in obese women: effects of jejunoileal bypass. Gastroenterol Clin North Am. 1987;16(3):483-491.
PK: pharmacokinetic
Table
Weights of dissolved portions of antidepressants before and after simulated RYGB
| Simulated pre-RYGB environment | Simulated post-RYGB environment | ||||
|---|---|---|---|---|---|
| Drug | Median weight of dissolved portion (mg) | Percentage* | Median weight of dissolved portion (mg) | Percentage* | P† |
| Amitriptyline, 75 mg/d | 80 | 28% | 60 | 21% | <.04 |
| Fluoxetine, 20 mg/d | 110 | 30% | 40 | 11% | <.04 |
| Paroxetine, 20 mg/d | 30 | 9% | 10 | 3% | <.04 |
| Sertraline, 100 mg/d | 50 | 16% | 30 | 10% | <.04 |
| Bupropion, 100 mg/d | 320 | 52% | 450 | 73% | <.05 |
| Venlafaxine, 75 mg/d | 180 | 59% | 180 | 59% | Not significant |
| Citalopram, 20 mg/d | 70 | 27% | 80 | 31% | Not significant |
| *Relative to original pill weight †Mann-Whitney U test RYGB: Roux-en-Y gastric bypass Source: Adapted from reference 37 | |||||
Altering antidepressant doses
Current PK data are insufficient to make clinical recommendations regarding appropriate postsurgical adjustment of dose or alternate dosage formulations (liquid, extended-release, etc.). However, based on theoretical considerations, Miller and Smith suggest that patients avoid extended-release preparations whenever possible after bariatric surgery, citing the rationale that decreased intestinal length and surface area leads to reduced absorption.33 No data are available to advise clinicians regarding the appropriateness of switching patients from extended-release products to immediate-release or liquid preparations following surgery.
Presently, increased medication monitoring may be the most appropriate clinical approach. If appropriate doses have little or no effect, consider the possibility of decreased medication absorption.33 Monitoring plasma levels of medications that have therapeutic ranges also is advisable.
Areas for future research
Before specific clinical recommendations for managing antidepressants following RYGB can be proposed, the extent to which the absorption, volume of distribution, drug metabolism, and other measures change after surgery need to be quantified. It is also unclear whether changes in medication absorption are subject to inter-patient variability, whether predictive characteristics can be identified, and whether any observed changes remain stable over time. Similarly, the extent to which variability in surgical procedures (eg, surgeon preference regarding remnant intestinal length) affects medication absorption is unknown. Data regarding medication absorption following AGB and other bariatric procedures also will be needed.
- American Society for Metabolic and Bariatric Surgery. Fact sheet: Metabolic and bariatric surgery. www.asbs.org/Newsite07/media/asmbs_fs_surgery.pdf.
- American Society for Metabolic and Bariatric Surgery. Suggestions for the pre-surgical psychological assessment of bariatric surgery candidates. www.asmbs.org/html/pdf/PsychPreSurgicalAssessment.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atorvastatin • Lipitor
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Cyclosporine • Sandimmune
- Digoxin • Lanoxin
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sulfisoxazole • Truxazole
- Tacrolimus • Prograf
- Venlafaxine • Effexor
Disclosures
Dr. Sarwer receives grant/research support from the National Institutes of Health, the American Society for Metabolic and Bariatric Surgery, and Ethicon Endo-Surgery, Inc., is consultant to Allergan, BAROnova, Inc., EnteroMedics, and Ethocon Endo-Surgery, Inc., and is on the board of directors of Surgical Review Corporation.
Dr. Roerig receives grant/research support from Eli Lilly and Company.
Drs. Faulconbridge, Steffen, and Mitchell report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/01/bariatric-procedures-managing-patients.html#comments
Bariatric surgery is the most effective treatment for obesity (defined as a body mass index [BMI] >30 kg/m2) and is recommended for extremely obese individuals (BMI >40 kg/m2) age >18.1,2 Most patients experience significant weight loss accompanied by improvements in mood, physical comorbidities, and quality of life (Box).3-8 Despite these favorable outcomes, several aspects of postoperative care—such as management of mental health issues—remain unclear. Bariatric surgery candidates show high rates of preoperative psychopathology, particularly depression and dysphoria. Little is known about how bariatric surgery affects absorption of psychiatric medications, leaving prescribing clinicians with minimal guidance when a postoperative patient reports changes in mood symptoms.
