User login
Basal cell carcinoma (BCC) is the most prevalent malignancy in white individuals and its incidence is rapidly increasing. Despite its low mortality rate, BCC can cause severe morbidity and remains a serious health problem with a high economic burden for health care systems. The incidence of BCC is higher in individuals who have red or blonde hair, light eye color, and/or Fitzpatrick skin types I and II. The risk for developing BCC also increases with age, and men are more frequently affected than women.1,2 Although several factors have been implicated in the etiology of this condition, such as exposure to ionizing radiation, trauma, chemical carcinogenesis, immunosuppression, predisposing syndromes, and host factors (eg, traits that affect susceptibility to disease),3-5 exposure to UV radiation is considered to be a major risk factor, with most BCCs presenting in sun-exposed areas of the body (eg, face, neck). Prolongate suberythrodermal UV doses, which do not burn the skin but cause erythema in the histological level, can lead to formation of pyrimidine dimers in the dermal and epidermal tissues and cause DNA mutation with potential carcinogenic effects. Due to a large number of outdoor occupations, it is likely that outdoor workers (OWs) with a history of UV exposure may develop BCCs with different features than those seen in indoor workers (IWs). However, there has been debate about the relevance of occupational UV exposure as a risk factor for BCC development.6,7 The aim of this study was to compare the clinical and histological features of BCCs in OWs versus IWs at a referral hospital in southern Spain.
Methods
Using the electronic pathology records at a referral hospital in southern Spain, we identified medical records between May 1, 2010, and May 1, 2011, of specimens containing the term skin in the specimen box and basal cell carcinoma in the diagnosis box. We excluded patients with a history of or concomitant squamous cell carcinoma. Reexcision of incompletely excised lesions; punch, shave or incisional biopsies; and palliative excisions also were excluded. The specimens were reviewed and classified according to the differentiation pattern of BCC (ie, nodular, superficial, morpheic, micronodular). Basal cell carcinomas with mixed features were classified according to the most predominant subtype.
We also gathered information regarding the patients’ work history (ie, any job held during their lifetime with a minimum duration of 6 months). Patients were asked about the type of work and start/end dates. In patients who performed OW, we evaluated hours per day and months as well as the type of clothing worn (eg, head covering, socks/stockings during work in the summer months).
Each patient was classified as an OW or IW based on his/her stated occupation. The OWs included those who performed all or most of their work (≥6 hours per day for at least 6 months) outdoors in direct sunlight. Most patients in this group included farmers and fishermen. Indoor workers were those who performed most of their work in an indoor environment (eg, shop, factory, office, hospital, library, bank, school, laboratory). Most patients in this group included mechanics and shop assistants. A small group of individuals could not be classified as OWs or IWs and therefore were excluded from the study. Individuals with a history of exposure to ionizing radiation, chemical carcinogenesis, immunosuppression, or predisposing syndromes also were excluded.
We included variables that could be considered independent risk factors for BCC, including age, sex, eye color, natural hair color, Fitzpatrick skin type, history of sunburns, and family history. All data were collected via a personal interview performed by a single dermatologist (H.H-E.) during the follow-up with the patients conducted after obtaining all medical records and contacting eligible patients; none of the patients were lost on follow-up.
The study was approved by the hospital’s ethics committee and written consent was obtained from all recruited patients for analyzing the data acquired and accessing the relevant diagnostic documents (eg, pathology reports).
The cohorts were compared by a χ2 test and Student t test, which were performed using the SPSS software version 15. Statistical significance was determined using α=.05, and all tests were 2-sided.
Results
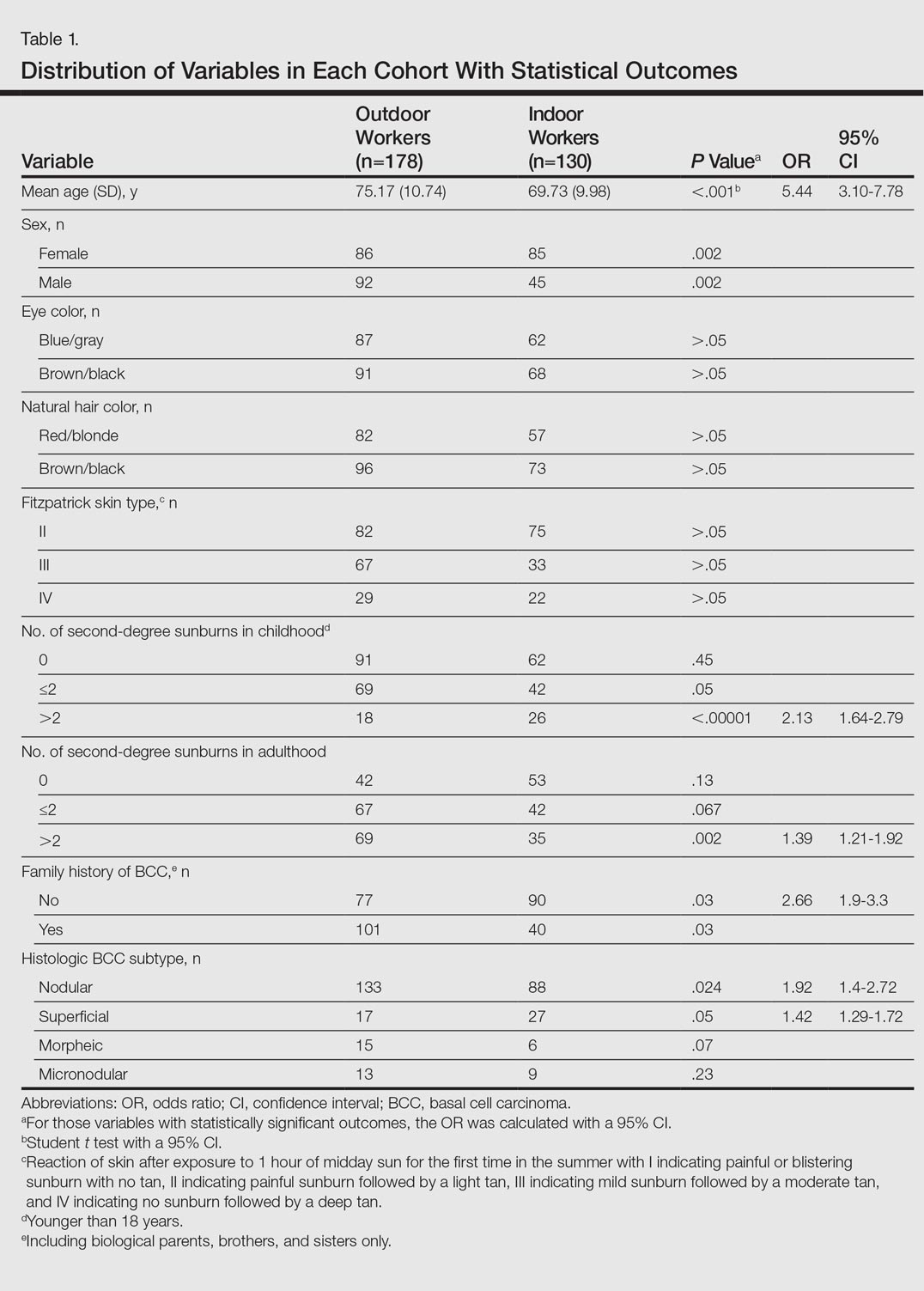
A total of 308 patients were included in the study, comprising 178 (58%) OWs and 130 (42%) IWs. Table 1 summarizes the characteristics of each cohort with the statistical outcomes.