This article discusses the psychosocial status of bariatric surgery candidates and presents a rationale for increased medication monitoring after surgery.
Weight loss after bariatric surgery is associated with significant improvements in obesity-related comorbidities, including diabetes and cardiovascular disease, and decreased mortality.3,4
Many patients are able to reduce or discontinue many of their nonpsychiatric preoperative medications as their comorbid conditions improve.5 Symptoms of depression and anxiety, health-related quality of life, self-esteem, and body image often improve dramatically in the first year after surgery and endure for several years.6,7
Psychosocial improvements, however, may not translate into changes in psychotropic use. In a sample of 114 bariatric surgery patients, 43% were taking a selective serotonin reuptake inhibitor before surgery, 40% at 12 months postsurgery, and 31% at 24 months.8 These percentages do not account for patients who were taking other types of antidepressants.
Surgical treatment of obesity
The most common surgical procedures for weight loss are adjustable gastric banding (AGB) and Roux-en-Y gastric bypass (RYGB); each can be performed laparoscopically. With both procedures, food intake is restricted by creating a gastric pouch at the base of the esophagus. RYGB (Figure)9,10 also is thought to induce weight loss through selective malabsorption and favorable effects on gut peptides11,12 and currently is the procedure of choice in the United States.13
Figure Roux-en-Y: Bariatric procedure of choice
Source: References 9,10Bariatric surgery patients typically lose 25% to 35% of their initial body weight within 12 to 18 months of surgery.3,4 However, 20% to 30% of patients fail to achieve typical postoperative weight loss or regain large amounts of weight within a few years.14-16 Suboptimal results have been attributed to multiple factors, including problematic dietary intake, disordered eating, low levels of physical activity, preoperative psychopathology, and poor follow-up.6,17,18
Preop psychopathology
Twenty percent to 60% of extremely obese persons who pursue bariatric surgery have a psychiatric illness.6,7 In a study of 288 bariatric surgery candidates assessed with the Structured Clinical Interview for DSMIV, 38% received a current axis I diagnosis and 66% were given a lifetime diagnosis.19 In a separate study of 174 individuals seeking bariatric surgery, 24% met criteria for a current axis I or axis II disorder and 37% were found to have ≥1 lifetime diagnoses.20 The most common lifetime diagnoses were affective disorders (22%), anxiety disorders (16%), and eating disorders (14%).20
Psychopathology could negatively impact postoperative outcome. In an observational study, patients with a lifetime diagnosis of any axis I disorder—particularly mood and anxiety disorders—experienced less weight loss 6 months after RYGB compared with those who never received an axis I diagnosis.21 Bariatric surgery patients with ≥2 psychiatric diagnoses were more likely to stop losing weight or regain weight after 1 year compared with those with 1 or no diagnosis.22 Psychiatric illness also appears to impact longer term weight loss.23
Most bariatric surgery programs in the United States require a mental health evaluation as part of the patient selection process.24 These assessments may include evaluating a patient’s behavior patterns, motivation, expectations, and cognitive and emotional functioning, and performing psychological testing (see Related Resources). Psychiatric problems such as substance abuse, active psychosis, bulimia nervosa, and severe, uncontrolled depression1,9,25 are widely considered contraindications to bariatric surgery.24,26
Postsurgery considerations
At the time of bariatric surgery 16% to 40% of patients are receiving mental health treatment, which often includes antidepressants.27-29 Unfortunately, little is known about how medications interact with these surgical procedures. Dramatic changes in medication absorption may occur because of reduced gastrointestinal (GI) surface area. Rapid reduction in body weight and fat mass and postoperative complications also may impact the efficacy and tolerability of antidepressants.
Pharmacokinetics. Anatomic and physiologic changes with bariatric surgery may lead to changes in the pharmacokinetic (PK) parameters of certain medications, particularly after RYGB. PK studies typically are conducted by collecting a series of plasma samples at predetermined intervals after a patient takes a medication. The blood levels of the medication and its active metabolites are used to compute multiple PK parameters that illustrate drug absorption, distribution, and metabolism. Theoretically, a bariatric surgery patient may experience changes in the rate and/or extent of:
- medication absorption from the GI tract into systemic circulation
- distribution throughout the body as fat mass and total body water change after surgery
- drug metabolism.