The mean age (SD) of the OWs was significantly higher than the IWs (75.17 [10.74] vs 69.73 [9.98] years; P<.001). The sex distribution among the 2 cohorts was significantly different (P=.002); the OW group featured a slightly higher proportion of men than women (92 [52%] vs 86 [48%]), whereas women were clearly more prevalent in the IW group than men (85 [65%] vs 45 [35%]).
No significant differences regarding eye color (blue/gray vs brown/black) between the 2 cohorts were found (P>.05). In the same way, the 2 cohorts did not show differences in the natural hair color (red/blonde vs brown/black)(P>.05).
Fitzpatrick skin type II was the most common between both cohorts (82 [46%] OWs and 75 [58%] IWs), but no statistical differences regarding the proportions of each skin type were found (P>.05).
History of sunburns (>2 episodes) was significantly different between the 2 cohorts. The incidence of second-degree sunburns in childhood was higher in IWs (P<.00001), while the incidence of second-degree sunburns in adulthood was higher in OWs (P=.002).
Most OWs had a positive family history of BCC (101 [57%]), while the majority of IWs had a negative family history of BCC (90 [69%]). This difference was statistically significant (P=.03).
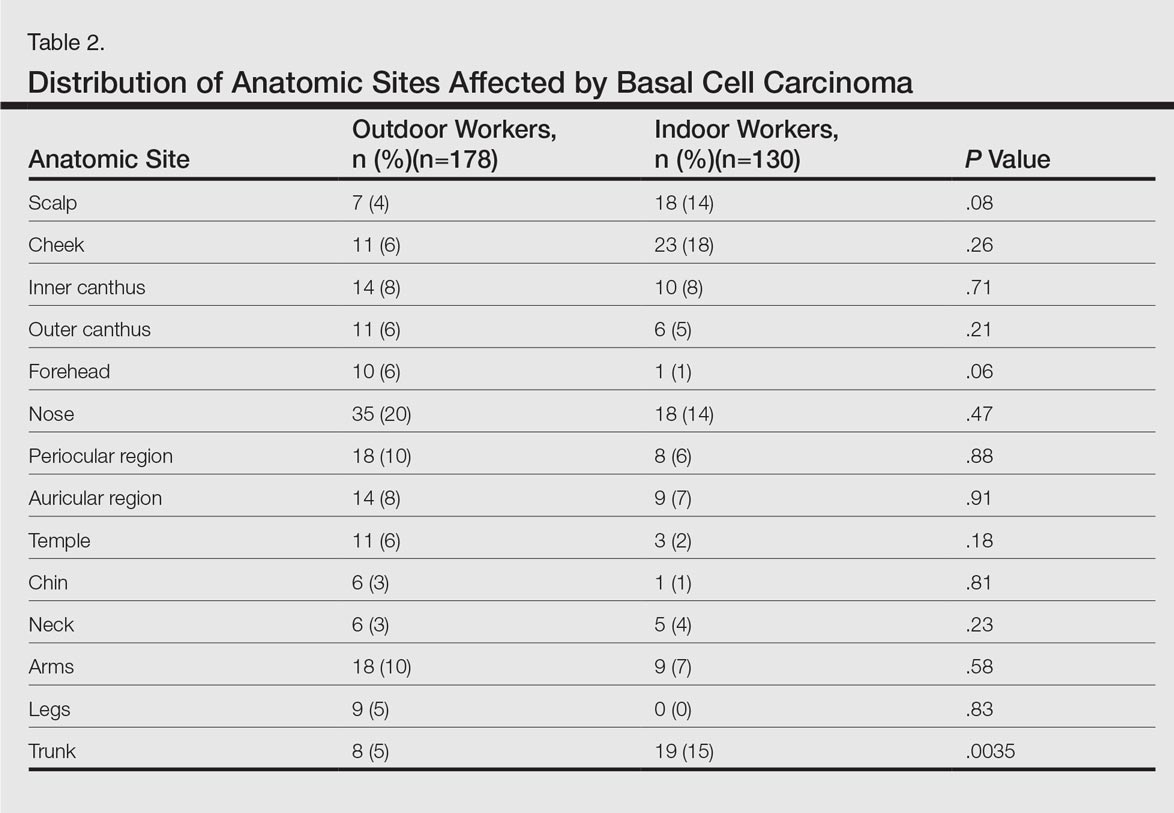
Table 2 shows the distribution of anatomic sites of BCCs in OWs and IWs. The nose was the most frequently affected area in OWs (35 cases [20%]), while the cheek was the most common location (23 [18%]) in IWs. Comparison of the frequency of BCC incidence for each anatomic location revealed that only the rate for truncal BCC was significantly different; IWs had a higher incidence of truncal BCCs than OWs (P=.0035). Although the differences between groups were not statistically significant, there was a trend toward a higher incidence of BCCs on the forehead in OW (P=.06).
In both cohorts, the most prevalent histologic subtype was nodular BCC (133 [75%] OWs and 88 [68%] IWs), followed by superficial BCC (17 [10%] OWs and 27 [21%] IWs). The incidence rate of nodular BCCs was statistically different between the 2 cohorts, with OWs showing a higher incidence compared to IWs (P=.024). Regarding the superficial subtype, the opposite was observed: IWs had significantly increased risk compared to OWs (P=.05). There was a trend toward a higher incidence of morpheic BCCs in OWs than IWs, but the difference was not statistically significant (P=.07).
Comment
Skin cancer due to occupational UV exposure is more common than is generally recognized,6,7 but occupational UV exposure as a risk factor for BCC is still an ongoing debate. In this study, we analyzed the different clinical and histological features of BCC in OWs versus IWs.
The geographic area where this study was performed is characterized by a subtropical Mediterranean climate with irregular rainfall; a short, cool to mild winter; and long, dry, hot summers. Summer temperatures usually are hot and regularly exceed 35°C (95°F). UV index (UVI) is a measure of the amount of skin-damaging UV radiation expected to reach the earth’s surface when the sun is highest in the sky (around midday) and ranges from 1 (low risk) to 10 (maximum risk). In southern Spain, the mean UVI is approximately 6 and can reach up to 9 or sometimes 10 in the summer months. Although Fitzpatrick skin types II and III are most common, the elevated UVI indicates that the general population in southern Spain is at a high risk for developing skin cancer.
In our study the mean age of IWs was lower than OWs, which suggests that IWs may develop BCC at a younger age than OWs. This finding is consistent with studies showing that cumulative occupational UV exposure has been associated with development of BCCs in older age groups, while acute intermittent recreational sun exposure, particularly sustained in childhood and adolescence, is linked with BCC in younger patients.6
The role of sex as a risk factor for BCC remains unclear. Some reports show that BCC is more common in men than in women.8-10 In our study, sex distribution was statistically significant (P=.002); there were more women in the IW cohort and more men in the OW cohort. These differences may be explained by cultural and lifestyle patterns, as women who are IWs tend to have office jobs in urban settings and wear modern fashion clothes at work and for recreation. In rural settings, women have agricultural jobs and tend to wear more traditional clothes that offer sun protection.
Positive family history has been suggested to be a constitutional risk factor for BCC development.8,11,12 In our study, we observed that positive family history was more common in OWs, while most IWs had a negative family history. These differences were significant (P=.03), and OWs had a 2.6-fold increased likelihood of having a positive family history of BCC compared to IWs. Cultural and lifestyle patterns may partially explain this finding. In rural settings, workers tend to have the same job as their parents as a traditional way of life and therefore have similar patterns of UV exposure; in urban settings, individuals may have different jobs than their parents and therefore the pattern of UV exposure may be different. However, a genetic predisposition for developing BCC cannot be excluded. In addition, we have to consider that the information on family history of BCC in the patients was self-reported and not validated, which may limit the results.
The difference in history of second-degree sunburn in childhood was significantly higher in IWs than in OWs (P<.00001). The OW group had a significant rate of sunburns in adulthood (P=.002). The relationship between UV radiation and BCC is complex, and the patterns of sun exposure and their occurrence in different periods of lifetime (ie, childhood vs adulthood) remain controversial.13 The overall history of severe sunburns seems to be more important than simply the tendency to burn or tan,14,15 and a history of sunburns in childhood and adolescence has been associated with early-onset BCC.6 Our findings were consistent in that the age of onset of BCCs was lower in IWs who had a history of sunburns in childhood. Basal cell carcinomas developed at older ages in OWs who had a higher incidence of sunburns in adulthood. However, we have to consider that the retrospective nature of the data collection on sunburns in childhood and adulthood was potentially limited, as the information was based on the patients’ memory. Additionally, other non-UV risk factors for BCC, such as ionizing radiation exposure, were not analyzed.
The majority of BCCs developed in sun-exposed areas of the head and neck in both cohorts, and only 35 (20%) and 28 (22%) BCCs were located on the trunk, arms, or legs in OWs and IWs, respectively. In our study, the rate of BCCs on the trunk was significantly lower in OWs than in IWs (P=.0035). Basal cell carcinomas on the trunk have been suggested to be linked to genetic susceptibility16,17 and reduced DNA repair capacity18 rather than sun exposure. Our findings support this hypothesis and suggest that occupational sun exposure has no direct relation with truncal BCC. This outcome is consistent with the result of a case-control study conducted by Pelucchi et al19 (N=1040). The authors concluded that occupational UV exposure was not associated with truncal BCC development but with head/neck BCC, indicating that there may be different etiological mechanisms between truncal and head/neck BCC.19 In the largest BCC case series published in the literature with 13,457 specimens, the authors stated that tumors on the trunk may represent a particular variant of BCC, in which the theory of chronic versus intermittent UV exposure cannot be simply extrapolated as it is for the rest of BCC sites. Other factors such as genetic predisposition could be involved in the development of truncal BCC.20 Similarly, Ramos et al21 suggested that nonmelanoma skin cancers in sun-protected anatomic sites may occur in individuals with impairment in the DNA repair process.
The classification of histological subtypes of BCC helps to predict tumor behavior,22 which can impact the prognosis. In our study, nodular BCC was the most common subtype in both cohorts, followed by superficial BCC. The nodular subtype was increased in OWs compared to IWs, while the superficial subtype was most common in IWs. Bastiaens et al23 and McCormack et al24 have suggested that the most frequent subtypes of BCC (nodular and superficial) may represent different tumors with distinct causal factors. According to these authors, nodular subtypes are associated with cumulative UV exposure, while superficial subtypes are associated with more intense and intermittent UV exposure. The results of the current study support this hypothesis, as the OW cohort with cumulative UV exposure showed more incidence of nodular BCC than IWs, while the patients with intense and intermittent sun exposure (the IWs) showed more risk of superficial BCC.