The effects of bariatric surgery on medication PK appears to be drug-specific.30-33
The bypassed portion of the GI tract is the primary absorption site for most medications; therefore, the length of the Roux limb may affect the extent of drug absorption impairment. However, the duodenum wall is one of many locations of the cytochrome P450 (CYP) isoenzymes CYP3A4 and CYP3A5,34 which are the primary metabolic enzymes for drugs such as atorvastatin. Eliminating this portion of the bowel could increase rather than decrease bioavailability.35 Alterations in drug absorption also may result from changes in gastric emptying rate, reduced exposure to absorptive mucosal surfaces, and alterations in gastric pH that can impair drug dissolution and solubility.30 These changes could reduce medication bioavailability.33 The impact of such changes may differ according to the characteristics of the specific drug. It has been theorized that drugs that are intrinsically poorly absorbed, are highly lipophilic, and undergo enterohepatic circulation carry the highest risk of malabsorption.30 Antidepressants vary in the extent to which they demonstrate these characteristics. Progressive changes in the volume of distribution as weight is lost also could affect the blood levels of some antidepressants.
A series of small studies and case reports of PK changes in medications such as digoxin, oral contraceptives, cyclosporine, sulfisoxazole, and tacrolimus after jejunoileal bypass—an older, obsolete bariatric procedure—reveal variability in the surgery’s effect on PK parameters, although most reported reduced absorption. Data specific to RYGB consist of small studies and case series that show reduced absorption after surgery with significant variability among agents (see our bibliography of studies describing PK changes in nonpsychiatric medications after bariatric surgery). In a systematic literature review, Padwal et al found evidence for a decreased magnitude of absorption in 15 of 22 studies of jejunoileal bypass patients, 1 of 3 studies of gastric bypass/gastroplasty, and no studies examining biliopancreatic diversion.30
It is unclear if antidepressant absorption is impaired after RYGB because currently only 1 case report presents in-vivo data. Hamad et al describe an obese patient (BMI 46 kg/m2) taking sertraline, 100 mg/d, for depression.36 Researchers measured sertraline levels before and 1 month after RYGB, at which time the patient’s depression worsened. After surgery, sertraline maximum concentration was lower (14.4 ng/ml vs 41.6 ng/ml), trough concentration was lower (11.1 ng/ml vs 17.5 ng/ml), and time to maximum concentration was shorter (240 vs 300 minutes). This suggests that a shift in sertraline absorption after surgery may have contributed to the patient’s worsened mood symptoms.
An in-vitro study that simulated preand post-RYGB GI environments found that 12 of 22 psychotropic drugs tested dissolved differently between the models.37 Whereas the dissolved fractions of amitriptyline, fluoxetine, paroxetine, and sertraline were significantly less in the post-RYGB environment, bupropion dissolved to a greater extent in the pre-RYGB environment, and venlafaxine and citalopram were not different between the 2 conditions (Table).37 Although several limitations prevent translating these data into clinical recommendations, this study suggests that there may be significant variability among medications with regard to the implications of an altered GI environment.
Blouin RA, Bauer LA, Miller DD, et al. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575-580.
Blouin RA, Brouwer KL, Record KE, et al. Amikacin pharmacokinetics in morbidly obese patients undergoing gastric-bypass surgery. Clin Pharm. 1985;4:70-72.
Chenhsu RY, Wu Y, Katz D, et al. Dose-adjusted cyclosporine c2 in a patient with jejunoileal bypass as compared to seven other liver transplant recipients. Ther Drug Monit. 2003;25(6):665-670.
Fuller AK, Tingle D, DeVane CL, et al. Haloperidol pharmacokinetics following gastric bypass surgery. J Clin Psychopharmacol. 1986;6:376-378.
Garrett ER, Süverkrup RS, Eberst K, et al. Surgically affected sulfisoxazole pharmacokinetics in the morbidly obese. Biopharm Drug Dispos. 1981;2:329-365.
Gerson CD, Lowe EH, Lindenbaum J. Bioavailability of digoxin tablets in patients with gastrointestinal dysfunction. Am J Med. 1980;69:43-49.
Hamad GG, Kozak GM, Wisner KL, et al. The effect of gastric bypass on SSRI pharmacokinetics and pharmacodynamics. Abstract presented at: American Society for Metabolic and Bariatric Surgery 25th Annual Meeting; June 15-20, 2008; Washington, DC.
Kelley M, Jain A, Kashyap R, et al. Change in oral absorption of tacrolimus in a liver transplant recipient after reversal of jejunoileal bypass: case report. Transplant Proc. 2005; 37:3165-3167.
Knight GC, Macris MP, Peric M, et al. Cyclosporine A pharmacokinetics in a cardiac allograft recipient with a jejunoileal bypass. Transplant Proc. 1988;20:351-355.
Marcus FI, Quinn EJ, Horton H, et al. The effect of jejunoileal bypass on the pharmacokinetics of digoxin in man. Circulation. 1977;55:537-541.
Magee SR, Shih G, Hume A. Malabsorption of oral antibiotics in pregnancy after gastric bypass surgery. J Am Board Fam Med. 2007;20:310-313.
Marterre WF, Hariharan S, First MR, et al. Gastric bypass in morbidly obese kidney transplant recipients. Clin Transplant. 1996;10:414-419.
Prince RA, Pincheira JC, Mason EE, et al. Influence of bariatric surgery on erythromycin absorption. J Clin Pharmacol. 1984;24:523-527.
Rogers CC, Alloway RR, Alexander JW, et al. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281-291.
Shepherd MF, Rosborough TK, Schwartz ML. Heparin thrombophylaxis in gastric bypass surgery. Obes Surg. 2003;13(2):249-253.
Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311-318.
Victor A, Odlind V, Kral JG. Oral contraceptive absorption and sex hormone binding globulins in obese women: effects of jejunoileal bypass. Gastroenterol Clin North Am. 1987;16(3):483-491.
PK: pharmacokinetic
Table
Weights of dissolved portions of antidepressants before and after simulated RYGB
| Simulated pre-RYGB environment | Simulated post-RYGB environment | ||||
|---|---|---|---|---|---|
| Drug | Median weight of dissolved portion (mg) | Percentage* | Median weight of dissolved portion (mg) | Percentage* | P† |
| Amitriptyline, 75 mg/d | 80 | 28% | 60 | 21% | <.04 |
| Fluoxetine, 20 mg/d | 110 | 30% | 40 | 11% | <.04 |
| Paroxetine, 20 mg/d | 30 | 9% | 10 | 3% | <.04 |
| Sertraline, 100 mg/d | 50 | 16% | 30 | 10% | <.04 |
| Bupropion, 100 mg/d | 320 | 52% | 450 | 73% | <.05 |
| Venlafaxine, 75 mg/d | 180 | 59% | 180 | 59% | Not significant |
| Citalopram, 20 mg/d | 70 | 27% | 80 | 31% | Not significant |
| *Relative to original pill weight †Mann-Whitney U test RYGB: Roux-en-Y gastric bypass Source: Adapted from reference 37 | |||||
Altering antidepressant doses
Current PK data are insufficient to make clinical recommendations regarding appropriate postsurgical adjustment of dose or alternate dosage formulations (liquid, extended-release, etc.). However, based on theoretical considerations, Miller and Smith suggest that patients avoid extended-release preparations whenever possible after bariatric surgery, citing the rationale that decreased intestinal length and surface area leads to reduced absorption.33 No data are available to advise clinicians regarding the appropriateness of switching patients from extended-release products to immediate-release or liquid preparations following surgery.
Presently, increased medication monitoring may be the most appropriate clinical approach. If appropriate doses have little or no effect, consider the possibility of decreased medication absorption.33 Monitoring plasma levels of medications that have therapeutic ranges also is advisable.
Areas for future research
Before specific clinical recommendations for managing antidepressants following RYGB can be proposed, the extent to which the absorption, volume of distribution, drug metabolism, and other measures change after surgery need to be quantified. It is also unclear whether changes in medication absorption are subject to inter-patient variability, whether predictive characteristics can be identified, and whether any observed changes remain stable over time. Similarly, the extent to which variability in surgical procedures (eg, surgeon preference regarding remnant intestinal length) affects medication absorption is unknown. Data regarding medication absorption following AGB and other bariatric procedures also will be needed.
- American Society for Metabolic and Bariatric Surgery. Fact sheet: Metabolic and bariatric surgery. www.asbs.org/Newsite07/media/asmbs_fs_surgery.pdf.
- American Society for Metabolic and Bariatric Surgery. Suggestions for the pre-surgical psychological assessment of bariatric surgery candidates. www.asmbs.org/html/pdf/PsychPreSurgicalAssessment.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atorvastatin • Lipitor
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Cyclosporine • Sandimmune
- Digoxin • Lanoxin
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sulfisoxazole • Truxazole
- Tacrolimus • Prograf
- Venlafaxine • Effexor
Disclosures
Dr. Sarwer receives grant/research support from the National Institutes of Health, the American Society for Metabolic and Bariatric Surgery, and Ethicon Endo-Surgery, Inc., is consultant to Allergan, BAROnova, Inc., EnteroMedics, and Ethocon Endo-Surgery, Inc., and is on the board of directors of Surgical Review Corporation.