The importance of occupational UV exposure in OWs as a risk factor for BCC is still an ongoing discussion. Our data show that occupational UV exposure may be considered an etiological factor for BCC according to histological subtype and anatomic site. Our study is limited by the retrospective nature of the data collection regarding occupation and childhood sunburns, which were based on the patients’ memory and therefore potentially biased. Data regarding family history of BCC also was self-reported and not validated. Another limiting factor was that other non-UV risk factors for BCC, such as ionizing radiation exposure, were not considered. The limited sample size also may have impacted the study results. Among the strengths of the study are the complete response rate, the similar catchment area of OWs and IWs, the common hospital setting of the 2 cohorts, and the similar attention to medical history. All patients were obtained from the practice of a single referral dermatologist and are felt to be representative of our working area. The use of a single dermatologist reduces provider-associated variability.
Conclusion
According to the results of this study, OWs are more likely to develop nodular BCCs with no increased risk for superficial BCCs. The age of onset in OWs is older than in IWs. Some anatomical sites such as the trunk are more commonly affected in IWs. Truncal BCCs may have etiological factors other than UV exposure, such as a genetic predisposition. This study is useful to occupational safety representatives and physicians to stimulate the implementation of prevention strategies for this easily preventable malignancy and may encourage further research.
- de Vries E, van de Poll-Franse LV, Louwman WJ, et al. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6.
- Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004;113:e74-e94.
- Miller SJ. Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527-536.
- Dessinioti C, Tzannis K, Sypsa V, et al. Epidemiologic risk factors of basal cell carcinoma development and age at onset in a Southern European population from Greece. Exp Dermatol. 2011;20:622-626.
- Bauer A, Diepgen TL, Schmitt J. Is occupational solar UV-irradiation a relevant risk factor for basal cell carcinoma? a systematic review and meta-analysis of the epidemiologic literature. Br J Dermatol. 2011;165:612-625.
- Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149(suppl 66):50-52.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843-844.
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292-305.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. Br Med J. 2003;327:794-798.
- Dessinioti C, Antoniou C, Katsambas AD, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-468.
- Hunter DJ, Colditz GA, Stampfer MJ, et al. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13-23.
- Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354-358.
- Ramachandran S, Lear JT, Ramsay H, et al. Presentation with multiple cutaneous basal cell carcinomas: association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61-67.
- Wei Q, Matanoski GM, Farmer ER, et al. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614-1618.
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study [published online ahead of print Oct 19, 2006]. J Invest Dermatol. 2007;127:935-944.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Ramos J, Villa J, Ruiz A, et al. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006-2011.
- Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393-398.
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, et al. Differences in age, site distribution and sex between nodular and superficial basal cell carcinomas indicate different type of tumors. J Invest Dermatol. 1998;110:880-884.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of histological subtypes of basal cell carcinoma. a possible indicator of different causes. Arch Dermatol. 1997;133:593-596.
Basal cell carcinoma (BCC) is the most prevalent malignancy in white individuals and its incidence is rapidly increasing. Despite its low mortality rate, BCC can cause severe morbidity and remains a serious health problem with a high economic burden for health care systems. The incidence of BCC is higher in individuals who have red or blonde hair, light eye color, and/or Fitzpatrick skin types I and II. The risk for developing BCC also increases with age, and men are more frequently affected than women.1,2 Although several factors have been implicated in the etiology of this condition, such as exposure to ionizing radiation, trauma, chemical carcinogenesis, immunosuppression, predisposing syndromes, and host factors (eg, traits that affect susceptibility to disease),3-5 exposure to UV radiation is considered to be a major risk factor, with most BCCs presenting in sun-exposed areas of the body (eg, face, neck). Prolongate suberythrodermal UV doses, which do not burn the skin but cause erythema in the histological level, can lead to formation of pyrimidine dimers in the dermal and epidermal tissues and cause DNA mutation with potential carcinogenic effects. Due to a large number of outdoor occupations, it is likely that outdoor workers (OWs) with a history of UV exposure may develop BCCs with different features than those seen in indoor workers (IWs). However, there has been debate about the relevance of occupational UV exposure as a risk factor for BCC development.6,7 The aim of this study was to compare the clinical and histological features of BCCs in OWs versus IWs at a referral hospital in southern Spain.
Methods
Using the electronic pathology records at a referral hospital in southern Spain, we identified medical records between May 1, 2010, and May 1, 2011, of specimens containing the term skin in the specimen box and basal cell carcinoma in the diagnosis box. We excluded patients with a history of or concomitant squamous cell carcinoma. Reexcision of incompletely excised lesions; punch, shave or incisional biopsies; and palliative excisions also were excluded. The specimens were reviewed and classified according to the differentiation pattern of BCC (ie, nodular, superficial, morpheic, micronodular). Basal cell carcinomas with mixed features were classified according to the most predominant subtype.
We also gathered information regarding the patients’ work history (ie, any job held during their lifetime with a minimum duration of 6 months). Patients were asked about the type of work and start/end dates. In patients who performed OW, we evaluated hours per day and months as well as the type of clothing worn (eg, head covering, socks/stockings during work in the summer months).
Each patient was classified as an OW or IW based on his/her stated occupation. The OWs included those who performed all or most of their work (≥6 hours per day for at least 6 months) outdoors in direct sunlight. Most patients in this group included farmers and fishermen. Indoor workers were those who performed most of their work in an indoor environment (eg, shop, factory, office, hospital, library, bank, school, laboratory). Most patients in this group included mechanics and shop assistants. A small group of individuals could not be classified as OWs or IWs and therefore were excluded from the study. Individuals with a history of exposure to ionizing radiation, chemical carcinogenesis, immunosuppression, or predisposing syndromes also were excluded.
We included variables that could be considered independent risk factors for BCC, including age, sex, eye color, natural hair color, Fitzpatrick skin type, history of sunburns, and family history. All data were collected via a personal interview performed by a single dermatologist (H.H-E.) during the follow-up with the patients conducted after obtaining all medical records and contacting eligible patients; none of the patients were lost on follow-up.
The study was approved by the hospital’s ethics committee and written consent was obtained from all recruited patients for analyzing the data acquired and accessing the relevant diagnostic documents (eg, pathology reports).
The cohorts were compared by a χ2 test and Student t test, which were performed using the SPSS software version 15. Statistical significance was determined using α=.05, and all tests were 2-sided.
Results
A total of 308 patients were included in the study, comprising 178 (58%) OWs and 130 (42%) IWs. Table 1 summarizes the characteristics of each cohort with the statistical outcomes.