Dr. Roerig receives grant/research support from Eli Lilly and Company.
Drs. Faulconbridge, Steffen, and Mitchell report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic and Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318-336.
2. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956-961.
3. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
4. Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559.
5. Hodo DM, Waller JL, Martindale RG, et al. Medication use after bariatric surgery in a managed care cohort. Surg Obes Rel Dis. 2008;4:601-607.
6. Sarwer DB, Wadden TA, Fabricatore AN. Psychosocial and behavioral aspects of bariatric surgery. Obes Res. 2005;13:639-648.
7. Mitchell JE, de Zwaan M. Bariatric surgery: a guide for mental health professionals. New York, NY: Routledge; 2005.
8. Malone M, Alger-Mayer SA. Medication use patterns after gastric bypass surgery for weight management. Ann Pharmacopher. 2005;39:637-642.
9. Buchwald H. and the Consensus Conference Panel. Consensus conference statement: bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Rel Dis. 2005;1:371-381.
10. Needleman BJ, Happel LC. Bariatric surgery: choosing the optimal procedure. Surg Clin North Am. 2008;88:991-1007.
11. Albrecht RJ, Pories WJ. Surgical intervention for the severely obese. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:149-172.
12. Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793-2796.
13. Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909-1917.
14. Brolin RE, Kenler HA, Gorman RC, et al. The dilemma of outcome assessment after operations for morbid obesity. Surgery. 1989;105:337-346.
15. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693.
16. Sjöström L, Narbro K, Sjöström CD, et al. and the Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752.
17. Bocchieri LE, Meana M, Fisher BL. A review of psychosocial outcomes of surgery for morbid obesity. J Psychosom Res. 2002;52:155-165.
18. Herpertz S, Kielmann R, Wolf AM, et al. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obes Res. 2004;12:1554-1569.
19. Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164:328-334.
20. Rosenberger PH, Henderson KE, Grilo CM. Psychiatric disorder comorbidity and association with eating disorders in bariatric surgery patients: a cross-sectional study using structured interview based diagnosis. J Clin Psychiatry. 2006;67:1080-1085.
21. Kalarchian MA, Marcus MD, Levine MD, et al. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surg Obes Relat Dis. 2008;4:544-549.
22. Rutledge T, Groesz LM, Savu M. Psychiatric factors and weight loss patterns following gastric bypass surgery in a veteran population. Obes Surg. 2009;[Epub ahead of print].
23. Kinzl JF, Schrattenecker M, Traweger C, et al. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg. 2006;16:1609-1614.
24. Bauchowitz AU, Gonder-Frederick LA, Olbrisch ME, et al. Psychosocial evaluation of bariatric surgery candidates: a survey of present practices. Psychosom Med. 2005;67(5):825-832.
25. Wadden TA, Sarwer DB, Womble LG, et al. Psychosocial aspects of obesity and obesity surgery. Surg Clin North Am. 2001;81:1001-1024.
26. Fabricatore AN, Crerand CE, Wadden TA, et al. How do mental health professionals evaluate candidates for bariatric surgery? Survey results. Obes Surg. 2006;16:567-573.
27. Sarwer DB, Cohn NI, Gibbons LM, et al. Psychiatric diagnoses and psychiatric treatment among bariatric surgery candidates. Obes Surg. 2004;14(9):1148-1156.
28. Larsen JK, Greenen R, van Ramshorst B, et al. Psychosocial functioning before and after laparoscopic adjustable gastric banding: a cross-sectional study. Obes Surg. 2003;13(4):629-636.
29. Clark MM, Balsiger BM, Sletten CD, et al. Psychosocial factors and 2-year outcome following bariatric surgery for weight loss. Obes Surg. 2003;13(5):739-745.
30. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
31. Macgreggor AMC, Boggs L. Drug distribution in obesity and following bariatric surgery: a literature review. Obes Surg. 1996;6:17-27.
32. Malone M. Altered drug disposition in obesity and after bariatric surgery. Nutr Clin Pract. 2003;18:131-135.
33. Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm. 2006;63:1852-1857.
34. Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552-1562.
35. Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311-318.