The mean age (SD) of the OWs was significantly higher than the IWs (75.17 [10.74] vs 69.73 [9.98] years; P<.001). The sex distribution among the 2 cohorts was significantly different (P=.002); the OW group featured a slightly higher proportion of men than women (92 [52%] vs 86 [48%]), whereas women were clearly more prevalent in the IW group than men (85 [65%] vs 45 [35%]).
No significant differences regarding eye color (blue/gray vs brown/black) between the 2 cohorts were found (P>.05). In the same way, the 2 cohorts did not show differences in the natural hair color (red/blonde vs brown/black)(P>.05).
Fitzpatrick skin type II was the most common between both cohorts (82 [46%] OWs and 75 [58%] IWs), but no statistical differences regarding the proportions of each skin type were found (P>.05).
History of sunburns (>2 episodes) was significantly different between the 2 cohorts. The incidence of second-degree sunburns in childhood was higher in IWs (P<.00001), while the incidence of second-degree sunburns in adulthood was higher in OWs (P=.002).
Most OWs had a positive family history of BCC (101 [57%]), while the majority of IWs had a negative family history of BCC (90 [69%]). This difference was statistically significant (P=.03).
Table 2 shows the distribution of anatomic sites of BCCs in OWs and IWs. The nose was the most frequently affected area in OWs (35 cases [20%]), while the cheek was the most common location (23 [18%]) in IWs. Comparison of the frequency of BCC incidence for each anatomic location revealed that only the rate for truncal BCC was significantly different; IWs had a higher incidence of truncal BCCs than OWs (P=.0035). Although the differences between groups were not statistically significant, there was a trend toward a higher incidence of BCCs on the forehead in OW (P=.06).
In both cohorts, the most prevalent histologic subtype was nodular BCC (133 [75%] OWs and 88 [68%] IWs), followed by superficial BCC (17 [10%] OWs and 27 [21%] IWs). The incidence rate of nodular BCCs was statistically different between the 2 cohorts, with OWs showing a higher incidence compared to IWs (P=.024). Regarding the superficial subtype, the opposite was observed: IWs had significantly increased risk compared to OWs (P=.05). There was a trend toward a higher incidence of morpheic BCCs in OWs than IWs, but the difference was not statistically significant (P=.07).
Comment
Skin cancer due to occupational UV exposure is more common than is generally recognized,6,7 but occupational UV exposure as a risk factor for BCC is still an ongoing debate. In this study, we analyzed the different clinical and histological features of BCC in OWs versus IWs.
The geographic area where this study was performed is characterized by a subtropical Mediterranean climate with irregular rainfall; a short, cool to mild winter; and long, dry, hot summers. Summer temperatures usually are hot and regularly exceed 35°C (95°F). UV index (UVI) is a measure of the amount of skin-damaging UV radiation expected to reach the earth’s surface when the sun is highest in the sky (around midday) and ranges from 1 (low risk) to 10 (maximum risk). In southern Spain, the mean UVI is approximately 6 and can reach up to 9 or sometimes 10 in the summer months. Although Fitzpatrick skin types II and III are most common, the elevated UVI indicates that the general population in southern Spain is at a high risk for developing skin cancer.
In our study the mean age of IWs was lower than OWs, which suggests that IWs may develop BCC at a younger age than OWs. This finding is consistent with studies showing that cumulative occupational UV exposure has been associated with development of BCCs in older age groups, while acute intermittent recreational sun exposure, particularly sustained in childhood and adolescence, is linked with BCC in younger patients.6
The role of sex as a risk factor for BCC remains unclear. Some reports show that BCC is more common in men than in women.8-10 In our study, sex distribution was statistically significant (P=.002); there were more women in the IW cohort and more men in the OW cohort. These differences may be explained by cultural and lifestyle patterns, as women who are IWs tend to have office jobs in urban settings and wear modern fashion clothes at work and for recreation. In rural settings, women have agricultural jobs and tend to wear more traditional clothes that offer sun protection.
Positive family history has been suggested to be a constitutional risk factor for BCC development.8,11,12 In our study, we observed that positive family history was more common in OWs, while most IWs had a negative family history. These differences were significant (P=.03), and OWs had a 2.6-fold increased likelihood of having a positive family history of BCC compared to IWs. Cultural and lifestyle patterns may partially explain this finding. In rural settings, workers tend to have the same job as their parents as a traditional way of life and therefore have similar patterns of UV exposure; in urban settings, individuals may have different jobs than their parents and therefore the pattern of UV exposure may be different. However, a genetic predisposition for developing BCC cannot be excluded. In addition, we have to consider that the information on family history of BCC in the patients was self-reported and not validated, which may limit the results.
The difference in history of second-degree sunburn in childhood was significantly higher in IWs than in OWs (P<.00001). The OW group had a significant rate of sunburns in adulthood (P=.002). The relationship between UV radiation and BCC is complex, and the patterns of sun exposure and their occurrence in different periods of lifetime (ie, childhood vs adulthood) remain controversial.13 The overall history of severe sunburns seems to be more important than simply the tendency to burn or tan,14,15 and a history of sunburns in childhood and adolescence has been associated with early-onset BCC.6 Our findings were consistent in that the age of onset of BCCs was lower in IWs who had a history of sunburns in childhood. Basal cell carcinomas developed at older ages in OWs who had a higher incidence of sunburns in adulthood. However, we have to consider that the retrospective nature of the data collection on sunburns in childhood and adulthood was potentially limited, as the information was based on the patients’ memory. Additionally, other non-UV risk factors for BCC, such as ionizing radiation exposure, were not analyzed.
The majority of BCCs developed in sun-exposed areas of the head and neck in both cohorts, and only 35 (20%) and 28 (22%) BCCs were located on the trunk, arms, or legs in OWs and IWs, respectively. In our study, the rate of BCCs on the trunk was significantly lower in OWs than in IWs (P=.0035). Basal cell carcinomas on the trunk have been suggested to be linked to genetic susceptibility16,17 and reduced DNA repair capacity18 rather than sun exposure. Our findings support this hypothesis and suggest that occupational sun exposure has no direct relation with truncal BCC. This outcome is consistent with the result of a case-control study conducted by Pelucchi et al19 (N=1040). The authors concluded that occupational UV exposure was not associated with truncal BCC development but with head/neck BCC, indicating that there may be different etiological mechanisms between truncal and head/neck BCC.19 In the largest BCC case series published in the literature with 13,457 specimens, the authors stated that tumors on the trunk may represent a particular variant of BCC, in which the theory of chronic versus intermittent UV exposure cannot be simply extrapolated as it is for the rest of BCC sites. Other factors such as genetic predisposition could be involved in the development of truncal BCC.20 Similarly, Ramos et al21 suggested that nonmelanoma skin cancers in sun-protected anatomic sites may occur in individuals with impairment in the DNA repair process.
The classification of histological subtypes of BCC helps to predict tumor behavior,22 which can impact the prognosis. In our study, nodular BCC was the most common subtype in both cohorts, followed by superficial BCC. The nodular subtype was increased in OWs compared to IWs, while the superficial subtype was most common in IWs. Bastiaens et al23 and McCormack et al24 have suggested that the most frequent subtypes of BCC (nodular and superficial) may represent different tumors with distinct causal factors. According to these authors, nodular subtypes are associated with cumulative UV exposure, while superficial subtypes are associated with more intense and intermittent UV exposure. The results of the current study support this hypothesis, as the OW cohort with cumulative UV exposure showed more incidence of nodular BCC than IWs, while the patients with intense and intermittent sun exposure (the IWs) showed more risk of superficial BCC.
The importance of occupational UV exposure in OWs as a risk factor for BCC is still an ongoing discussion. Our data show that occupational UV exposure may be considered an etiological factor for BCC according to histological subtype and anatomic site. Our study is limited by the retrospective nature of the data collection regarding occupation and childhood sunburns, which were based on the patients’ memory and therefore potentially biased. Data regarding family history of BCC also was self-reported and not validated. Another limiting factor was that other non-UV risk factors for BCC, such as ionizing radiation exposure, were not considered. The limited sample size also may have impacted the study results. Among the strengths of the study are the complete response rate, the similar catchment area of OWs and IWs, the common hospital setting of the 2 cohorts, and the similar attention to medical history. All patients were obtained from the practice of a single referral dermatologist and are felt to be representative of our working area. The use of a single dermatologist reduces provider-associated variability.
Conclusion
According to the results of this study, OWs are more likely to develop nodular BCCs with no increased risk for superficial BCCs. The age of onset in OWs is older than in IWs. Some anatomical sites such as the trunk are more commonly affected in IWs. Truncal BCCs may have etiological factors other than UV exposure, such as a genetic predisposition. This study is useful to occupational safety representatives and physicians to stimulate the implementation of prevention strategies for this easily preventable malignancy and may encourage further research.
Basal cell carcinoma (BCC) is the most prevalent malignancy in white individuals and its incidence is rapidly increasing. Despite its low mortality rate, BCC can cause severe morbidity and remains a serious health problem with a high economic burden for health care systems. The incidence of BCC is higher in individuals who have red or blonde hair, light eye color, and/or Fitzpatrick skin types I and II. The risk for developing BCC also increases with age, and men are more frequently affected than women.1,2 Although several factors have been implicated in the etiology of this condition, such as exposure to ionizing radiation, trauma, chemical carcinogenesis, immunosuppression, predisposing syndromes, and host factors (eg, traits that affect susceptibility to disease),3-5 exposure to UV radiation is considered to be a major risk factor, with most BCCs presenting in sun-exposed areas of the body (eg, face, neck). Prolongate suberythrodermal UV doses, which do not burn the skin but cause erythema in the histological level, can lead to formation of pyrimidine dimers in the dermal and epidermal tissues and cause DNA mutation with potential carcinogenic effects. Due to a large number of outdoor occupations, it is likely that outdoor workers (OWs) with a history of UV exposure may develop BCCs with different features than those seen in indoor workers (IWs). However, there has been debate about the relevance of occupational UV exposure as a risk factor for BCC development.6,7 The aim of this study was to compare the clinical and histological features of BCCs in OWs versus IWs at a referral hospital in southern Spain.
Methods
Using the electronic pathology records at a referral hospital in southern Spain, we identified medical records between May 1, 2010, and May 1, 2011, of specimens containing the term skin in the specimen box and basal cell carcinoma in the diagnosis box. We excluded patients with a history of or concomitant squamous cell carcinoma. Reexcision of incompletely excised lesions; punch, shave or incisional biopsies; and palliative excisions also were excluded. The specimens were reviewed and classified according to the differentiation pattern of BCC (ie, nodular, superficial, morpheic, micronodular). Basal cell carcinomas with mixed features were classified according to the most predominant subtype.
We also gathered information regarding the patients’ work history (ie, any job held during their lifetime with a minimum duration of 6 months). Patients were asked about the type of work and start/end dates. In patients who performed OW, we evaluated hours per day and months as well as the type of clothing worn (eg, head covering, socks/stockings during work in the summer months).
Each patient was classified as an OW or IW based on his/her stated occupation. The OWs included those who performed all or most of their work (≥6 hours per day for at least 6 months) outdoors in direct sunlight. Most patients in this group included farmers and fishermen. Indoor workers were those who performed most of their work in an indoor environment (eg, shop, factory, office, hospital, library, bank, school, laboratory). Most patients in this group included mechanics and shop assistants. A small group of individuals could not be classified as OWs or IWs and therefore were excluded from the study. Individuals with a history of exposure to ionizing radiation, chemical carcinogenesis, immunosuppression, or predisposing syndromes also were excluded.
We included variables that could be considered independent risk factors for BCC, including age, sex, eye color, natural hair color, Fitzpatrick skin type, history of sunburns, and family history. All data were collected via a personal interview performed by a single dermatologist (H.H-E.) during the follow-up with the patients conducted after obtaining all medical records and contacting eligible patients; none of the patients were lost on follow-up.
The study was approved by the hospital’s ethics committee and written consent was obtained from all recruited patients for analyzing the data acquired and accessing the relevant diagnostic documents (eg, pathology reports).
The cohorts were compared by a χ2 test and Student t test, which were performed using the SPSS software version 15. Statistical significance was determined using α=.05, and all tests were 2-sided.
Results
A total of 308 patients were included in the study, comprising 178 (58%) OWs and 130 (42%) IWs. Table 1 summarizes the characteristics of each cohort with the statistical outcomes.