36. Hamad GG, Kozak GM, Wisner KL, et al. The effect of gastric bypass on SSRI pharmacokinetics and pharmacodynamics. Abstract presented at: American Society for Metabolic and Bariatric Surgery 25 Annual Meeting; June 15-20, 2008; Washington, DC.
37. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46:250-253.
1. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic and Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318-336.
2. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956-961.
3. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
4. Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559.
5. Hodo DM, Waller JL, Martindale RG, et al. Medication use after bariatric surgery in a managed care cohort. Surg Obes Rel Dis. 2008;4:601-607.
6. Sarwer DB, Wadden TA, Fabricatore AN. Psychosocial and behavioral aspects of bariatric surgery. Obes Res. 2005;13:639-648.
7. Mitchell JE, de Zwaan M. Bariatric surgery: a guide for mental health professionals. New York, NY: Routledge; 2005.
8. Malone M, Alger-Mayer SA. Medication use patterns after gastric bypass surgery for weight management. Ann Pharmacopher. 2005;39:637-642.
9. Buchwald H. and the Consensus Conference Panel. Consensus conference statement: bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Rel Dis. 2005;1:371-381.
10. Needleman BJ, Happel LC. Bariatric surgery: choosing the optimal procedure. Surg Clin North Am. 2008;88:991-1007.
11. Albrecht RJ, Pories WJ. Surgical intervention for the severely obese. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:149-172.
12. Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793-2796.
13. Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909-1917.
14. Brolin RE, Kenler HA, Gorman RC, et al. The dilemma of outcome assessment after operations for morbid obesity. Surgery. 1989;105:337-346.
15. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693.
16. Sjöström L, Narbro K, Sjöström CD, et al. and the Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752.
17. Bocchieri LE, Meana M, Fisher BL. A review of psychosocial outcomes of surgery for morbid obesity. J Psychosom Res. 2002;52:155-165.
18. Herpertz S, Kielmann R, Wolf AM, et al. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obes Res. 2004;12:1554-1569.
19. Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164:328-334.
20. Rosenberger PH, Henderson KE, Grilo CM. Psychiatric disorder comorbidity and association with eating disorders in bariatric surgery patients: a cross-sectional study using structured interview based diagnosis. J Clin Psychiatry. 2006;67:1080-1085.
21. Kalarchian MA, Marcus MD, Levine MD, et al. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surg Obes Relat Dis. 2008;4:544-549.
22. Rutledge T, Groesz LM, Savu M. Psychiatric factors and weight loss patterns following gastric bypass surgery in a veteran population. Obes Surg. 2009;[Epub ahead of print].
23. Kinzl JF, Schrattenecker M, Traweger C, et al. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg. 2006;16:1609-1614.
24. Bauchowitz AU, Gonder-Frederick LA, Olbrisch ME, et al. Psychosocial evaluation of bariatric surgery candidates: a survey of present practices. Psychosom Med. 2005;67(5):825-832.
25. Wadden TA, Sarwer DB, Womble LG, et al. Psychosocial aspects of obesity and obesity surgery. Surg Clin North Am. 2001;81:1001-1024.
26. Fabricatore AN, Crerand CE, Wadden TA, et al. How do mental health professionals evaluate candidates for bariatric surgery? Survey results. Obes Surg. 2006;16:567-573.
27. Sarwer DB, Cohn NI, Gibbons LM, et al. Psychiatric diagnoses and psychiatric treatment among bariatric surgery candidates. Obes Surg. 2004;14(9):1148-1156.
28. Larsen JK, Greenen R, van Ramshorst B, et al. Psychosocial functioning before and after laparoscopic adjustable gastric banding: a cross-sectional study. Obes Surg. 2003;13(4):629-636.
29. Clark MM, Balsiger BM, Sletten CD, et al. Psychosocial factors and 2-year outcome following bariatric surgery for weight loss. Obes Surg. 2003;13(5):739-745.
30. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
31. Macgreggor AMC, Boggs L. Drug distribution in obesity and following bariatric surgery: a literature review. Obes Surg. 1996;6:17-27.
32. Malone M. Altered drug disposition in obesity and after bariatric surgery. Nutr Clin Pract. 2003;18:131-135.
33. Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm. 2006;63:1852-1857.
34. Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552-1562.
35. Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311-318.
36. Hamad GG, Kozak GM, Wisner KL, et al. The effect of gastric bypass on SSRI pharmacokinetics and pharmacodynamics. Abstract presented at: American Society for Metabolic and Bariatric Surgery 25 Annual Meeting; June 15-20, 2008; Washington, DC.
37. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46:250-253.