The mean age (SD) of the OWs was significantly higher than the IWs (75.17 [10.74] vs 69.73 [9.98] years; P<.001). The sex distribution among the 2 cohorts was significantly different (P=.002); the OW group featured a slightly higher proportion of men than women (92 [52%] vs 86 [48%]), whereas women were clearly more prevalent in the IW group than men (85 [65%] vs 45 [35%]).
No significant differences regarding eye color (blue/gray vs brown/black) between the 2 cohorts were found (P>.05). In the same way, the 2 cohorts did not show differences in the natural hair color (red/blonde vs brown/black)(P>.05).
Fitzpatrick skin type II was the most common between both cohorts (82 [46%] OWs and 75 [58%] IWs), but no statistical differences regarding the proportions of each skin type were found (P>.05).
History of sunburns (>2 episodes) was significantly different between the 2 cohorts. The incidence of second-degree sunburns in childhood was higher in IWs (P<.00001), while the incidence of second-degree sunburns in adulthood was higher in OWs (P=.002).
Most OWs had a positive family history of BCC (101 [57%]), while the majority of IWs had a negative family history of BCC (90 [69%]). This difference was statistically significant (P=.03).
Table 2 shows the distribution of anatomic sites of BCCs in OWs and IWs. The nose was the most frequently affected area in OWs (35 cases [20%]), while the cheek was the most common location (23 [18%]) in IWs. Comparison of the frequency of BCC incidence for each anatomic location revealed that only the rate for truncal BCC was significantly different; IWs had a higher incidence of truncal BCCs than OWs (P=.0035). Although the differences between groups were not statistically significant, there was a trend toward a higher incidence of BCCs on the forehead in OW (P=.06).
In both cohorts, the most prevalent histologic subtype was nodular BCC (133 [75%] OWs and 88 [68%] IWs), followed by superficial BCC (17 [10%] OWs and 27 [21%] IWs). The incidence rate of nodular BCCs was statistically different between the 2 cohorts, with OWs showing a higher incidence compared to IWs (P=.024). Regarding the superficial subtype, the opposite was observed: IWs had significantly increased risk compared to OWs (P=.05). There was a trend toward a higher incidence of morpheic BCCs in OWs than IWs, but the difference was not statistically significant (P=.07).
Comment
Skin cancer due to occupational UV exposure is more common than is generally recognized,6,7 but occupational UV exposure as a risk factor for BCC is still an ongoing debate. In this study, we analyzed the different clinical and histological features of BCC in OWs versus IWs.
The geographic area where this study was performed is characterized by a subtropical Mediterranean climate with irregular rainfall; a short, cool to mild winter; and long, dry, hot summers. Summer temperatures usually are hot and regularly exceed 35°C (95°F). UV index (UVI) is a measure of the amount of skin-damaging UV radiation expected to reach the earth’s surface when the sun is highest in the sky (around midday) and ranges from 1 (low risk) to 10 (maximum risk). In southern Spain, the mean UVI is approximately 6 and can reach up to 9 or sometimes 10 in the summer months. Although Fitzpatrick skin types II and III are most common, the elevated UVI indicates that the general population in southern Spain is at a high risk for developing skin cancer.
In our study the mean age of IWs was lower than OWs, which suggests that IWs may develop BCC at a younger age than OWs. This finding is consistent with studies showing that cumulative occupational UV exposure has been associated with development of BCCs in older age groups, while acute intermittent recreational sun exposure, particularly sustained in childhood and adolescence, is linked with BCC in younger patients.6
The role of sex as a risk factor for BCC remains unclear. Some reports show that BCC is more common in men than in women.8-10 In our study, sex distribution was statistically significant (P=.002); there were more women in the IW cohort and more men in the OW cohort. These differences may be explained by cultural and lifestyle patterns, as women who are IWs tend to have office jobs in urban settings and wear modern fashion clothes at work and for recreation. In rural settings, women have agricultural jobs and tend to wear more traditional clothes that offer sun protection.
Positive family history has been suggested to be a constitutional risk factor for BCC development.8,11,12 In our study, we observed that positive family history was more common in OWs, while most IWs had a negative family history. These differences were significant (P=.03), and OWs had a 2.6-fold increased likelihood of having a positive family history of BCC compared to IWs. Cultural and lifestyle patterns may partially explain this finding. In rural settings, workers tend to have the same job as their parents as a traditional way of life and therefore have similar patterns of UV exposure; in urban settings, individuals may have different jobs than their parents and therefore the pattern of UV exposure may be different. However, a genetic predisposition for developing BCC cannot be excluded. In addition, we have to consider that the information on family history of BCC in the patients was self-reported and not validated, which may limit the results.
The difference in history of second-degree sunburn in childhood was significantly higher in IWs than in OWs (P<.00001). The OW group had a significant rate of sunburns in adulthood (P=.002). The relationship between UV radiation and BCC is complex, and the patterns of sun exposure and their occurrence in different periods of lifetime (ie, childhood vs adulthood) remain controversial.13 The overall history of severe sunburns seems to be more important than simply the tendency to burn or tan,14,15 and a history of sunburns in childhood and adolescence has been associated with early-onset BCC.6 Our findings were consistent in that the age of onset of BCCs was lower in IWs who had a history of sunburns in childhood. Basal cell carcinomas developed at older ages in OWs who had a higher incidence of sunburns in adulthood. However, we have to consider that the retrospective nature of the data collection on sunburns in childhood and adulthood was potentially limited, as the information was based on the patients’ memory. Additionally, other non-UV risk factors for BCC, such as ionizing radiation exposure, were not analyzed.
The majority of BCCs developed in sun-exposed areas of the head and neck in both cohorts, and only 35 (20%) and 28 (22%) BCCs were located on the trunk, arms, or legs in OWs and IWs, respectively. In our study, the rate of BCCs on the trunk was significantly lower in OWs than in IWs (P=.0035). Basal cell carcinomas on the trunk have been suggested to be linked to genetic susceptibility16,17 and reduced DNA repair capacity18 rather than sun exposure. Our findings support this hypothesis and suggest that occupational sun exposure has no direct relation with truncal BCC. This outcome is consistent with the result of a case-control study conducted by Pelucchi et al19 (N=1040). The authors concluded that occupational UV exposure was not associated with truncal BCC development but with head/neck BCC, indicating that there may be different etiological mechanisms between truncal and head/neck BCC.19 In the largest BCC case series published in the literature with 13,457 specimens, the authors stated that tumors on the trunk may represent a particular variant of BCC, in which the theory of chronic versus intermittent UV exposure cannot be simply extrapolated as it is for the rest of BCC sites. Other factors such as genetic predisposition could be involved in the development of truncal BCC.20 Similarly, Ramos et al21 suggested that nonmelanoma skin cancers in sun-protected anatomic sites may occur in individuals with impairment in the DNA repair process.
The classification of histological subtypes of BCC helps to predict tumor behavior,22 which can impact the prognosis. In our study, nodular BCC was the most common subtype in both cohorts, followed by superficial BCC. The nodular subtype was increased in OWs compared to IWs, while the superficial subtype was most common in IWs. Bastiaens et al23 and McCormack et al24 have suggested that the most frequent subtypes of BCC (nodular and superficial) may represent different tumors with distinct causal factors. According to these authors, nodular subtypes are associated with cumulative UV exposure, while superficial subtypes are associated with more intense and intermittent UV exposure. The results of the current study support this hypothesis, as the OW cohort with cumulative UV exposure showed more incidence of nodular BCC than IWs, while the patients with intense and intermittent sun exposure (the IWs) showed more risk of superficial BCC.
The importance of occupational UV exposure in OWs as a risk factor for BCC is still an ongoing discussion. Our data show that occupational UV exposure may be considered an etiological factor for BCC according to histological subtype and anatomic site. Our study is limited by the retrospective nature of the data collection regarding occupation and childhood sunburns, which were based on the patients’ memory and therefore potentially biased. Data regarding family history of BCC also was self-reported and not validated. Another limiting factor was that other non-UV risk factors for BCC, such as ionizing radiation exposure, were not considered. The limited sample size also may have impacted the study results. Among the strengths of the study are the complete response rate, the similar catchment area of OWs and IWs, the common hospital setting of the 2 cohorts, and the similar attention to medical history. All patients were obtained from the practice of a single referral dermatologist and are felt to be representative of our working area. The use of a single dermatologist reduces provider-associated variability.
Conclusion
According to the results of this study, OWs are more likely to develop nodular BCCs with no increased risk for superficial BCCs. The age of onset in OWs is older than in IWs. Some anatomical sites such as the trunk are more commonly affected in IWs. Truncal BCCs may have etiological factors other than UV exposure, such as a genetic predisposition. This study is useful to occupational safety representatives and physicians to stimulate the implementation of prevention strategies for this easily preventable malignancy and may encourage further research.
- de Vries E, van de Poll-Franse LV, Louwman WJ, et al. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6.
- Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004;113:e74-e94.
- Miller SJ. Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527-536.
- Dessinioti C, Tzannis K, Sypsa V, et al. Epidemiologic risk factors of basal cell carcinoma development and age at onset in a Southern European population from Greece. Exp Dermatol. 2011;20:622-626.
- Bauer A, Diepgen TL, Schmitt J. Is occupational solar UV-irradiation a relevant risk factor for basal cell carcinoma? a systematic review and meta-analysis of the epidemiologic literature. Br J Dermatol. 2011;165:612-625.
- Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149(suppl 66):50-52.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843-844.
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292-305.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. Br Med J. 2003;327:794-798.
- Dessinioti C, Antoniou C, Katsambas AD, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-468.
- Hunter DJ, Colditz GA, Stampfer MJ, et al. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13-23.
- Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354-358.
- Ramachandran S, Lear JT, Ramsay H, et al. Presentation with multiple cutaneous basal cell carcinomas: association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61-67.
- Wei Q, Matanoski GM, Farmer ER, et al. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614-1618.
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study [published online ahead of print Oct 19, 2006]. J Invest Dermatol. 2007;127:935-944.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Ramos J, Villa J, Ruiz A, et al. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006-2011.
- Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393-398.
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, et al. Differences in age, site distribution and sex between nodular and superficial basal cell carcinomas indicate different type of tumors. J Invest Dermatol. 1998;110:880-884.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of histological subtypes of basal cell carcinoma. a possible indicator of different causes. Arch Dermatol. 1997;133:593-596.
- de Vries E, van de Poll-Franse LV, Louwman WJ, et al. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6.
- Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004;113:e74-e94.
- Miller SJ. Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527-536.
- Dessinioti C, Tzannis K, Sypsa V, et al. Epidemiologic risk factors of basal cell carcinoma development and age at onset in a Southern European population from Greece. Exp Dermatol. 2011;20:622-626.
- Bauer A, Diepgen TL, Schmitt J. Is occupational solar UV-irradiation a relevant risk factor for basal cell carcinoma? a systematic review and meta-analysis of the epidemiologic literature. Br J Dermatol. 2011;165:612-625.
- Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149(suppl 66):50-52.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843-844.
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292-305.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. Br Med J. 2003;327:794-798.
- Dessinioti C, Antoniou C, Katsambas AD, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-468.
- Hunter DJ, Colditz GA, Stampfer MJ, et al. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13-23.
- Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354-358.
- Ramachandran S, Lear JT, Ramsay H, et al. Presentation with multiple cutaneous basal cell carcinomas: association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61-67.
- Wei Q, Matanoski GM, Farmer ER, et al. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614-1618.
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study [published online ahead of print Oct 19, 2006]. J Invest Dermatol. 2007;127:935-944.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Ramos J, Villa J, Ruiz A, et al. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006-2011.
- Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393-398.
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, et al. Differences in age, site distribution and sex between nodular and superficial basal cell carcinomas indicate different type of tumors. J Invest Dermatol. 1998;110:880-884.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of histological subtypes of basal cell carcinoma. a possible indicator of different causes. Arch Dermatol. 1997;133:593-596.
Practice Points
- Basal cell carcinoma (BCC) is the most common cancer in white individuals with rapidly increasing incidence rates and a high economic burden.
- Despite a large number of epidemiologic studies and the known importance of UV exposure in BCC carcinogenesis, there are no clear conclusions regarding the role of chronic and acute sun exposure related to BCC subtypes.
- It is reasonable to assume that outdoor workers with a history of UV exposure may develop BCCs with different features than those observed in indoor workers.